Discordant Humoral and T-Cell Response to mRNA SARS-CoV-2 Vaccine and the Risk of Breakthrough Infections in Women with Breast Cancer, Receiving Cyclin-Dependent Kinase 4 and 6 Inhibitors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
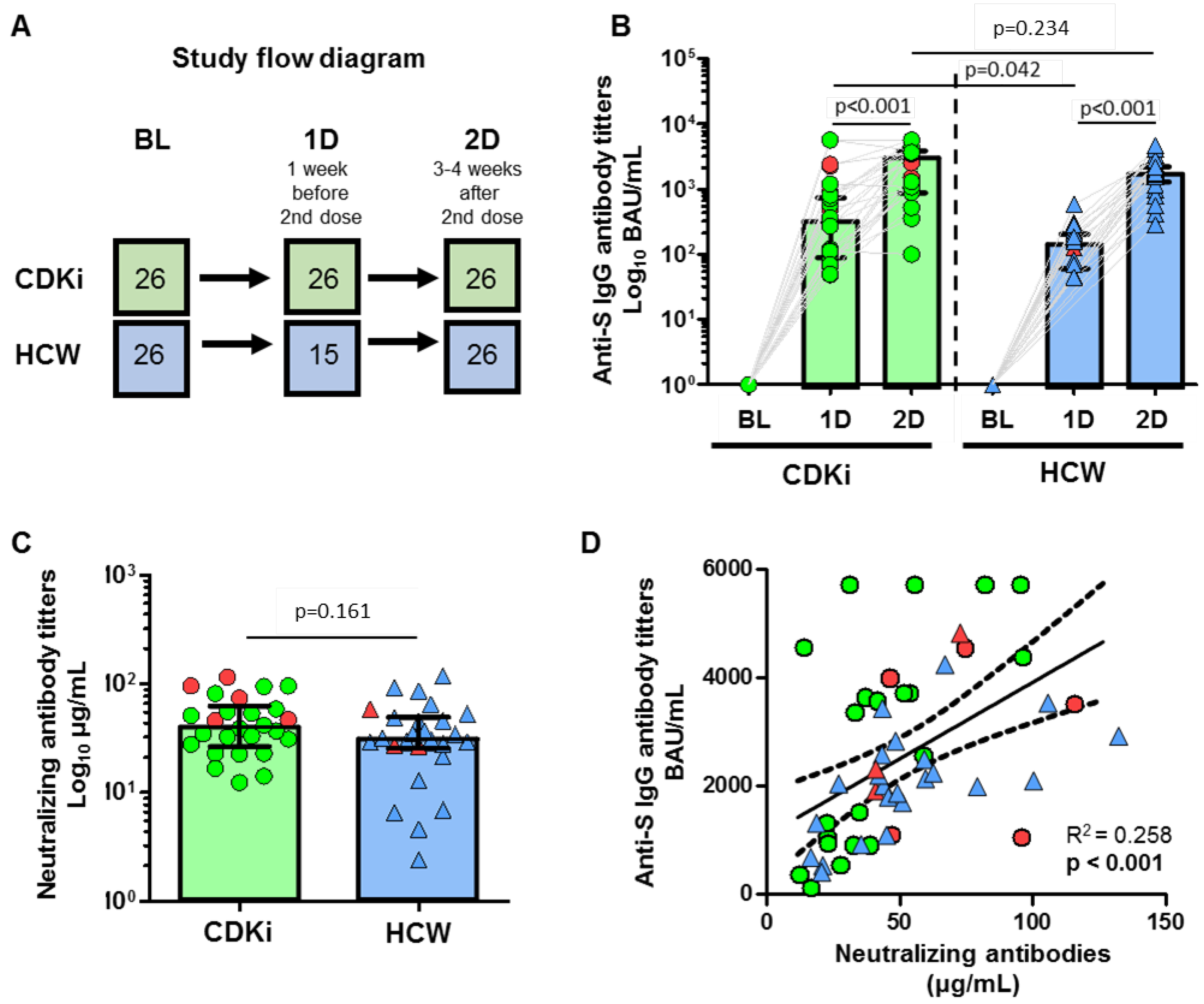
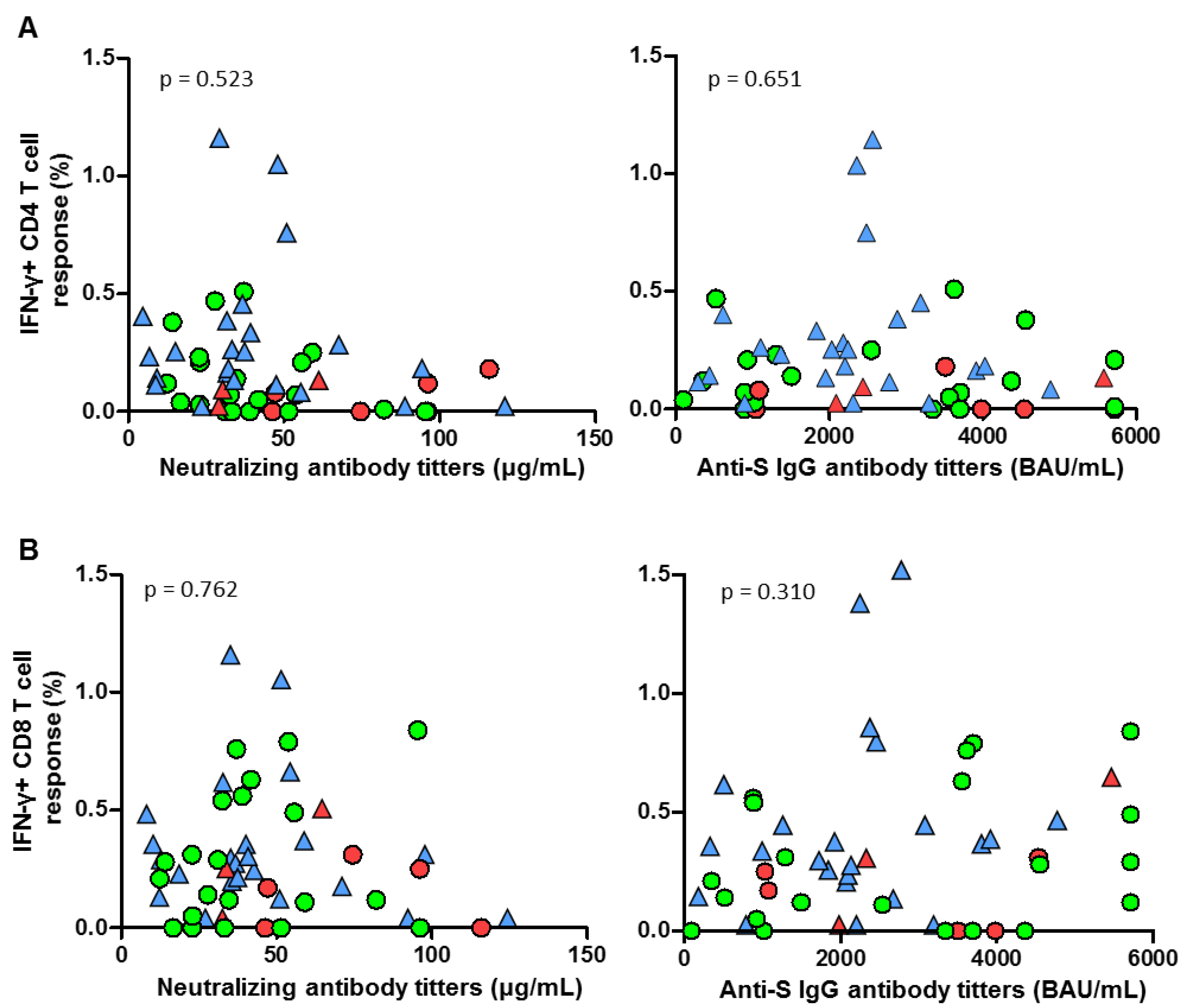
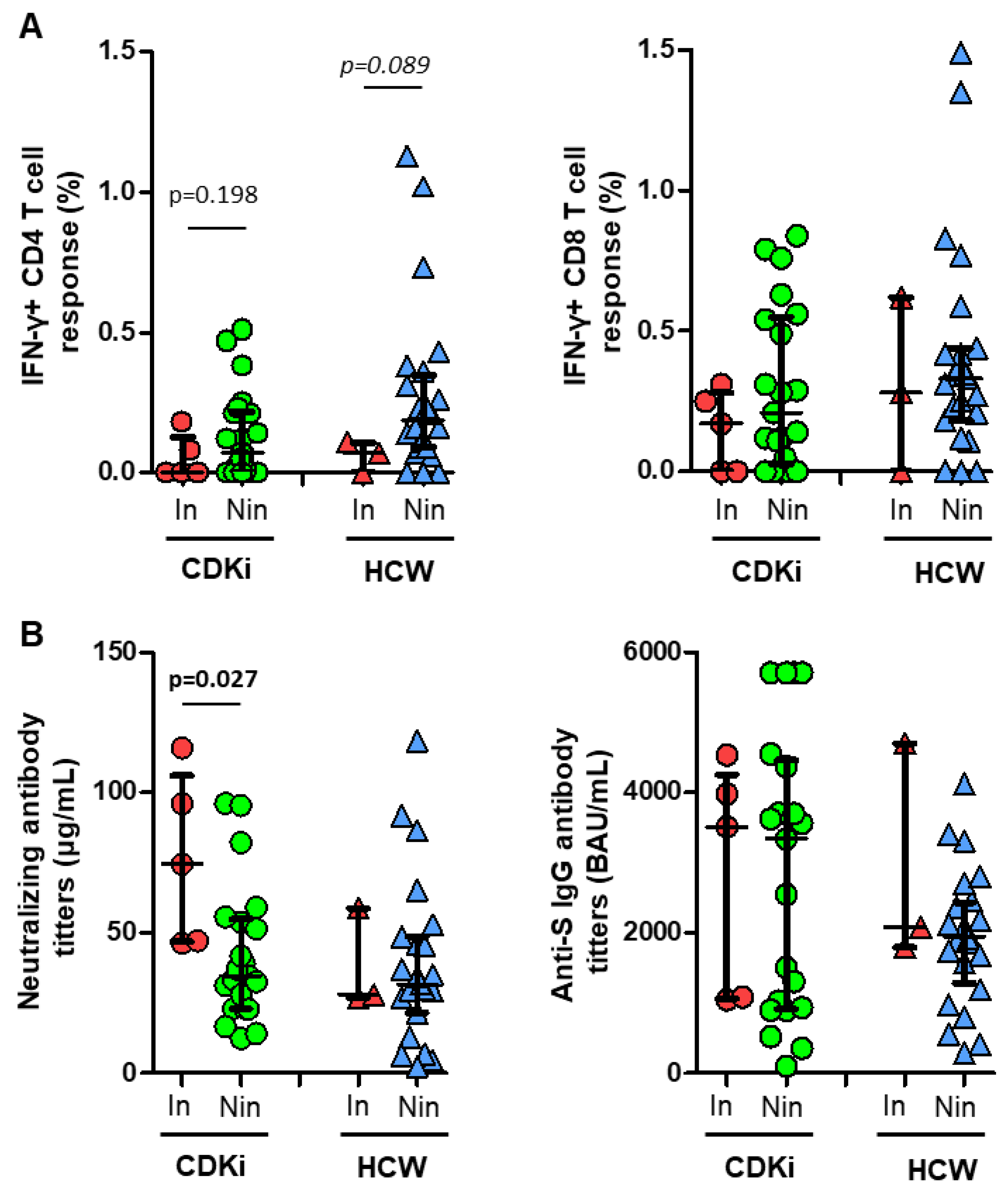
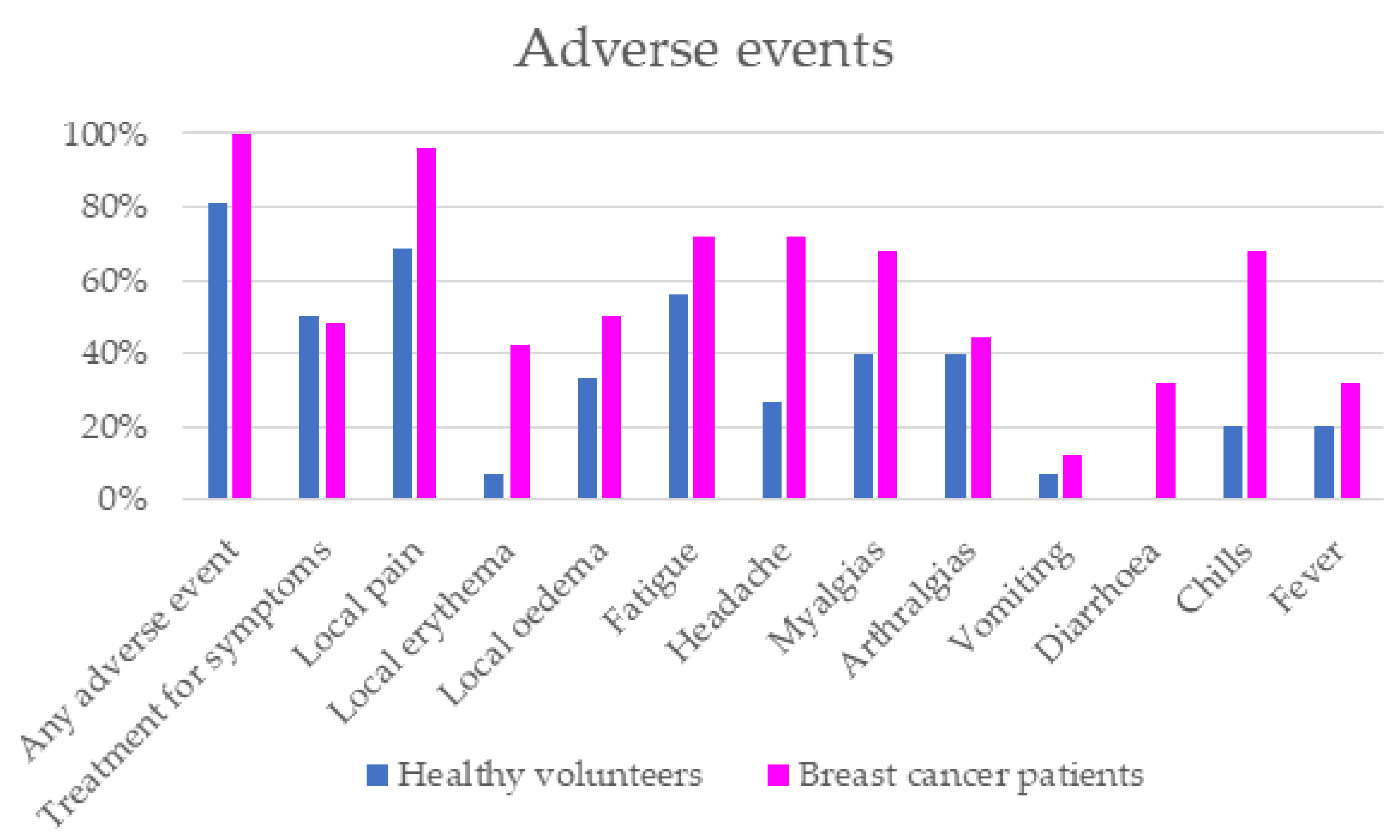
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDK4/6i | cyclin-dependent kinases 4/6 inhibitors |
| HCWs | healthcare workers |
| AEs | adverse events |
| BL | baseline |
| 1D | first vaccine dose |
| 2D | second vaccine dose |
| RUs | relative units |
| AUs | arbitrary units |
| BAUs | binding antibody units |
| INF | interferon |
| PBMC | peripheral blood mononuclear cell |
| PBS | phosphate-buffered saline |
| FBS | fetal bovine serum |
| DMSO | dimethyl sulfoxide |
| Rb | retinoblastoma |
References
- WHO COVID-19 Dashboard; World Health Organization: Geneva, Switzerland. 2020. Available online: https://covid19.who.int/ (accessed on 26 March 2023).
- Addeo, A.; Shah, P.K.; Bordry, N.; Hudson, R.D.; Albracht, B.; Di Marco, M.; Kaklamani, V.; Dietrich, P.-Y.; Taylor, B.S.; Simand, P.-F.; et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell 2021, 39, 1091–1098.e2. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.T.; Chalasani, P.; Wei, R.; Pennington, D.; Quirk, G.; Schoenle, M.V.; Peyton, K.L.; Uhrlaub, J.L.; Ripperger, T.J.; Jergović, M.; et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat. Med. 2021, 27, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- Barrière, J.; Chamorey, E.; Adjtoutah, Z.; Castelnau, O.; Mahamat, A.; Marco, S.; Petit, E.; Leysalle, A.; Raimondi, V.; Carles, M. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann. Oncol. 2021, 32, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Palich, R.; Veyri, M.; Marot, S.; Vozy, A.; Gligorov, J.; Maingon, P.; Marcelin, A.-G.; Spano, J.-P. Weak immunogenicity after a single dose of SARS-CoV-2 mRNA vaccine in treated cancer patients. Ann. Oncol. 2021, 32, 1051–1053. [Google Scholar] [CrossRef] [PubMed]
- Monin, L.; Laing, A.G.; Muñoz-Ruiz, M.; McKenzie, D.R.; del Molino del Barrio, I.; Alaguthurai, T.; Domingo-Vila, C.; Hayday, T.S.; Graham, C.; Seow, J.; et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: Interim analysis of a prospective observational study. Lancet Oncol. 2021, 22, 765–778. [Google Scholar] [CrossRef]
- Mansi, L.; Spehner, L.; Daguindau, E.; Bouiller, K.; Almotlak, H.; Stein, U.; Bouard, A.; Kim, S.; Klajer, E.; Jary, M.; et al. Study of the SARS-CoV-2-specific immune T-cell responses in COVID-19-positive cancer patients. Eur. J. Cancer 2021, 150, 1–9. [Google Scholar] [CrossRef]
- Schmidt, A.L.; Labaki, C.; Hsu, C.-Y.; Bakouny, Z.; Balanchivadze, N.; Berg, S.A.; Blau, S.; Daher, A.; El Zarif, T.; Friese, C.R.; et al. COVID-19 and Cancer Consortium. COVID-19 vaccination and breakthrough infections in patients with cancer. Ann Oncol. 2022, 33, 340–346. [Google Scholar] [CrossRef]
- Long, Q.-X.; Tang, X.-J.; Shi, Q.-L.; Li, Q.; Deng, H.-J.; Yuan, J.; Hu, J.-L.; Xu, W.; Zhang, Y.; Lv, F.-J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef]
- Burstein, H.J.; Somerfield, M.R.; Barton, D.L.; Dorris, A.; Fallowfield, L.J.; Jain, D.; Johnston, S.R.D.; Korde, L.A.; Litton, J.K.; Macrae, E.R.; et al. Endocrine Treatment and Targeted Therapy for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 3959–3977. [Google Scholar] [CrossRef]
- Spring, L.M.; Zangardi, M.L.; Moy, B.; Bardia, A. Clinical Management of Potential Toxicities and Drug Interactions Related to Cyclin-Dependent Kinase 4/6 Inhibitors in Breast Cancer: Practical Considerations and Recommendations. Oncologist 2017, 22, 1039–1048. [Google Scholar] [CrossRef]
- Petroni, G.; Formenti, S.C.; Chen-Kiang, S.; Galluzzi, L. Immunomodulation by anticancer cell cycle inhibitors. Nat. Rev. Immunol. 2020, 20, 669–679. [Google Scholar] [CrossRef]
- Deng, J.; Wang, E.S.; Jenkins, R.W.; Li, S.; Dries, R.; Yates, K.; Chhabra, S.; Huang, W.; Liu, H.; Aref, A.R.; et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov. 2018, 8, 216–233. [Google Scholar] [CrossRef]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- Saker, K.; Escuret, V.; Pitiot, V.; Massardier-Pilonchéry, A.; Paul, S.; Mokdad, B.; Langlois-Jacques, C.; Rabilloud, M.; Goncalves, D.; Fabien, N.; et al. Evaluation of Commercial Anti-SARS-CoV-2 Antibody Assays and Comparison of Standardized Titers in Vaccinated Health Care Workers. J. Clin. Microbiol. 2022, 60, e0174621. [Google Scholar] [CrossRef]
- Wu, F.; Wang, A.; Liu, M.; Wang, Q.; Chen, J.; Xia, S.; Ling, Y.; Zhang, Y.; Xun, J.; Lu, L.; et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv 2020. [Google Scholar] [CrossRef]
- Zagouri, F.; Terpos, E.; Fiste, O.; Liontos, M.; Briasoulis, A.; Katsiana, I.; Skafida, E.; Markellos, C.; Kunadis, E.; Andrikopoulou, A.; et al. SARS-CoV-2 neutralizing antibodies after first vaccination dose in breast cancer patients receiving CDK4/6 inhibitors. Breast 2021, 60, 58–61. [Google Scholar] [CrossRef]
- Hu, W.; Sung, T.; Jessen, B.A.; Thibault, S.; Finkelstein, M.B.; Khan, N.K.; Sacaan, A.I. Mechanistic Investigation of Bone Marrow Suppression Associated with Palbociclib and its Differentiation from Cytotoxic Chemotherapies. Clin. Cancer Res. 2016, 22, 2000–2008. [Google Scholar] [CrossRef]
- Kassem, L.; Shohdy, K.S.; Lasheen, S.; Abdel-Rahman, O.; Bachelot, T. Hematological adverse effects in breast cancer patients treated with cyclin-dependent kinase 4 and 6 inhibitors: A systematic review and meta-analysis. Breast Cancer 2018, 25, 17–27. [Google Scholar] [CrossRef]
- Massarweh, A.; Eliakim-Raz, N.; Stemmer, A.; Levy-Barda, A.; Yust-Katz, S.; Zer, A.; Benouaich-Amiel, A.; Ben-Zvi, H.; Moskovits, N.; Brenner, B.; et al. Evaluation of Seropositivity following BNT162b2 Messenger RNA Vaccination for SARS-CoV-2 in Patients Undergoing Treatment for Cancer. JAMA Oncol. 2021, 7, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Gallais, F.; Gantner, P.; Bruel, T.; Velay, A.; Planas, D.; Wendling, M.-J.; Bayer, S.; Solis, M.; Laugel, E.; Reix, N.; et al. Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection. EBioMedicine 2021, 71, 103561. [Google Scholar] [CrossRef]
- Lelliott, E.J.; Kong, I.Y.; Zethoven, M.; Ramsbottom, K.M.; Martelotto, L.G.; Meyran, D.; Zhu, J.J.; Costacurta, M.; Kirby, L.; Sandow, J.J.; et al. CDK4/6 Inhibition Promotes Antitumor Immunity through the Induction of T-cell Memory. Cancer Discov. 2021, 11, 2582–2601. [Google Scholar] [CrossRef] [PubMed]
- Heckler, M.; Ali, L.R.; Clancy-Thompson, E.; Qiang, L.; Ventre, K.S.; Lenehan, P.; Roehle, K.; Luoma, A.; Boelaars, K.; Peters, V.; et al. Inhibition of CDK4/6 Promotes CD8 T-cell Memory Formation. Cancer Discov. 2021, 11, 2564–2581. [Google Scholar] [CrossRef] [PubMed]
- Alidjinou, E.K.; Gaillot, O.; Guigon, A.; Tinez, C.; Lazrek, M.; Bocket, L.; Engelmann, I.; Hober, D. SARS-CoV-2 infection long time after full vaccination is related to a lack of neutralizing antibodies. Diagn. Microbiol. Infect. Dis. 2022, 102, 115565. [Google Scholar] [CrossRef] [PubMed]
- Haggenburg, S.; Lissenberg-Witte, B.I.; van Binnendijk, R.S.; den Hartog, G.; Bhoekhan, M.S.; Haverkate, N.J.E.; De Rooij, D.M.; van Meerloo, J.; Cloos, J.; Kootstra, N.A.; et al. Quantitative analysis of mRNA-1273 COVID-19 vaccination response in immunocompromised adult hematology patients. Blood Adv. 2022, 6, 1537–1546. [Google Scholar] [CrossRef]
- Barrière, J.; Carles, M.; Audigier-Valette, C.; Re, D.; Adjtoutah, Z.; Seitz-Polski, B.; Gounant, A.; Descamps, D.; Zalcman, C. Third dose of anti-SARS-CoV-2 vaccine for patients with cancer: Should humoral responses be monitored? A position article. Eur. J. Cancer 2022, 162, 182–193. [Google Scholar] [CrossRef]
- Loyal, L.; Braun, J.; Henze, L.; Kruse, B.; Dingeldey, M.; Reimer, U.; Kern, F.; Schwarz, T.; Mangold, M.; Unger, C.; et al. Cross-reactive CD4+ T cells enhance SARS-CoV-2 immune responses upon infection and vaccination. medRxiv 2021. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]

| CDK4/6 Inhibitors (n = 26) | Healthy Control Workers (n = 26) | p-Value | ||
|---|---|---|---|---|
| Age | 53.3 (29.1–77) | 48.6 (33.6–65.9) | 0.19 | |
| Female | 100% | 100% | 1 | |
| Stage IV | 96% | - | ||
| Comorbidities | ||||
| 27% | 8% | 0.14 | |
| 7.70% | 4% | 1 | |
| 15% | 24% | 0.499 | |
| 7.70% | 4% | 1 | |
| 0% | 8% | 0.235 | |
| 31% | 52% | 0.16 | |
| Anti-S IgG titers (BAU/mL) at BL | 0.85 (0–2.6) | 4.94 (3.79–6.01) | 0.001 | |
| Humoral immunogenicity (anti-S IgG, BAU/mL) | ||||
| 366 (111–806) 100% | 164 (70–236) 100% | 0.042 1 | |
| 3427 (1026–4372) 100% | 1946 (1573–2455) 100% | 0.2 1 | |
| Neutralizing antibodies 2D (IgG, µg/mL) | 40 (27–59) | 31 (26–48) | 0.158 | |
| Lymphocytes (%) at BL | 66.9 (50.9–77.2) | 63.4 (58.5–68.7) | 0.742 | |
| CD4 T cells (%) | 63.7 (56.4–69.4) | 64.3 (59.1–70.3) | 0.756 | |
| CD8 T cells (%) | 28.9 (20.7–34.3) | 23.1 (18.8–31.1) | 0.122 | |
| T-cell cross-reactivity at BL | ||||
| 23.10% | 30.80% | 0.755 | |
| 34.60% | 34.60% | 1 | |
| Lymphocytes (%) after 2D | 74.1 (64.4–78.2) | 67.9 (61.1–73.6) | 0.129 | |
| CD4 T cells (%) | 67.4 (62.7–73.9) | 66.7 (63.7–70.2) | 0.552 | |
| CD8 T cells (%) | 27.2 (21.3–32.1) | 28.1 (24.7–31.6) | 0.647 | |
| T-cell response after 2D | ||||
| Anti-S CD4 (%) | 0.12 (0–0.25) | 0.16 (0.09–0.31) | 0.269 | |
| Individuals with CD4 response | 69% | 85% | 0.324 | |
| Anti-S CD8 (%) | 0.155 (0–0.31) | 0.32 (0.18–0.44) | 0.083 | |
| Individuals with CD8 response | 69% | 85% | 0.324 | |
| CDK4/6 Inhibitors (n = 26) | Healthy Control Workers (n = 26) | p-Value | |
|---|---|---|---|
| Any adverse event | 100% | 81.25% | 0.049 |
| Treatment for symptoms | 48% | 50% | >0.05 |
| Local pain | 96.15% | 68.75% | 0.023 |
| Local erythema | 42.31% | 6.67% | 0.03 |
| Local oedema | 50% | 33.33% | >0.05 |
| Fatigue | 72% | 56.25% | >0.05 |
| Headache | 72% | 26.67% | 0.009 |
| Myalgias | 68% | 40% | >0.05 |
| Arthralgias | 44% | 40% | >0.05 |
| Vomiting | 12% | 6.67% | >0.05 |
| Diarrhea | 32% | - | - |
| Chills | 68% | 20% | 0.008 |
| Fever | 32% | 20% | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saavedra, C.; Vallejo, A.; Longo, F.; Serrano, J.J.; Fernández, M.; Gion, M.; López-Miranda, E.; Martínez-Jáñez, N.; Guerra, E.; Chamorro, J.; et al. Discordant Humoral and T-Cell Response to mRNA SARS-CoV-2 Vaccine and the Risk of Breakthrough Infections in Women with Breast Cancer, Receiving Cyclin-Dependent Kinase 4 and 6 Inhibitors. Cancers 2023, 15, 2000. https://doi.org/10.3390/cancers15072000
Saavedra C, Vallejo A, Longo F, Serrano JJ, Fernández M, Gion M, López-Miranda E, Martínez-Jáñez N, Guerra E, Chamorro J, et al. Discordant Humoral and T-Cell Response to mRNA SARS-CoV-2 Vaccine and the Risk of Breakthrough Infections in Women with Breast Cancer, Receiving Cyclin-Dependent Kinase 4 and 6 Inhibitors. Cancers. 2023; 15(7):2000. https://doi.org/10.3390/cancers15072000
Chicago/Turabian StyleSaavedra, Cristina, Alejandro Vallejo, Federico Longo, Juan José Serrano, María Fernández, María Gion, Elena López-Miranda, Noelia Martínez-Jáñez, Eva Guerra, Jesús Chamorro, and et al. 2023. "Discordant Humoral and T-Cell Response to mRNA SARS-CoV-2 Vaccine and the Risk of Breakthrough Infections in Women with Breast Cancer, Receiving Cyclin-Dependent Kinase 4 and 6 Inhibitors" Cancers 15, no. 7: 2000. https://doi.org/10.3390/cancers15072000
APA StyleSaavedra, C., Vallejo, A., Longo, F., Serrano, J. J., Fernández, M., Gion, M., López-Miranda, E., Martínez-Jáñez, N., Guerra, E., Chamorro, J., Rosero, D., Velasco, H., Martín, A., Carrato, A., Casado, J. L., & Cortés, A. (2023). Discordant Humoral and T-Cell Response to mRNA SARS-CoV-2 Vaccine and the Risk of Breakthrough Infections in Women with Breast Cancer, Receiving Cyclin-Dependent Kinase 4 and 6 Inhibitors. Cancers, 15(7), 2000. https://doi.org/10.3390/cancers15072000






