Outcomes of Rural Men with Breast Cancer: A Multicenter Population Based Retrospective Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
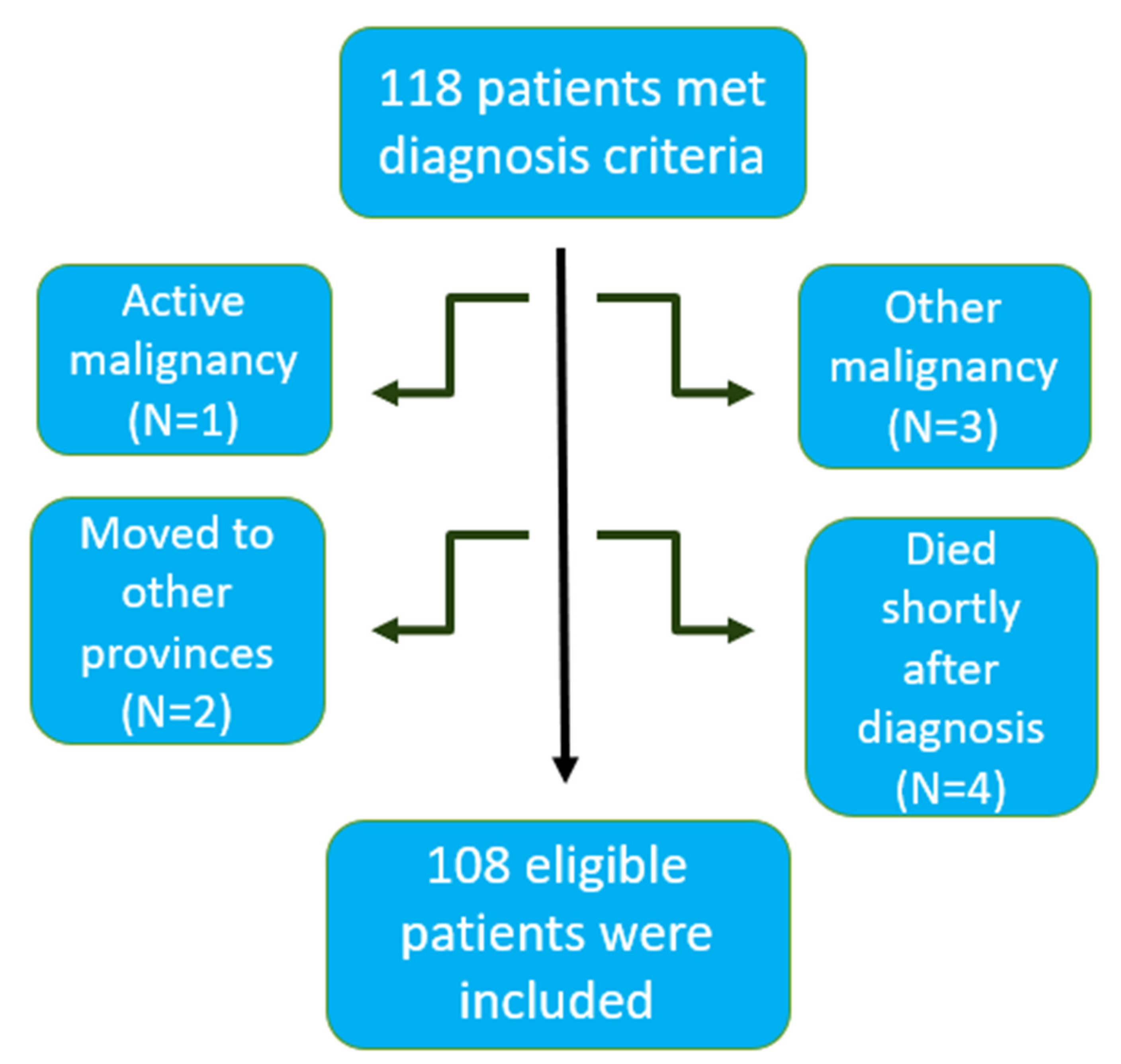
2.1. Study Population
2.2. Definitions
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Intervention and Survival
3.3. The Cox Proportional Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Government of Canada. Breast Cancer—Canada.ca./Gouvernement du Canada. 2021. Available online: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/breast-cancer.html (accessed on 9 January 2023).
- Lei, S.; Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Zhou, J.; Wei, W. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 2021, 41, 1183–1194. [Google Scholar] [CrossRef]
- Coughlin, S.S. Epidemiology of Breast Cancer in Women. Adv. Exp. Med. Biol. 2019, 1152, 9–29. [Google Scholar] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortman, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Prim. 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Gucalp, A.; Traina, T.A.; Eisner, J.R.; Parker, J.S.; Selitsky, S.R.; Park, B.H.; Elias, A.D.; Baskin-Bey, E.S.; Cardoso, F. Male breast cancer: A disease distinct from female breast cancer. Breast Cancer Res. Treat. 2018, 173, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Jene, N.; Investigators, K.; Fox, S.B. Genotypic and phenotypic analysis of familial male breast cancer shows under representation of the HER2 and basal subtypes in BRCA-associated carcinomas. BMC Cancer 2012, 12, 510. [Google Scholar] [CrossRef]
- Gómez-Raposo, C.; Zambrana Tévar, F.; Sereno Moyano, M.; López Gómez, M.; Casado, E. Male breast cancer. Cancer Treat. Rev. 2010, 36, 451–457. [Google Scholar] [CrossRef]
- Deb, S.; Lakhani, S.R.; Ottini, L.; Fox, S.B. The cancer genetics and pathology of male breast cancer. Histopathology 2015, 68, 110–118. [Google Scholar] [CrossRef]
- Tong, C.W.; Wu, M.; Cho, W.C.; To, K.K. Recent advances in the treatment of breast cancer. Front. Oncol. 2018, 8, 227. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast cancer treatment. JAMA 2019, 321, 288. [Google Scholar] [CrossRef]
- Henley, S.J.; Anderson, R.N.; Thomas, C.C.; Massetti, G.M.; Peaker, B.; Richardson, L.C. Invasive cancer incidence, 2004–2013, and deaths, 2006–2015, in Nonmetropolitan and Metropolitan counties—United states. MMWR Surveill. Summ. 2017, 66, 1–13. [Google Scholar] [CrossRef]
- Zahnd, W.E.; James, A.S.; Jenkins, W.D.; Izadi, S.R.; Fogleman, A.J.; Steward, D.E.; Colditz, G.A.; Brard, L. Rural–urban differences in cancer incidence and trends in the United States. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Huang, Z.L.; Yu, P.; Li, K. Trends in cancer mortality in China: An update. Ann. Oncol. 2012, 23, 2755–2762. [Google Scholar] [CrossRef] [PubMed]
- Malvia, S.; Bagadi, S.A.; Dubey, U.S.; Saxena, S. Epidemiology of breast cancer in Indian women. Asia-Pac. J. Clin. Oncol. 2017, 13, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Q.; Luo, Q.; Kahn, C.; O’Connell, D.L.; Houssami, N. Temporal trends show improved breast cancer survival in Australia but widening urban–rural differences. Breast 2015, 24, 524–527. [Google Scholar] [CrossRef]
- Gray, R.E.; James, P.; Manthorne, J.; Gould, J.; Fitch, M.I. A consultation with Canadian Rural Women with Breast Cancer. Health Expect. 2004, 7, 40–50. [Google Scholar] [CrossRef]
- Sprague, B.L.; Ahern, T.P.; Herschorn, S.D.; Sowden, M.; Weaver, D.L.; Wood, M.E. Identifying key barriers to effective breast cancer control in rural settings. Prev. Med. 2021, 152, 106741. [Google Scholar] [CrossRef]
- Government of Canada SC. Rural and Small Town Canada Analysis Bulletin Catalogue No. 21-006-XIE Vol. 3, No. 3. Government of Canada, Statistics Canada, 2001. Available online: https://www150.statcan.gc.ca/n1/en/catalogue/21-006-X (accessed on 9 January 2023).
- Dikshit, R.; Gupta, P.C.; Ramasundarahettige, C.; Gajalakshmi, V.; Aleksandrowicz, L.; Badwe, R.; Roy, S.; Suraweera, W.; Bray, F.; Mallath, M.; et al. Cancer mortality in India: A nationally representative survey. Lancet 2012, 379, 1807–1816. [Google Scholar] [CrossRef]
- Johnson, A.E.; Coopey, S.B.; Spring, L.M.; Horick, N.K.; Leone, J.P.; Lin, N.U.; Dominici, L.S.; Hughes, K.S.; Jimenez, R.B. Management and outcomes of men diagnosed with primary breast cancer. Breast Cancer Res. Treat. 2021, 188, 56–569. [Google Scholar] [CrossRef]
- Masci, G.; Caruso, M.; Caruso, F.; Salvini, P.; Carnaghi, C.; Giordano, L.; Miserocchi, V.; Losurdo, A.; Zuradelli, M.; Torrisi, R.; et al. Clinicopathological and immunohistochemical characteristics in male breast cancer: A retrospective case series. Oncologist 2015, 20, 586–592. [Google Scholar] [CrossRef]
- Sarmiento, S.; McColl, M.; Musavi, L.; Gani, F.; Canner, J.K.; Jacobs, L.; Fu, F.; Siotos, C.; Habibi, M. Male breast cancer: A closer look at patient and tumor characteristics and factors that affect survival using the National Cancer Database. Breast Cancer Res. Treat. 2020, 180, 471–479. [Google Scholar] [CrossRef]
- Scomersi, S.; Giudici, F.; Cacciatore, G.; Losurdo, P.; Fracon, S.; Cortinovis, S.; Ceccherini, R.; Zanconati, F.; Tonutti, M.; Bortul, M. Comparison between male and female breast cancer survival using propensity score matching analysis. Sci. Rep. 2021, 11, 11639. [Google Scholar] [CrossRef] [PubMed]
- McKinley, N.; McCain, S.; Kirk, S. Long Term follow up of Male Breast Cancer. Ulst. Med. J. 2017, 86, 177–180. [Google Scholar]
- Wan, B.A.; Ganesh, V.; Zhang, L.; Sousa, P.; Drost, L.; Lorentz, J.; Vesprini, D.; Lee, J.; Rakovitch, E.; Lu, F.-I.; et al. Treatment outcomes in male breast cancer: A retrospective analysis of 161 patients. Clin. Oncol. 2018, 30, 354–365. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Xue, Y. Clinicopathological features and prognosis of male breast cancer. J. Int. Med. Res. 2021, 49, 030006052110499. [Google Scholar] [CrossRef]
- Jang, R.W.; Caraiscos, V.B.; Swami, N.; Banerjee, S.; Mak, E.; Kaya, E.; Rodin, G.; Bryson, J.; Ridley, J.Z.; Le, L.W.; et al. Simple prognostic model for patients with advanced cancer based on performance status. J. Oncol. Pract. 2014, 10, e335–e341. [Google Scholar] [CrossRef]
- Hanna, T.P.; King, W.D.; Thibodeau, S.; Jalink, M.; Paulin, G.A.; Harvey-Jones, E.; O’Sullivan, D.E.; Booth, C.M.; Sullivan, R.; Aggarwal, A. Mortality due to cancer treatment delay: Systematic review and meta-analysis. BMJ 2020, 371, m4087. [Google Scholar] [CrossRef] [PubMed]
- Raphael, M.J.; Biagi, J.J.; Kong, W.; Mates, M.; Booth, C.M.; Mackillop, W.J. The relationship between time to initiation of adjuvant chemotherapy and survival in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2016, 160, 17–28. [Google Scholar] [CrossRef]
- Corti, C.; Crimini, E.; Criscitiello, C.; Trapani, D.; Curigliano, G. Adjuvant treatment of early male breast cancer. Curr. Opin. Oncol. 2020, 32, 594–602. [Google Scholar] [CrossRef]
- Hassett, M.J.; Somerfield, M.R.; Baker, E.R.; Cardoso, F.; Kansal, K.J.; Kwait, D.C.; Plichta, J.K.; Ricker, C.; Roshal, A.; Ruddy, K.J.; et al. Management of Male Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 38, 1849–1863. [Google Scholar] [CrossRef]

| Variables | All N = 108 (%) | Rural N = 66 (%) | Urban N = 42 (%) | p Value |
|---|---|---|---|---|
| Median Age (range) | 69 (30–91) | 69 (30–89) | 69 (36–91) | 0.62 |
| Age ≥ 65 years | 69 (64) | 41 (62) | 28 (67) | 0.68 |
| Time period (2000–2009) | 45 (42) | 30 (45) | 15 (36) | 0.42 |
| Presence of comorbid illnesses a | 81 (75) | 47 (71) | 34 (81) | 0.36 |
| Past history of a secondary cancer | 27 (26) | 17 (26) | 10 (24) | 1.0 |
| WHO Performance Status b | ||||
| 58 (56) | 37 (59) | 21 (51) | 0.55 |
| 30 (29) | 17 (27) | 13 (32) | 0.66 |
| 13 (13) | 7 (11) | 6 (14) | 0.56 |
| 3 (3) | 2 (3) | 1 (2) | 1.0 |
| Active smoker | 59 (55) | 37 (56) | 22 (52) | 0.21 |
| Stage c | ||||
| 8 (7) | 6 (9) | 2 (5) | 0.77 |
| 33 (31) | 17 (26) | 16 (38) | 0.20 |
| 46 (42) | 28 (42) | 18 (43) | 1.0 |
| 12 (11) | 10 (15) | 2 (5) | 0.12 |
| 9 (8) | 5 (8) | 4 (10) | 0.73 |
| Site | ||||
| 48 (44) | 32 (49) | 16 (38) | 0.32 |
| 59 (55) | 33 (50) | 26 (62) | 0.24 |
| 1 (1) | 1 (2) | 0 | 1.0 |
| Surgery | ||||
| 1 (1) | 1 (2) | 0 | 1.0 |
| 95 (88) | 58 (88) | 37 (88) | 1.0 |
| 4 (4) | 4 (6) | 0 | 0.15 |
| 8 (7) | 3 (4) | 5 (12) | 0.25 |
| Axillary nodal dissection | 66 (61) | 44 (66) | 22 (52) | 0.16 |
| Multifocal disease | 9 (8) | 4 (6) | 5 (12) | 0.30 |
| T status d | ||||
| 8 (7) | 6 (9) | 2 (5) | 0.48 |
| 48 (45) | 28 (42) | 20 (49) | 0.69 |
| 35 (33) | 23 (35) | 12 (29) | 0.53 |
| 3 (3) | 2 (3) | 1 (2) | 1.0 |
| 13 (12) | 7 (11) | 6 (15) | 0.56 |
| Grade | ||||
| 15 (14) | 7 (11) | 8 (19) | 0.26 |
| 45 (42) | 31 (48) | 14 (33) | 0.23 |
| 39 (36) | 24 (37) | 15 (38) | 1.0 |
| 9 (8) | 4 (6) | 5 (12) | 0.31 |
| Nodal status | ||||
| 52 (48) | 31 (47) | 21 (50) | 0.84 |
| 33 (31) | 21 (32) | 12 (29) | 0.83 |
| 9 (8) | 6 (9) | 3 (7) | 1.0 |
| 4 (4) | 3 (5) | 1 (2) | 1.0 |
| 10 (9) | 5 (8) | 5 (12) | 0.51 |
| Positive resection margin | 9 (8) | 4 (6) | 5 (12) | 0.31 |
| Lymphovascular invasive | 34 (31) | 24 (36) | 10 (24) | 0.21 |
| Underlying DCIS | 35 (32) | 22 (33) | 13 (31) | 0.84 |
| Receptor status e | ||||
| 98 (98) | 59 (98) | 39 (98) | 0.74 |
| 12 (12) | 5 (8) | 7 (18) | 0.21 |
| 1 (1) | 0 | 1 (2) | 0.38 |
| Mean creatinine μmol/L | 97 ± 33 | 97 ± 36 | 97 ± 28 | 0.92 |
| Mean Albumin g/L | 38 ± 4 | 39 ± 4 | 38 ± 3 | 0.13 |
| Mean Alkaline phosphatase U/L | 86 ± 40 | 83 ± 31 | 90 ± 50 | 0.43 |
| Mean bilirubin μm/L | 10 ± 5 | 10 ± 8 | 9 ± 5 | 0.22 |
| Mean ALT U/L | 28 ± 26 | 31 ± 31 | 24 ± 15 | 0.20 |
| Mean AST U/L | 26 ± 11 | 26 ± 10 | 27 ± 14 | 0.75 |
| Mean WBC × 109/L | 7.5 ± 2.0 | 7.2 ± 2.1 | 7.8 ± 1.9 | 0.17 |
| Mean hemoglobin g/L | 144 ± 18 | 146 ± 20 | 142 ± 16 | 0.18 |
| Mean platelets × 109/L | 235 ± 72 | 230 ± 68 | 242 ± 78 | 0.44 |
| Median NLR (IQR) | 2.6 (2.0–3.7) | 2.9 (2.0–3.7) | 2.4 (1.9–3.5) | 0.52 |
| Variables | All N = 108 (%) | Rural N = 66 (%) | Urban N = 42 (%) | p Value |
|---|---|---|---|---|
| Median follow up in months (IQR) | 75 (39–127) | 74 (42–135) | 80 (34–118) | 0.58 |
| Received Systemic therapy for early stage disease | 81 of 99 (81) | 48 of 61 (79) | 33 of 38 (87) | 0.42 |
| Adjuvant | 73 (90) | 45 (94) | 28 (85) | 0.26 |
| Neoadjuvant | 8 (10) | 3 (6) | 5 (15) | 0.26 |
| Type of adjuvant treatment a | ||||
| Endocrine therapy alone b | 45 (55) | 24 (50) | 21 (64) | 0.26 |
| Chemotherapy plus endocrine c | 33 (41) | 22 (46) | 11 (33) | 0.35 |
| Chemotherapy alone | 2 (2) | 1 (2) | 1 (3) | 1.0 |
| Trastuzumab with chemotherapy | 11 (14) | 5 (10) | 6 (18) | 0.34 |
| Completed planned chemotherapy d | 26 (74) | 17 (74) | 9 (75) | 1.0 |
| Adjuvant radiation | 32 of 99 (32) | 21 of 61 (34) | 11 of 38 (29) | 0.66 |
| Disease-Free Survival events | 60 of 99 (61) | 38 of 61 (62) | 22 of 38 (58) | 0.67 |
| Recurrent breast cancer | 15 (25) | 8 (21) | 7 (32) | 0.37 |
| Distant metastases | 10 (66) | 7 (88) | 3 (43) | 0.73 |
| New invasive breast cancer | 1 (2) | 0 | 1 (5) | 1.0 |
| New secondary cancer | 20 (33) | 14 (37) | 6 (27) | 0.57 |
| Death | 24 (40) | 16 (42) | 8 (36) | 0.78 |
| Advanced disease (de novo/relapse) | 19 (18) | 12 (18) | 7 (17) | 1.0 |
| Endocrine therapy | 15 (79) | 8 (67) | 7 (100) | 0.45 |
| CDK 4/6 inhibitor | 11 (58) | 4 (34) | 7 (100) | 0.45 |
| Chemotherapy for advanced disease | 4 (21) | 2 (17) | 2 (29) | 0.60 |
| Median line of therapy for advanced disease (range) | 2 (0–6) | 2 (1–5) | 2 (0–6) | 1.0 |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (CI) | p Value | HR (CI) | p Value | |
| Age < 65 years | 1 | 1 | ||
| Age ≥ 65 years | 2.45 (1.27–4.72) | 0.007 | 1.32 (0.63–2.76) | 0.45 |
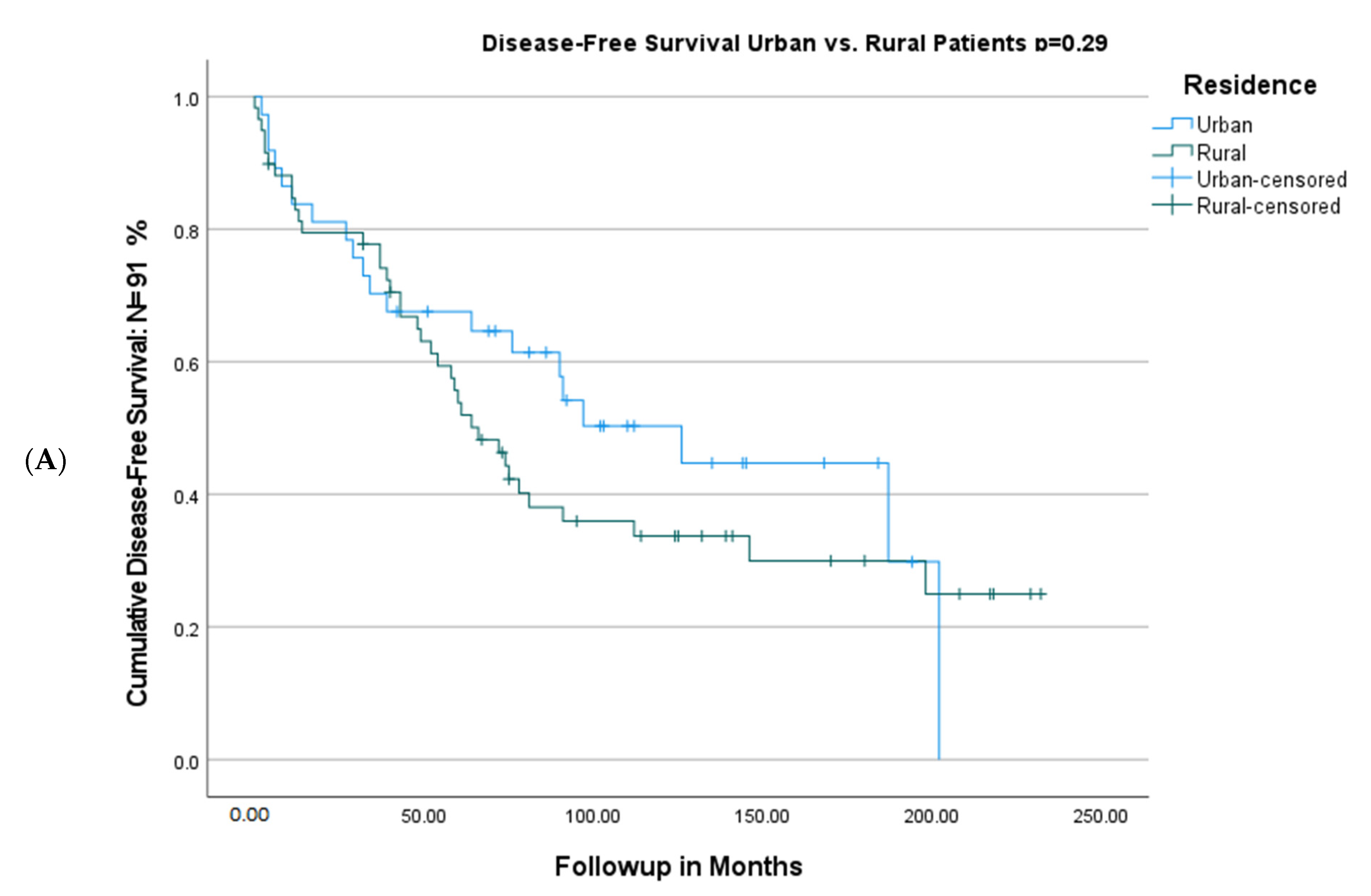
| Urban residence | 1 | …… | ||
| Rural residence | 1.35 (0.77–2.38) | 0.29 | …… | |
| Time period (2010–2019) | 1 | …… | ||
| Time period (2000–2009) | 1.22 (0.70–2.12) | 0.47 | …… | |
| No comorbid illness | 1 | …… | ||
| Comorbid illness | 1.15 (0.59–2.21) | 0.68 | …… | |
| No history of a secondary cancer | 1 | …… | ||
| Past history of a secondary cancer | 1.41 (0.80–2.47) | 0.23 | …… | |
| WHO performance status < 2 | 1 | 1 | ||
| WHO performance status ≥ 2 | 3.0 (1.44–8.32) | 0.003 | 2.82 (1.14–6.94) | 0.025 |
| ≤T2 disease | 1 | …… | ||
| >T2 disease | 1.42 (0.56–3.57) | 0.46 | …… | |
| Node negative disease | 1 | 1 | ||
| Node positive disease | 1.59 (0.91–2.76) | 0.10 | 2.32 (1.22–4.40) | 0.01 |
| Grade I or II tumor | 1 | …… | ||
| Grade III tumor | 1.26 (0.74–2.15) | 0.40 | …… | |
| Negative resection margin | 1 | …… | ||
| Positive resection margin | 1.58 (0.63–4.0) | 0.32 | …… | |
| HER2 negative disease | 1 | 1 | ||
| HER2 positive disease | 0.35 (1.09–1.14) | 0.08 | 0.38 (0.12–1.27) | 0.11 |
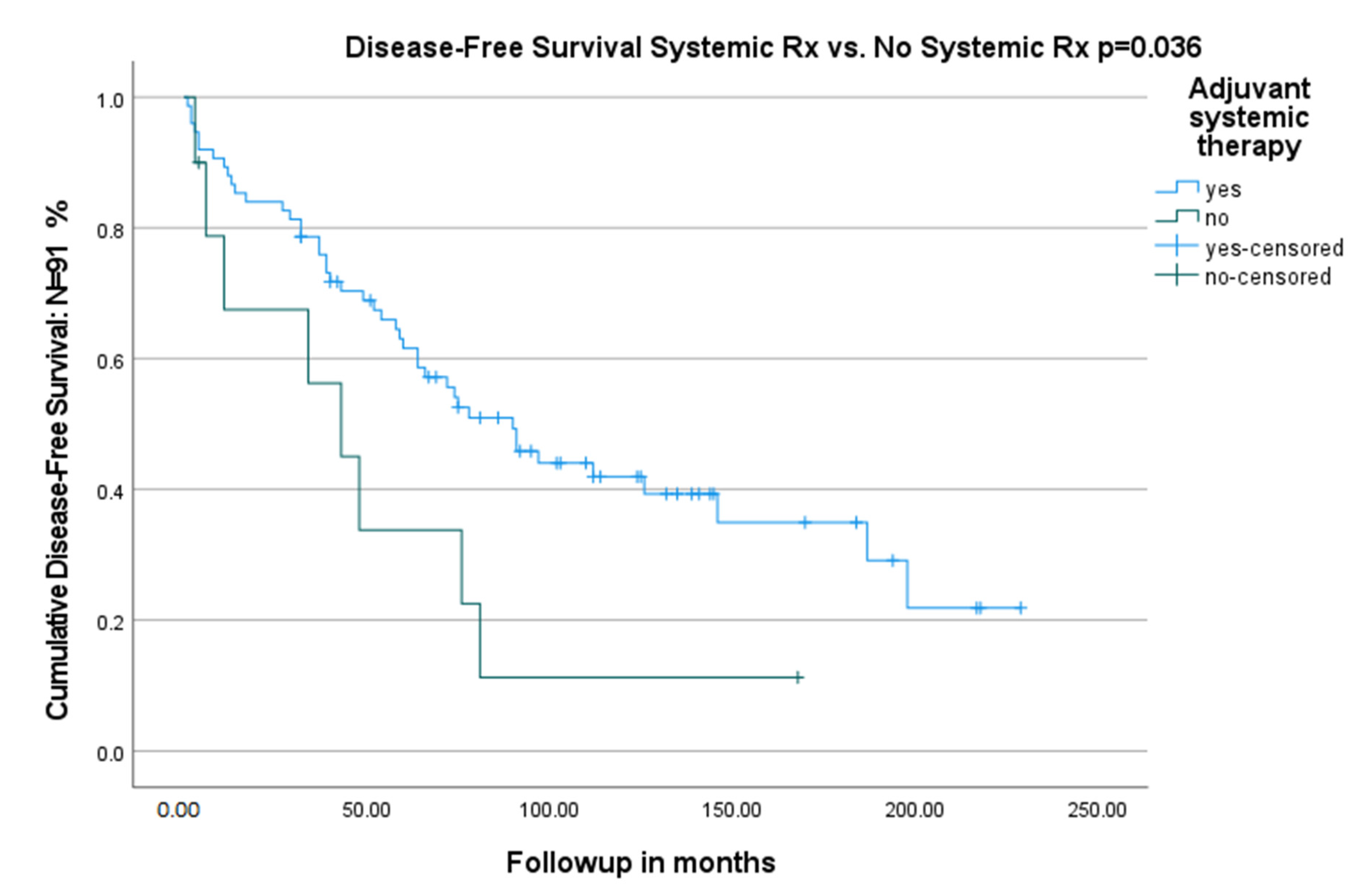
| Adjuvant systemic therapy | 1 | 1 | ||
| No adjuvant systemic therapy | 2.20 (1.03–4.71) | 0.04 | 2.47 (1.03–5.92) | 0.04 |
| Adjuvant chemotherapy | 1 | …… | ||
| No adjuvant chemotherapy | 1.29 (0.75–2.22) | 0.35 | …… | |
| Adjuvant radiation | 1 | …… | ||
| No adjuvant radiation | 0.72 (0.53–2.25) | 0.38 | …… | |
| Neutrophil:Lymphocyte ≤ 2.5 | 1 | …… | ||
| Neutrophil:Lymphocyte > 2.5 | 1.31 (0.75–2.28) | 0.34 | …… | |
| Alkaline phosphatase ≤ 110 U/L | 1 | …… | ||
| Alkaline phosphatase > 110 U/L | 1.72 (0.94–3.15) | 0.07 | …… | |
| Albumin ≥ 35 g/L | 1 | …… | ||
| Albumin < 35 g/L | 1.47 (0.68–3.15) | 0.32 | …… | |
| Creatinine < 105 μmol/L | 1 | 1 | ||
| Creatinine ≥ 105 μmol/L | 2.10 (1.21–3.64) | 0.008 | 1.94 (0.93–4.06) | 0.08 |
| Hemoglobin ≥ 135 g/L | 1 | 1 | ||
| Hemoglobin < 135 g/L | 1.72 (0.98–3.05) | 0.06 | 1.28 (0.52–3.14) | 0.59 |
| WBC > 11 × 109/L | 1 | 1 | ||
| WBC > 11 × 109/L | 1.69 (0.90–3.17) | 0.10 | 1.45 (0.56–3.75) | 0.44 |
| Platelets > 400 × 109/L | 1 | …… | ||
| Platelets > 400 × 109/L | 1.52 (0.79–2.91) | 0.21 | …… | |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age < 65 years | 1 | 1 | ||
| Age ≥ 65 years | 3.28 (1.68–6.39) | <0.001 | 2.37 (1.13–5.0) | 0.02 |
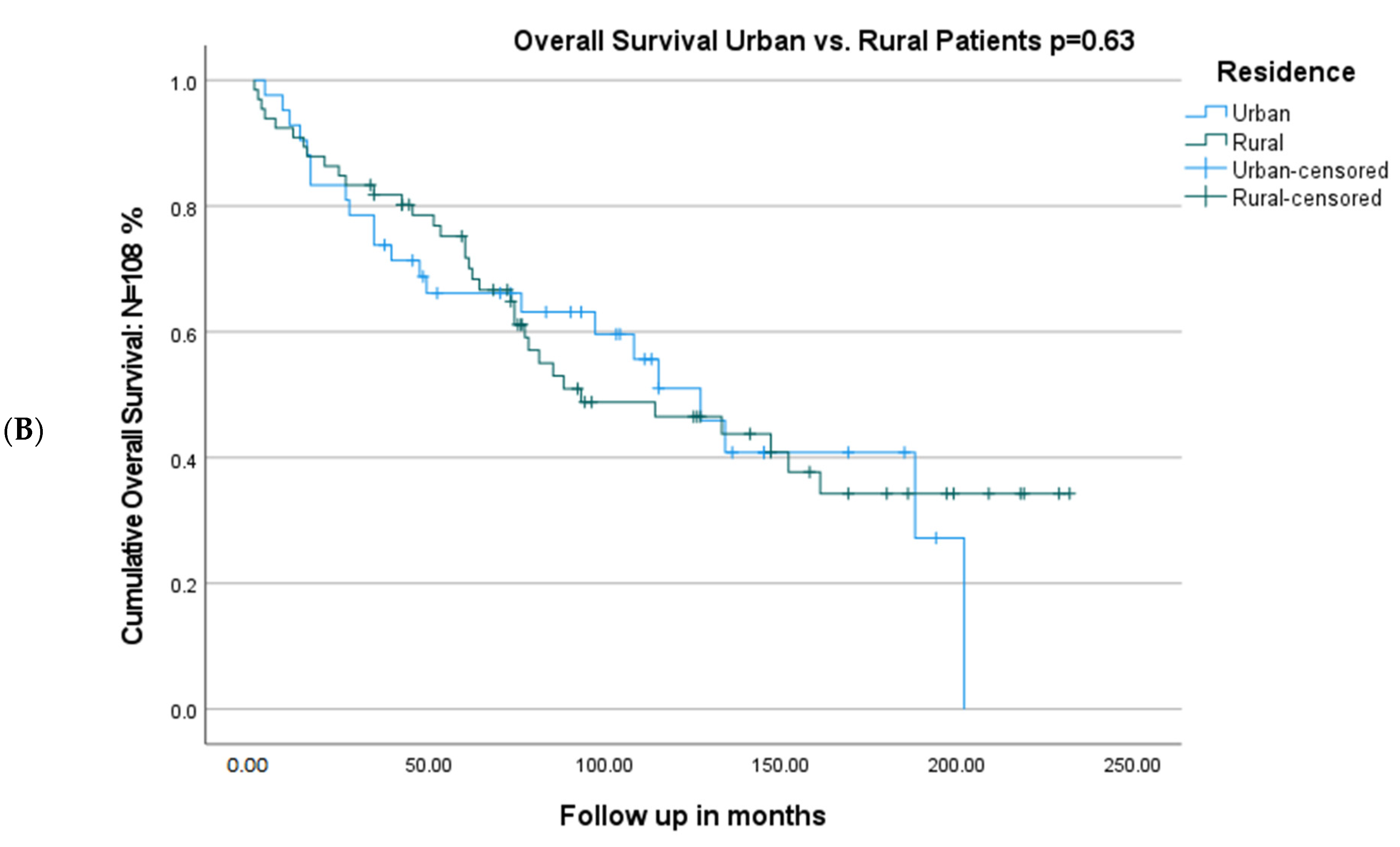
| Urban residence | 1 | …… | ||
| Rural residence | 0.95 (0.56–1.63) | 0.87 | ..... | |
| No comorbid illness | 1 | …… | ||
| Comorbid illness | 1.25 (0.65–2.40) | 0.50 | …… | |
| No history of a secondary cancer | 1 | 1 | ||
| Past history of a secondary cancer | 1.64 (0.92–2.90) | 0.09 | 1.69 (0.90-3.19) | 0.10 |
| Stage I to III disease | 1 | 1 | ||
| Stage IV disease | 4.78 (2.3–9.90) | <0.001 | 7.82 (3.14–19.48) | <0.001 |
| HER2 negative disease | 1 | …… | ||
| HER2 positive disease | 0.46 (0.14–1.50) | 0.20 | …… | |
| WHO performance status < 2 | 1 | 1 | ||
| WHO performance status ≥ 2 | 4.35 (2.24–8.49) | <0.001 | 3.25 (1.57–6.71) | 0.001 |
| Neutrophil:Lymphocyte ≤ 2.5 | 1 | 1 | ||
| Neutrophil:Lymphocyte > 2.5 | 1.66 (0.94–2.94) | 0.08 | 1.54 (0.82–2.91) | 0.18 |
| Time period (2010–2019) | 1 | …… | ||
| Time period (2000–2009) | 1.11 (0.64–1.93) | 0.70 | …… | |
| Alkaline phosphatase ≤ 110 | 1 | 1 | ||
| Alkaline phosphatase > 110 | 1.94 (1.15–3.30) | 0.014 | 2.13 (0.97–4.66) | 0.06 |
| Albumin ≥ 35 g/L | 1 | …… | ||
| Albumin < 35 g/L | 1.34 (0.60–3.0) | 0.47 | …… | |
| Creatinine < 105 | 1 | 1 | ||
| Creatinine ≥ 105 | 2.40 (1.42–4.10) | 0.001 | 1.74 (0.92–3.29) | 0.09 |
| Hemoglobin ≥ 135 g/L | 1 | 1 | ||
| Hemoglobin < 135 g/L | 2.0 (1.20–3.42) | 0.008 | 1.87 (0.88–3.95) | 0.10 |
| WBC ≤ 11 × 109/L | 1 | …… | ||
| WBC > 11 × 109/L | 1.23 (0.68–2.22) | 0.50 | …… | |
| Platelets ≤ 400 × 109/L | 1 | …… | ||
| Platelets > 400 × 109/L | 1.47 (0.82–2.63) | 0.19 | …… | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fisher, L.A.B.; Ahmed, O.; Chalchal, H.I.; Deobald, R.; El-Gayed, A.; Graham, P.; Groot, G.; Haider, K.; Iqbal, N.; Johnson, K.; et al. Outcomes of Rural Men with Breast Cancer: A Multicenter Population Based Retrospective Cohort Study. Cancers 2023, 15, 1995. https://doi.org/10.3390/cancers15071995
Fisher LAB, Ahmed O, Chalchal HI, Deobald R, El-Gayed A, Graham P, Groot G, Haider K, Iqbal N, Johnson K, et al. Outcomes of Rural Men with Breast Cancer: A Multicenter Population Based Retrospective Cohort Study. Cancers. 2023; 15(7):1995. https://doi.org/10.3390/cancers15071995
Chicago/Turabian StyleFisher, Lucas A. B., Osama Ahmed, Haji Ibraheem Chalchal, Ray Deobald, Ali El-Gayed, Peter Graham, Gary Groot, Kamal Haider, Nayyer Iqbal, Kate Johnson, and et al. 2023. "Outcomes of Rural Men with Breast Cancer: A Multicenter Population Based Retrospective Cohort Study" Cancers 15, no. 7: 1995. https://doi.org/10.3390/cancers15071995
APA StyleFisher, L. A. B., Ahmed, O., Chalchal, H. I., Deobald, R., El-Gayed, A., Graham, P., Groot, G., Haider, K., Iqbal, N., Johnson, K., Le, D., Mahmood, S., Manna, M., Meiers, P., Pauls, M., Salim, M., Sami, A., Wright, P., Younis, M., & Ahmed, S. (2023). Outcomes of Rural Men with Breast Cancer: A Multicenter Population Based Retrospective Cohort Study. Cancers, 15(7), 1995. https://doi.org/10.3390/cancers15071995









