Pediatric Extracranial Germ Cell Tumors: Review of Clinics and Perspectives in Application of Autologous Stem Cell Transplantation
Abstract
Simple Summary
Abstract
1. Introduction
2. Pediatric Extracranial Germ Cell Tumor
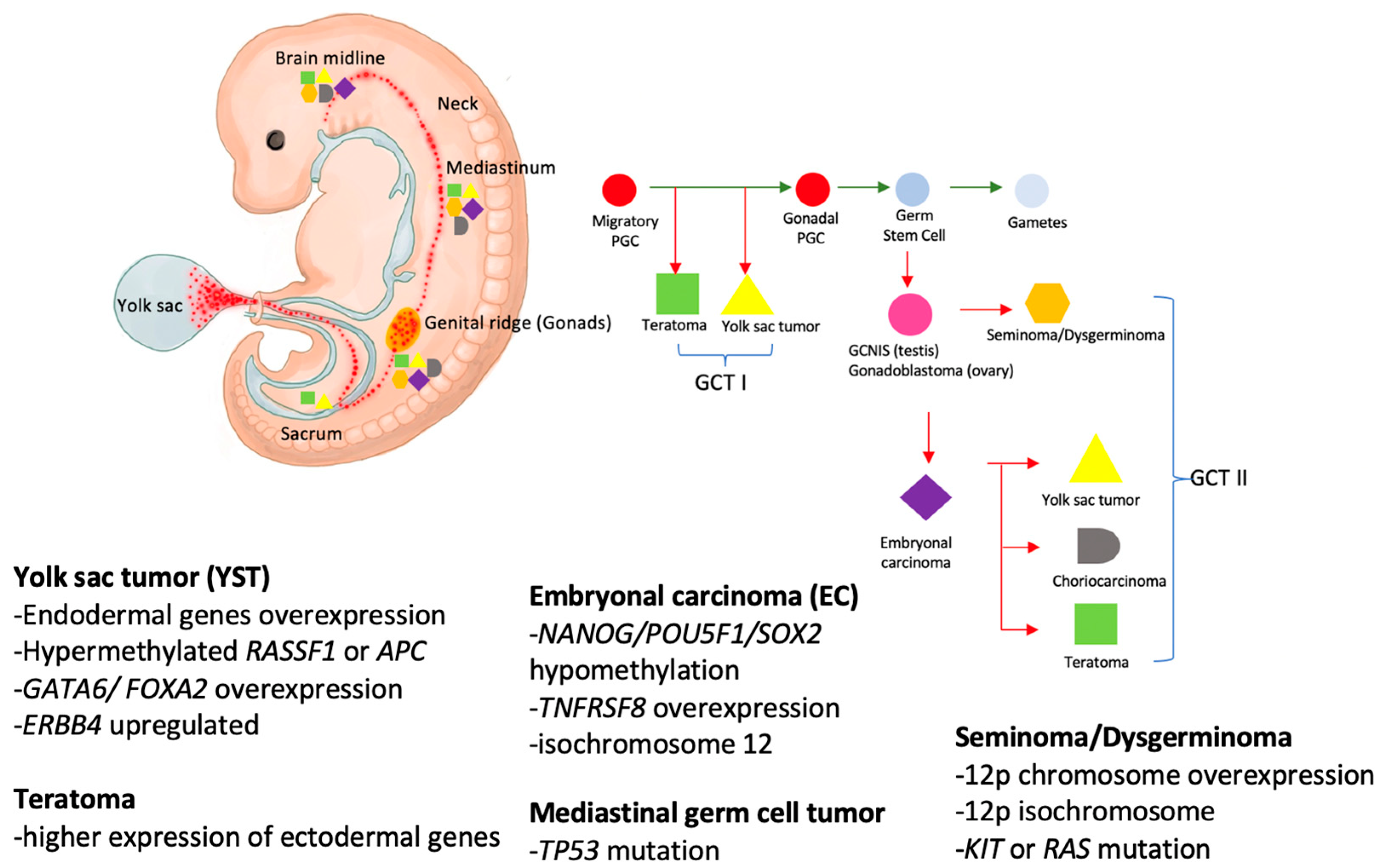
2.1. Prevalence and Embryogenesis
2.2. Genetics and Molecular Biology
2.3. Histology Classification and Staging System
2.3.1. Seminoma/Dysgerminoma
2.3.2. Teratoma/Immature Teratoma
2.3.3. Yolk Sac Tumor (YST)
2.3.4. Embryonal Carcinoma (EC)
2.3.5. Choriocarcinoma (CC)
2.3.6. Mixed Germ Cell Tumors
2.3.7. Staging System
2.4. Risk Classification
2.5. Treatment Overview
2.5.1. Surgical Treatment
2.5.2. Conventional Chemotherapy and Toxicity
2.5.3. Radiotherapy
2.5.4. Targeted-Therapy and Novel Therapy
3. High Dose Chemotherapy and Autologous Stem Cell Transplantation in Pediatric GCTS
3.1. Overview
3.2. Autologous Stem Cell Transplantation Studies in Adult Patients
3.3. Autologous Stem Cell Transplantation Studies in Pediatric Patients
3.4. Conditioning Regimen
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olson, T.A.; Murray, M.J.; Rodriguez-Galindo, C.; Nicholson, J.C.; Billmire, D.F.; Krailo, M.D.; Dang, H.M.; Amatruda, J.F.; Thornton, C.M.; Arul, G.S.; et al. Pediatric and Adolescent Extracranial Germ Cell Tumors: The Road to Collaboration. J. Clin. Oncol. 2015, 33, 3018–3028. [Google Scholar] [CrossRef] [PubMed]
- Poynter, J.N.; Amatruda, J.F.; Ross, J.A. Trends in incidence and survival of pediatric and adolescent patients with germ cell tumors in the United States, 1975 to 2006. Cancer 2010, 116, 4882–4891. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.Y.; Liu, H.C.; Yeh, T.C.; Sheu, J.C.; Chen, K.H.; Chang, C.Y.; Liang, D.C. Treatment Results of Extracranial Malignant Germ Cell Tumor with Regimens of Cisplatin, Vinblastine, Bleomycin or Carboplatin, Etoposide, and Bleomycin with Special Emphasis on the Sites of Vagina and Testis. Pediatr. Neonatol. 2015, 56, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Frazier, A.L.; Hale, J.P.; Rodriguez-Galindo, C.; Dang, H.; Olson, T.; Murray, M.J.; Amatruda, J.F.; Thornton, C.; Arul, G.S.; Billmire, D.; et al. Revised risk classification for pediatric extracranial germ cell tumors based on 25 years of clinical trial data from the United Kingdom and United States. J. Clin. Oncol. 2015, 33, 195–201. [Google Scholar] [CrossRef]
- Einhorn, L.H.; Williams, S.D.; Chamness, A.; Brames, M.J.; Perkins, S.M.; Abonour, R. High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumors. N. Engl. J. Med. 2007, 357, 340–348. [Google Scholar] [CrossRef]
- Liu, Y.L.; Lo, W.C.; Chiang, C.J.; Yang, Y.W.; Lu, M.Y.; Hsu, W.M.; Ho, W.L.; Li, M.J.; Miser, J.S.; Lin, D.T.; et al. Incidence of cancer in children aged 0–14 years in Taiwan, 1996–2010. Cancer Epidemiol. 2015, 39, 21–28. [Google Scholar] [CrossRef]
- Plant, A.S.; Chi, S.N.; Frazier, L. Pediatric malignant germ cell tumors: A comparison of the neuro-oncology and solid tumor experience. Pediatr. Blood Cancer 2016, 63, 2086–2095. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Arya, M.; Muneer, A.; Mushtaq, I.; Sebire, N.J. Testicular and paratesticular tumours in the prepubertal population. Lancet Oncol. 2010, 11, 476–483. [Google Scholar] [CrossRef]
- Znaor, A.; Skakkebaek, N.E.; Rajpert-De Meyts, E.; Kulis, T.; Laversanne, M.; Gurney, J.; Sarfati, D.; McGlynn, K.A.; Bray, F. Global patterns in testicular cancer incidence and mortality in 2020. Int. J. Cancer 2022, 151, 692–698. [Google Scholar] [CrossRef]
- Pettersson, A.; Richiardi, L.; Nordenskjold, A.; Kaijser, M.; Akre, O. Age at surgery for undescended testis and risk of testicular cancer. N. Engl. J. Med. 2007, 356, 1835–1841. [Google Scholar] [CrossRef]
- Akre, O.; Pettersson, A.; Richiardi, L. Risk of contralateral testicular cancer among men with unilaterally undescended testis: A meta analysis. Int. J. Cancer 2009, 124, 687–689. [Google Scholar] [CrossRef]
- De Backer, A.; Madern, G.C.; Oosterhuis, J.W.; Hakvoort-Cammel, F.G.; Hazebroek, F.W. Ovarian germ cell tumors in children: A clinical study of 66 patients. Pediatr. Blood Cancer 2006, 46, 459–464. [Google Scholar] [CrossRef]
- Hubbard, A.K.; Poynter, J.N. Global incidence comparisons and trends in ovarian germ cell tumors by geographic region in girls, adolescents and young women: 1988–2012. Gynecol. Oncol. 2019, 154, 608–615. [Google Scholar] [CrossRef]
- McIntyre, A.; Gilbert, D.; Goddard, N.; Looijenga, L.; Shipley, J. Genes, chromosomes and the development of testicular germ cell tumors of adolescents and adults. Genes Chromosomes Cancer 2008, 47, 547–557. [Google Scholar] [CrossRef]
- Oosterhuis, J.W.; Looijenga, L.H.J. Human germ cell tumours from a developmental perspective. Nat. Rev. Cancer 2019, 19, 522–537. [Google Scholar] [CrossRef]
- Williams, L.A.; Pankratz, N.; Lane, J.; Krailo, M.; Roesler, M.; Richardson, M.; Frazier, A.L.; Amatruda, J.F.; Poynter, J.N. Klinefelter syndrome in males with germ cell tumors: A report from the Children’s Oncology Group. Cancer 2018, 124, 3900–3908. [Google Scholar] [CrossRef]
- Pierce, J.L.; Frazier, A.L.; Amatruda, J.F. Pediatric Germ Cell Tumors: A Developmental Perspective. Adv. Urol. 2018, 2018, 9059382. [Google Scholar] [CrossRef]
- Shaikh, F.; Murray, M.J.; Amatruda, J.F.; Coleman, N.; Nicholson, J.C.; Hale, J.P.; Pashankar, F.; Stoneham, S.J.; Poynter, J.N.; Olson, T.A.; et al. Paediatric extracranial germ-cell tumours. Lancet Oncol. 2016, 17, e149–e162. [Google Scholar] [CrossRef]
- Stamatiou, K.; Papadopoulos, P.; Perlepes, G.; Galariotis, N.; Olympitis, M.; Moschouris, H.; Vasilakaki, T. Mixed germ cell tumor of the testicle with ravdomuosarcomatous component: A case report. Cases J. 2009, 2, 9299. [Google Scholar] [CrossRef]
- Bonouvrie, K.; van der Werff Ten Bosch, J.; van den Akker, M. Klinefelter syndrome and germ cell tumors: Review of the literature. Int. J. Pediatr. Endocrinol. 2020, 2020, 18. [Google Scholar] [CrossRef]
- Palmer, R.D.; Foster, N.A.; Vowler, S.L.; Roberts, I.; Thornton, C.M.; Hale, J.P.; Schneider, D.T.; Nicholson, J.C.; Coleman, N. Malignant germ cell tumours of childhood: New associations of genomic imbalance. Br. J. Cancer 2007, 96, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Seki, M.; Kawai, T.; Isobe, T.; Yoshida, M.; Sekiguchi, M.; Kimura, S.; Watanabe, K.; Sato-Otsubo, A.; Yoshida, K.; et al. Comprehensive genetic analysis of pediatric germ cell tumors identifies potential drug targets. Commun. Biol. 2020, 3, 544. [Google Scholar] [CrossRef] [PubMed]
- Ulbright, T.M. Germ cell tumors of the gonads: A selective review emphasizing problems in differential diagnosis, newly appreciated, and controversial issues. Mod. Pathol. 2005, 18 (Suppl. S2), S61–S79. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Devesa, S.S.; Sigurdson, A.J.; Brown, L.M.; Tsao, L.; Tarone, R.E. Trends in the incidence of testicular germ cell tumors in the United States. Cancer 2003, 97, 63–70. [Google Scholar] [CrossRef]
- Schrader, M.; Kempkensteffen, C.; Christoph, F.; Hinz, S.; Weikert, S.; Lein, M.; Krause, H.; Stephan, C.; Jung, K.; Hoepfner, M.; et al. Germ cell tumors of the gonads: A selective review emphasizing problems in drug resistance and current therapy options. Oncology 2009, 76, 77–84. [Google Scholar] [CrossRef]
- Matthew, R.; Christopher, O.; Philippa, S. Severe malignancy-associated hypercalcemia in dysgerminoma. Pediatr. Blood Cancer 2006, 47, 621–623. [Google Scholar] [CrossRef]
- Heifetz, S.A.; Cushing, B.; Giller, R.; Shuster, J.J.; Stolar, C.J.; Vinocur, C.D.; Hawkins, E.P. Immature teratomas in children: Pathologic considerations: A report from the combined Pediatric Oncology Group/Children’s Cancer Group. Am. J. Surg. Pathol. 1998, 22, 1115–1124. [Google Scholar] [CrossRef]
- Schneider, D.T.; Wessalowski, R.; Calaminus, G.; Pape, H.; Bamberg, M.; Engert, J.; Waag, K.; Gadner, H.; Gobel, U. Treatment of recurrent malignant sacrococcygeal germ cell tumors: Analysis of 22 patients registered in the German protocols MAKEI 83/86, 89, and 96. J. Clin. Oncol. 2001, 19, 1951–1960. [Google Scholar] [CrossRef]
- Rescorla, F.J.; Sawin, R.S.; Coran, A.G.; Dillon, P.W.; Azizkhan, R.G. Long-term outcome for infants and children with sacrococcygeal teratoma: A report from the Childrens Cancer Group. J. Pediatr. Surg. 1998, 33, 171–176. [Google Scholar] [CrossRef]
- Schindewolffs, L.; Dierks, C.; Heppelmann, M.; Gahle, M.; Piechotta, M.; Beineke, A.; Brehm, R.; Distl, O. Testicular yolk sac tumor and impaired spermatogenesis in a Holstein Friesian calf. Syst. Biol. Reprod. Med. 2015, 61, 314–319. [Google Scholar] [CrossRef]
- Ross, J.H.; Rybicki, L.; Kay, R. Clinical behavior and a contemporary management algorithm for prepubertal testis tumors: A summary of the Prepubertal Testis Tumor Registry. J. Urol. 2002, 168, 1675–1678; discussion 1678–1679. [Google Scholar] [CrossRef]
- Al-Obaidy, K.I.; Idrees, M.T. Testicular Tumors: A Contemporary Update on Morphologic, Immunohistochemical and Molecular Features. Adv. Anat. Pathol. 2021, 28, 258–275. [Google Scholar] [CrossRef]
- Cornejo, K.M.; Frazier, L.; Lee, R.S.; Kozakewich, H.P.; Young, R.H. Yolk Sac Tumor of the Testis in Infants and Children: A Clinicopathologic Analysis of 33 Cases. Am. J. Surg. Pathol. 2015, 39, 1121–1131. [Google Scholar] [CrossRef]
- Muller, M.R.; Skowron, M.A.; Albers, P.; Nettersheim, D. Molecular and epigenetic pathogenesis of germ cell tumors. Asian J. Urol. 2021, 8, 144–154. [Google Scholar] [CrossRef]
- Huang, T.H.; Hung, G.Y.; Weng, T.F.; Wang, F.M.; Lee, C.Y.; Lin, D.T.; Chen, B.W.; Lin, K.H.; Wu, K.H.; Liu, H.C.; et al. Surgical treatment confers prognostic significance in pediatric malignant mediastinal germ cell tumors. Cancer 2022, 128, 4139–4149. [Google Scholar] [CrossRef]
- Qiu, Z.; Wu, Y.; Wang, Y.; Hu, C. Male primary mediastinal choriocarcinoma with diffuse metastases: A case report. Medicine 2019, 98, e16411. [Google Scholar] [CrossRef]
- Jiang, F.; Xiang, Y.; Feng, F.Z.; Ren, T.; Cui, Z.M.; Wan, X.R. Clinical analysis of 13 males with primary choriocarcinoma and review of the literature. OncoTargets Ther. 2014, 7, 1135–1141. [Google Scholar] [CrossRef]
- Cushing, B.; Giller, R.; Cullen, J.W.; Marina, N.M.; Lauer, S.J.; Olson, T.A.; Rogers, P.C.; Colombani, P.; Rescorla, F.; Billmire, D.F.; et al. Randomized comparison of combination chemotherapy with etoposide, bleomycin, and either high-dose or standard-dose cisplatin in children and adolescents with high-risk malignant germ cell tumors: A pediatric intergroup study—Pediatric Oncology Group 9049 and Children’s Cancer Group 8882. J. Clin. Oncol. 2004, 22, 2691–2700. [Google Scholar] [CrossRef]
- Frazier, A.L.; Rumcheva, P.; Olson, T.; Giller, R.; Cushing, B.; Cullen, J.; Marina, N.; London, W.B.; Children’s Oncology, G. Application of the adult international germ cell classification system to pediatric malignant non-seminomatous germ cell tumors: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2008, 50, 746–751. [Google Scholar] [CrossRef]
- Fizazi, K.; Culine, S.; Kramar, A.; Amato, R.J.; Bouzy, J.; Chen, I.; Droz, J.P.; Logothetis, C.J. Early predicted time to normalization of tumor markers predicts outcome in poor-prognosis nonseminomatous germ cell tumors. J. Clin. Oncol. 2004, 22, 3868–3876. [Google Scholar] [CrossRef]
- Fizazi, K.; Pagliaro, L.; Laplanche, A.; Flechon, A.; Mardiak, J.; Geoffrois, L.; Kerbrat, P.; Chevreau, C.; Delva, R.; Rolland, F.; et al. Personalised chemotherapy based on tumour marker decline in poor prognosis germ-cell tumours (GETUG 13): A phase 3, multicentre, randomised trial. Lancet Oncol. 2014, 15, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Faure-Conter, C.; Orbach, D.; Cropet, C.; Baranzelli, M.C.; Martelli, H.; Thebaud, E.; Verite, C.; Rome, A.; Fasola, S.; Corradini, N.; et al. Salvage therapy for refractory or recurrent pediatric germ cell tumors: The French SFCE experience. Pediatr. Blood Cancer 2014, 61, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.T.; Calaminus, G.; Reinhard, H.; Gutjahr, P.; Kremens, B.; Harms, D.; Gobel, U. Primary mediastinal germ cell tumors in children and adolescents: Results of the German cooperative protocols MAKEI 83/86, 89, and 96. J. Clin. Oncol. 2000, 18, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Gobel, U.; Schneider, D.T.; Calaminus, G.; Jurgens, H.; Spaar, H.J.; Sternschulte, W.; Waag, K.; Harms, D. Multimodal treatment of malignant sacrococcygeal germ cell tumors: A prospective analysis of 66 patients of the German cooperative protocols MAKEI 83/86 and 89. J. Clin. Oncol. 2001, 19, 1943–1950. [Google Scholar] [CrossRef]
- Einhorn, L.H.; Donohue, J.P. Improved chemotherapy in disseminated testicular cancer. J. Urol. 1977, 117, 65–69. [Google Scholar] [CrossRef]
- Williams, S.D.; Birch, R.; Einhorn, L.H.; Irwin, L.; Greco, F.A.; Loehrer, P.J. Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N. Engl. J. Med. 1987, 316, 1435–1440. [Google Scholar] [CrossRef]
- O’Sullivan, J.M.; Huddart, R.A.; Norman, A.R.; Nicholls, J.; Dearnaley, D.P.; Horwich, A. Predicting the risk of bleomycin lung toxicity in patients with germ-cell tumours. Ann. Oncol. 2003, 14, 91–96. [Google Scholar] [CrossRef]
- Sleijfer, S. Bleomycin-induced pneumonitis. Chest 2001, 120, 617–624. [Google Scholar] [CrossRef]
- Ranganath, P.; Kesler, K.A.; Einhorn, L.H. Perioperative Morbidity and Mortality Associated With Bleomycin in Primary Mediastinal Nonseminomatous Germ Cell Tumor. J. Clin. Oncol. 2016, 34, 4445–4446. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Kohrmann, O.; Tischler, J.; Weissbach, L.; Rath, U.; Haupt, A.; Schoffski, P.; Harstrick, A.; Schmoll, H.J. A randomized trial of cisplatin, etoposide and bleomycin (PEB) versus carboplatin, etoposide and bleomycin (CEB) for patients with ‘good-risk’ metastatic non-seminomatous germ cell tumors. Ann. Oncol. 1996, 7, 1015–1021. [Google Scholar] [CrossRef]
- Barton, C.D.; Pizer, B.; Jones, C.; Oni, L.; Pirmohamed, M.; Hawcutt, D.B. Identifying cisplatin-induced kidney damage in paediatric oncology patients. Pediatr. Nephrol. 2018, 33, 1467–1474. [Google Scholar] [CrossRef]
- Meijer, A.J.M.; Li, K.H.; Brooks, B.; Clemens, E.; Ross, C.J.; Rassekh, S.R.; Hoetink, A.E.; van Grotel, M.; van den Heuvel-Eibrink, M.M.; Carleton, B.C. The cumulative incidence of cisplatin-induced hearing loss in young children is higher and develops at an early stage during therapy compared with older children based on 2052 audiological assessments. Cancer 2022, 128, 169–179. [Google Scholar] [CrossRef]
- Wei, M.; Yuan, X. Cisplatin-induced Ototoxicity in Children With Solid Tumor. J. Pediatr. Hematol. Oncol. 2019, 41, e97–e100. [Google Scholar] [CrossRef]
- Frazier, A.L.; Stoneham, S.; Rodriguez-Galindo, C.; Dang, H.; Xia, C.; Olson, T.A.; Murray, M.J.; Amatruda, J.F.; Shaikh, F.; Pashankar, F.; et al. Comparison of carboplatin versus cisplatin in the treatment of paediatric extracranial malignant germ cell tumours: A report of the Malignant Germ Cell International Consortium. Eur. J. Cancer 2018, 98, 30–37. [Google Scholar] [CrossRef]
- Mann, J.R.; Raafat, F.; Robinson, K.; Imeson, J.; Gornall, P.; Sokal, M.; Gray, E.; McKeever, P.; Hale, J.; Bailey, S.; et al. The United Kingdom Children’s Cancer Study Group’s second germ cell tumor study: Carboplatin, etoposide, and bleomycin are effective treatment for children with malignant extracranial germ cell tumors, with acceptable toxicity. J. Clin. Oncol. 2000, 18, 3809–3818. [Google Scholar] [CrossRef]
- Nichols, C.R.; Catalano, P.J.; Crawford, E.D.; Vogelzang, N.J.; Einhorn, L.H.; Loehrer, P.J. Randomized comparison of cisplatin and etoposide and either bleomycin or ifosfamide in treatment of advanced disseminated germ cell tumors: An Eastern Cooperative Oncology Group, Southwest Oncology Group, and Cancer and Leukemia Group B Study. J. Clin. Oncol. 1998, 16, 1287–1293. [Google Scholar] [CrossRef]
- Pashankar, F.; Frazier, A.L.; Krailo, M.; Xia, C.; Pappo, A.S.; Malogolowkin, M.; Olson, T.A.; Rodriguez-Galindo, C. Treatment of refractory germ cell tumors in children with paclitaxel, ifosfamide, and carboplatin: A report from the Children’s Oncology Group AGCT0521 study. Pediatr. Blood Cancer 2018, 65, e27111. [Google Scholar] [CrossRef]
- Stutzman, R.E.; McLeod, D.G. Radiation therapy: A primary treatment modality for seminoma. Urol. Clin. North Am. 1980, 7, 757–764. [Google Scholar] [CrossRef]
- Nichols, C.R. Mediastinal germ cell tumors. Clinical features and biologic correlates. Chest 1991, 99, 472–479. [Google Scholar] [CrossRef]
- Wang, J.; Bi, N.; Wang, X.; Hui, Z.; Liang, J.; Lv, J.; Zhou, Z.; Feng, Q.F.; Xiao, Z.; Chen, D.; et al. Role of radiotherapy in treating patients with primary malignant mediastinal non-seminomatous germ cell tumor: A 21-year experience at a single institution. Thorac. Cancer 2015, 6, 399–406. [Google Scholar] [CrossRef]
- MacDonald, S.M.; Trofimov, A.; Safai, S.; Adams, J.; Fullerton, B.; Ebb, D.; Tarbell, N.J.; Yock, T.I. Proton radiotherapy for pediatric central nervous system germ cell tumors: Early clinical outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Kalavska, K.; Schmidtova, S.; Chovanec, M.; Mego, M. Immunotherapy in Testicular Germ Cell Tumors. Front. Oncol. 2020, 10, 573977. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Carvajal, L.; Sanchez-Munoz, A.; Ribelles, N.; Saez, M.; Baena, J.; Ruiz, S.; Ithurbisquy, C.; Alba, E. Targeted treatment approaches in refractory germ cell tumors. Crit. Rev. Oncol. Hematol. 2019, 143, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Nieto, Y.; Tu, S.M.; Bassett, R.; Jones, R.B.; Gulbis, A.M.; Tannir, N.; Kingham, A.; Ledesma, C.; Margolin, K.; Holmberg, L.; et al. Bevacizumab/high-dose chemotherapy with autologous stem-cell transplant for poor-risk relapsed or refractory germ-cell tumors. Ann. Oncol. 2015, 26, 2507–2508. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, C.D.; Honecker, F.; Beyer, J.; Bode, P.K. Emerging Therapeutic Targets for Male Germ Cell Tumors. Curr. Oncol. Rep. 2015, 17, 54. [Google Scholar] [CrossRef]
- Chi, E.A.; Schweizer, M.T. Durable Response to Immune Checkpoint Blockade in a Platinum-Refractory Patient With Nonseminomatous Germ Cell Tumor. Clin. Genitourin. Cancer 2017, 15, e855–e857. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Vo, H.H.; Subbiah, V.; Janku, F.; Piha-Paul, S.; Yilmaz, B.; Gong, J.; Naqvi, M.F.; Tu, S.M.; Campbell, M.; et al. Pembrolizumab in Patients with Advanced Metastatic Germ Cell Tumors. Oncologist 2021, 26, 558.e1098. [Google Scholar] [CrossRef]
- Singh, R.; Fazal, Z.; Freemantle, S.J.; Spinella, M.J. Mechanisms of cisplatin sensitivity and resistance in testicular germ cell tumors. Cancer Drug Resist. 2019, 2, 580–594. [Google Scholar] [CrossRef]
- Albany, C.; Fazal, Z.; Singh, R.; Bikorimana, E.; Adra, N.; Hanna, N.H.; Einhorn, L.H.; Perkins, S.M.; Sandusky, G.E.; Christensen, B.C.; et al. A phase 1 study of combined guadecitabine and cisplatin in platinum refractory germ cell cancer. Cancer Med. 2021, 10, 156–163. [Google Scholar] [CrossRef]
- Wessalowski, R.; Schneider, D.T.; Mils, O.; Friemann, V.; Kyrillopoulou, O.; Schaper, J.; Matuschek, C.; Rothe, K.; Leuschner, I.; Willers, R.; et al. Regional deep hyperthermia for salvage treatment of children and adolescents with refractory or recurrent non-testicular malignant germ-cell tumours: An open-label, non-randomised, single-institution, phase 2 study. Lancet Oncol. 2013, 14, 843–852. [Google Scholar] [CrossRef]
- Oing, C.; Lorch, A. The Role of Salvage High-Dose Chemotherapy in Relapsed Male Germ Cell Tumors. Oncol. Res. Treat. 2018, 41, 365–369. [Google Scholar] [CrossRef]
- De Pasquale, M.D.; D’Angelo, P.; Crocoli, A.; Boldrini, R.; Conte, M.; Bisogno, G.; Spreafico, F.; Inserra, A.; Biasoni, D.; Dall’Igna, P.; et al. Salvage treatment for children with relapsed/refractory germ cell tumors: The Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP) experience. Pediatr. Blood Cancer 2020, 67, e28125. [Google Scholar] [CrossRef]
- Agarwal, R.; Dvorak, C.C.; Stockerl-Goldstein, K.E.; Johnston, L.; Srinivas, S. High-dose chemotherapy followed by stem cell rescue for high-risk germ cell tumors: The Stanford experience. Bone Marrow Transplant. 2009, 43, 547–552. [Google Scholar] [CrossRef]
- De Giorgi, U.; Rosti, G.; Slavin, S.; Yaniv, I.; Harousseau, J.L.; Ladenstein, R.; Demirer, T.; Dini, G.; European Group for, B.; Marrow Transplantation Solid, T.; et al. Salvage high-dose chemotherapy for children with extragonadal germ-cell tumours. Br. J. Cancer 2005, 93, 412–417. [Google Scholar] [CrossRef]
- International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: A prognostic factor-based staging system for metastatic germ cell cancers. J. Clin. Oncol. 1997, 15, 594–603. [Google Scholar] [CrossRef]
- Lorch, A.; Kleinhans, A.; Kramar, A.; Kollmannsberger, C.K.; Hartmann, J.T.; Bokemeyer, C.; Rick, O.; Beyer, J. Sequential versus single high-dose chemotherapy in patients with relapsed or refractory germ cell tumors: Long-term results of a prospective randomized trial. J. Clin. Oncol. 2012, 30, 800–805. [Google Scholar] [CrossRef]
- Bin Riaz, I.; Umar, M.; Zahid, U.; Husnain, M.; Iftikhar, A.; McBride, A.; Bilal, J.; Javed, A.; Akbar, S.; Singh, P.; et al. Role of one, two and three doses of high-dose chemotherapy with autologous transplantation in the treatment of high-risk or relapsed testicular cancer: A systematic review. Bone Marrow Transplant. 2018, 53, 1242–1254. [Google Scholar] [CrossRef]
- Motzer, R.J.; Nichols, C.J.; Margolin, K.A.; Bacik, J.; Richardson, P.G.; Vogelzang, N.J.; Bajorin, D.F.; Lara, P.N., Jr.; Einhorn, L.; Mazumdar, M.; et al. Phase III randomized trial of conventional-dose chemotherapy with or without high-dose chemotherapy and autologous hematopoietic stem-cell rescue as first-line treatment for patients with poor-prognosis metastatic germ cell tumors. J. Clin. Oncol. 2007, 25, 247–256. [Google Scholar] [CrossRef]
- Papiani, G.; Einhorn, L.H. Salvage chemotherapy with high-dose carboplatin plus etoposide and autologous peripheral blood stem cell transplant in male pure choriocarcinoma: A retrospective analysis of 13 cases. Bone Marrow Transplant. 2007, 40, 235–237. [Google Scholar] [CrossRef]
- Gossi, F.; Spahn, M.; Samaras, P.; Beyer, J.; Schardt, J.; Pabst, T. Response to first-line treatment and histology are associated with achieving complete remission after the first salvage high-dose chemotherapy in relapsing germ cell tumor patients. Bone Marrow Transplant. 2018, 53, 820–825. [Google Scholar] [CrossRef]
- Trimble, E.L.; Adams, J.D.; Vena, D.; Hawkins, M.J.; Friedman, M.A.; Fisherman, J.S.; Christian, M.C.; Canetta, R.; Onetto, N.; Hayn, R.; et al. Paclitaxel for platinum-refractory ovarian cancer: Results from the first 1,000 patients registered to National Cancer Institute Treatment Referral Center 9103. J. Clin. Oncol. 1993, 11, 2405–2410. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.R.; Sheinfeld, J.; Bajorin, D.F.; Fischer, P.; Turkula, S.; Ishill, N.; Patil, S.; Bains, M.; Reich, L.M.; Bosl, G.J.; et al. TI-CE high-dose chemotherapy for patients with previously treated germ cell tumors: Results and prognostic factor analysis. J. Clin. Oncol. 2010, 28, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Collinson, K.; Murray, M.J.; Orsi, N.M.; Cummings, M.; Shipley, J.; Joffe, J.K.; Coleman, N.; Stark, D. Age-related biological features of germ cell tumors. Genes Chromosomes Cancer 2014, 53, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, F.; Stark, D.; Fonseca, A.; Dang, H.; Xia, C.; Krailo, M.; Pashankar, F.; Rodriguez-Galindo, C.; Olson, T.A.; Nicholson, J.C.; et al. Outcomes of adolescent males with extracranial metastatic germ cell tumors: A report from the Malignant Germ Cell Tumor International Consortium. Cancer 2021, 127, 193–202. [Google Scholar] [CrossRef]
- Blohm, M.E.; Gobel, U. Unexplained anaemia and failure to thrive as initial symptoms of infantile choriocarcinoma: A review. Eur. J. Pediatr. 2004, 163, 1–6. [Google Scholar] [CrossRef]
- Mora, J. Autologous Stem-Cell Transplantation for High-Risk Neuroblastoma: Historical and Critical Review. Cancers 2022, 14, 2572. [Google Scholar] [CrossRef]
- Grimison, P.S.; Stockler, M.R.; Chatfield, M.; Thomson, D.B.; Gebski, V.; Friedlander, M.; Boland, A.L.; Houghton, B.; Gurney, H.; Rosenthal, M.; et al. Accelerated BEP for metastatic germ cell tumours: A multicenter phase II trial by the Australian and New Zealand Urogenital and Prostate Cancer Trials Group (ANZUP). Ann. Oncol. 2014, 25, 143–148. [Google Scholar] [CrossRef]
- Ussowicz, M.; Mielcarek-Siedziuk, M.; Musial, J.; Stachowiak, M.; Weclawek-Tompol, J.; Sega-Pondel, D.; Fraczkiewicz, J.; Trelinska, J.; Raciborska, A. Melphalan, Etoposide, and Carboplatin Megatherapy with Autologous Stem Cell Transplantation in Children with Relapsing or Therapy-Resistant Extracranial Germ-Cell Tumors-A Retrospective Analysis. Cancers 2020, 12, 3841. [Google Scholar] [CrossRef]
- McHugh, D.J.; Feldman, D.R. Conventional-Dose versus High-Dose Chemotherapy for Relapsed Germ Cell Tumors. Adv. Urol. 2018, 2018, 7272541. [Google Scholar] [CrossRef]
| Disease Status | 5-Year Overall Survival (%) |
|---|---|
| Ovarian stage IV disease and age ≥ 11 years | 67 [4] |
| Extragonadal disease and age ≥ 11 years | |
| Stage II–III | 65 [4] |
| Stage IV | 40 [4] |
| Primary mediastinal germ cell tumors | 54 [35] |
| Primary choriocarcinoma | - * [37] |
| Relapsed or refractory disease | 32 [42] |
| Unfavorable tumor markers decline | - ** [41] |
| Study | Country | Study Type | No. of Patients (n) | Median Age | HDC Regimen | Outcome (%) | Adverse Effects (%) |
|---|---|---|---|---|---|---|---|
| U De Giorgi et al. [74] | UK | Retrospective | 23 | 12 years | CarboPEC | CR:16/23 (70) | TRM = 0 (0) |
| EBMT database | CE | 1y DFS: 52% | Grade 3 stomatitis = 9 (39) | ||||
| Thiotepa, VP-16 | 1y OS: 74% | Fever = 21 (81) | |||||
| CarboPETM | 5y DFS:10/23 (43) | Infection = 13 (50) | |||||
| Grade 3 pulmonary toxicity = 2 (9) | |||||||
| Grade 3 neurotoxicity = 1 (4) | |||||||
| Psychosis = 1 (4) | |||||||
| Veno-occlusion disease = 2 (9) | |||||||
| Cecile Faure-Conter et al. [42] | French | Prospective study | 10 | <20 years-old | CarboPEC* or | 5y OS: 4/10 (40) | Not documented |
| VP-16 and thiotepa | |||||||
| De Pasquale et al. [72] | Italy | Retrospective study | 16 | 21 months | VP-16, thiotepa, CY ** | OS: 13/16 (81) | Grade 3 mucositis = 2 (13) |
| Thiotepa, melphalan ** | |||||||
| Marek Ussowicz et al. [88] | Poland | Cohort study | 18 | <18 years-old | MEC1: 9 | 5y EFS: 70.8% | Leukopenia/neutropenia = 18 (100) |
| MEC2: 9 | 5y OS: 76% | Fever = 15 (88) | |||||
| Grade 3 mucositis = 16 (88) | |||||||
| Sepsis = 4 (22) | |||||||
| Bacteremia = 1 (5) | |||||||
| Veno-occlusion disease = 11 (61) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lew, C.-Z.; Liu, H.-C.; Hou, J.-Y.; Huang, T.-H.; Yeh, T.-C. Pediatric Extracranial Germ Cell Tumors: Review of Clinics and Perspectives in Application of Autologous Stem Cell Transplantation. Cancers 2023, 15, 1998. https://doi.org/10.3390/cancers15071998
Lew C-Z, Liu H-C, Hou J-Y, Huang T-H, Yeh T-C. Pediatric Extracranial Germ Cell Tumors: Review of Clinics and Perspectives in Application of Autologous Stem Cell Transplantation. Cancers. 2023; 15(7):1998. https://doi.org/10.3390/cancers15071998
Chicago/Turabian StyleLew, Chong-Zhi, Hsi-Che Liu, Jen-Yin Hou, Ting-Huan Huang, and Ting-Chi Yeh. 2023. "Pediatric Extracranial Germ Cell Tumors: Review of Clinics and Perspectives in Application of Autologous Stem Cell Transplantation" Cancers 15, no. 7: 1998. https://doi.org/10.3390/cancers15071998
APA StyleLew, C.-Z., Liu, H.-C., Hou, J.-Y., Huang, T.-H., & Yeh, T.-C. (2023). Pediatric Extracranial Germ Cell Tumors: Review of Clinics and Perspectives in Application of Autologous Stem Cell Transplantation. Cancers, 15(7), 1998. https://doi.org/10.3390/cancers15071998






