AFP Response to Locoregional Therapy Can Stratify the Risk of Tumor Recurrence in HCC Patients after Living Donor Liver Transplantation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
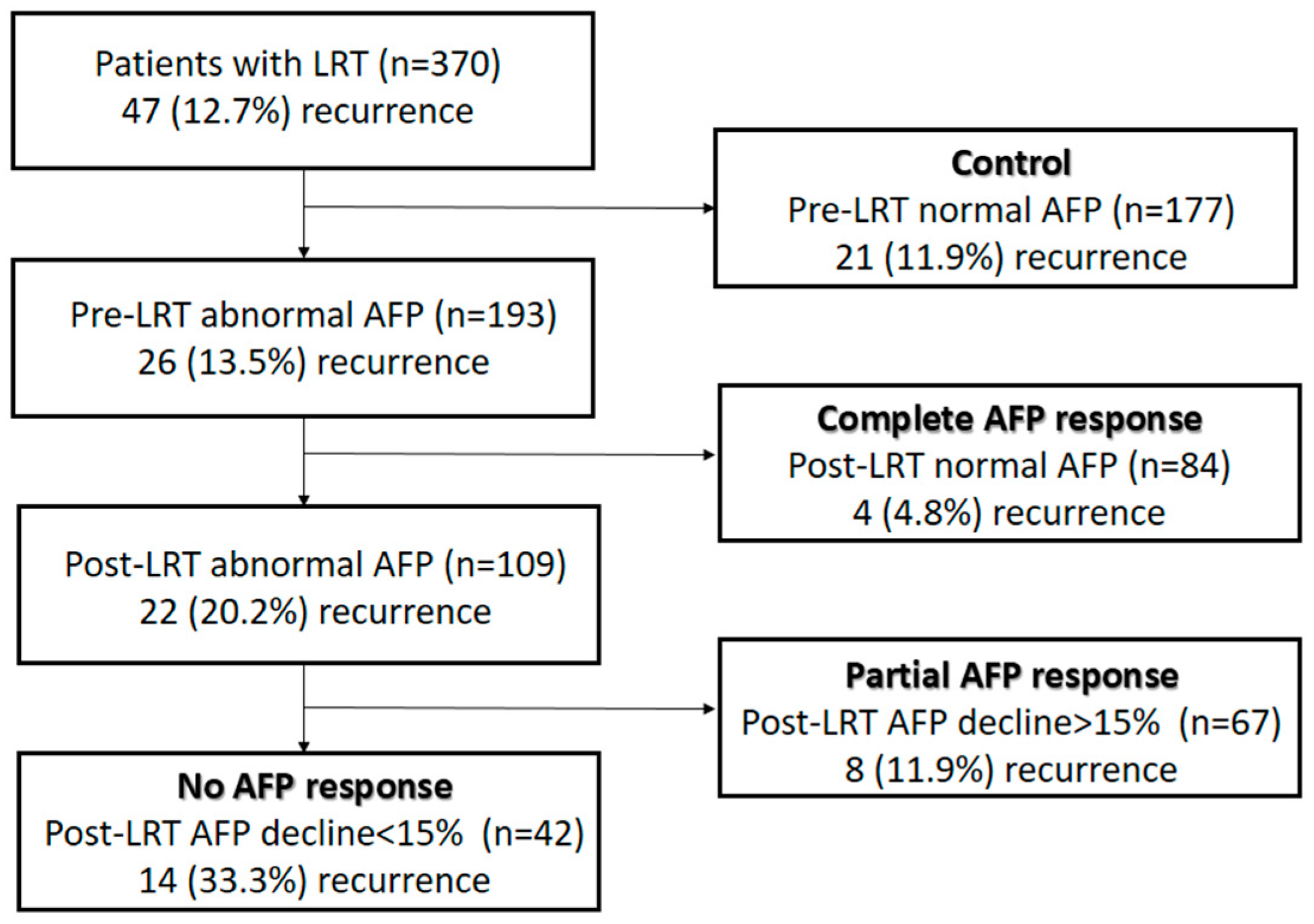
2.2. Patient Grouping
2.3. Immunosuppression and Follow-Up Protocol
2.4. Statistical Analysis
2.5. Ethics Statement
3. Results
3.1. Patient Characteristics and Their Association with Recurrence
3.2. Cutoff Value of AFP Response to LRT
3.3. Recurrence Rates According to Pre-LRT AFP and AFP-Response Groups
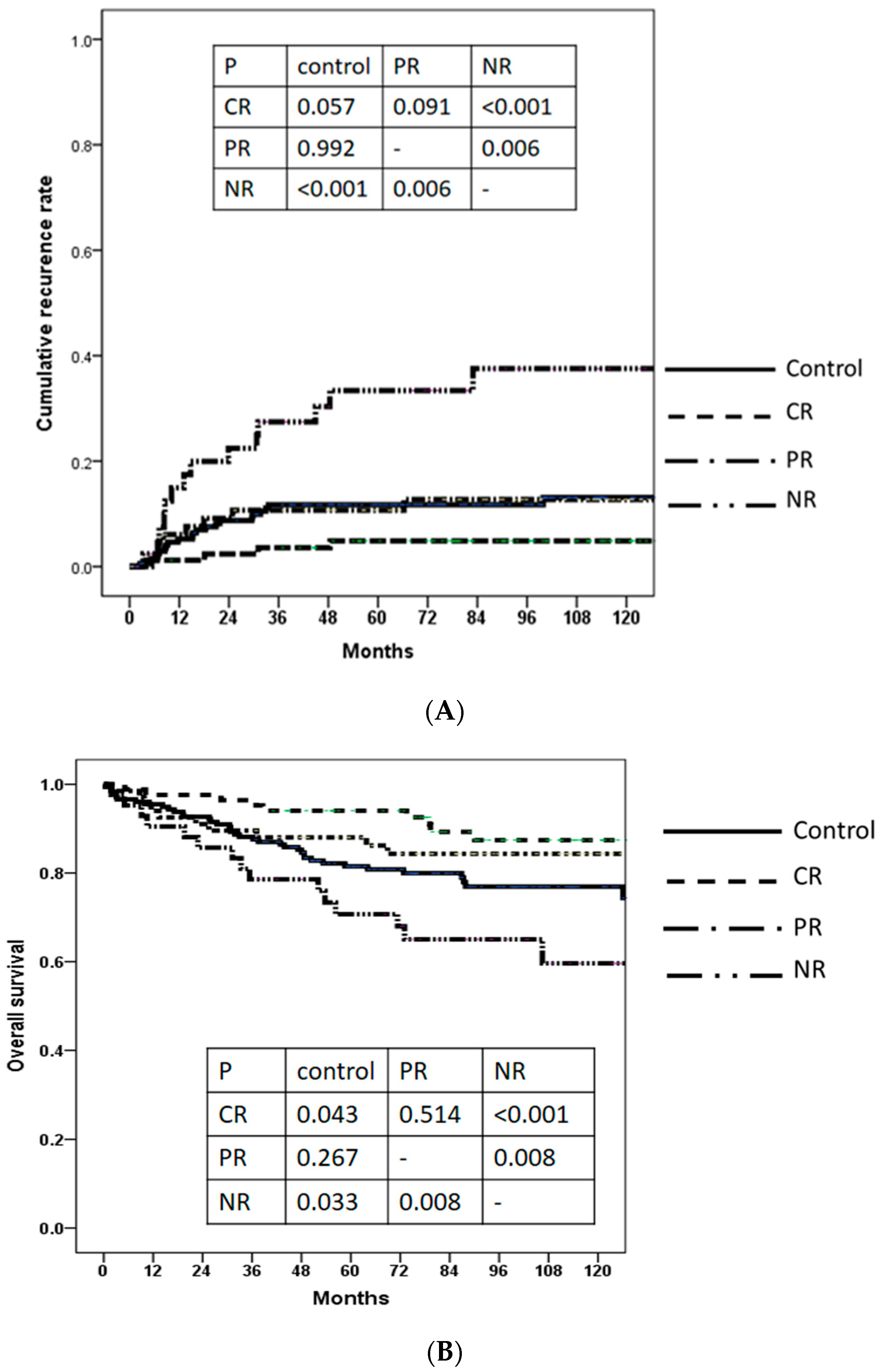
3.4. Cumulative Recurrence Rate and Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marrero, J.A. Multidisciplinary Management of Hepatocellular Carcinoma: Where Are We Today? Semin. Liver Dis. 2013, 33 (Suppl. 1), S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Concejero, A.; Chen, C.-L.; Wang, C.-C.; Wang, S.-H.; Lin, C.-C.; Liu, Y.-W.; Yang, C.-H.; Yong, C.-C.; Lin, T.-S.; Jawan, B.; et al. Living Donor Liver Transplantation for Hepatocellular Carcinoma: A Single-Center Experience in Taiwan. Transplantation 2008, 85, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, F.; Baskiran, A. The Importance of AFP in Liver Transplantation for HCC. J. Gastrointest. Cancer 2020, 51, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.Y.; Kerlan, R.K., Jr.; Hirose, R.; Davern, T.J., 3rd; Bass, N.M.; Feng, S.; Peters, M.; Terrault, N.; Freise, C.E.; Ascher, N.L.; et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: An intention-to-treat analysis. Hepatology 2008, 48, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, M.; Grazi, G.L.; Piscaglia, F.; Trevisani, F.; Cescon, M.; Ercolani, G.; Vivarelli, M.; Golfieri, R.; Grigioni, A.D.; Panzini, I.; et al. Liver Transplantation for Hepatocellular Carcinoma: Results of Down-Staging in Patients Initially Outside the Milan Selection Criteria. Am. J. Transplant. 2008, 8, 2547–2557. [Google Scholar] [CrossRef]
- Toso, C.; Asthana, S.; Bigam, D.L.; Shapiro, A.M.J.; Kneteman, N.M. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the scientific registry of transplant recipients database. Hepatology 2009, 49, 832–838. [Google Scholar] [CrossRef]
- Mazzaferro, V.M.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef]
- Pomfret, E.A.; Washburn, K.; Wald, C.; Nalesnik, M.A.; Douglas, D.; Russo, M.; Roberts, J.; Reich, D.J.; Schwartz, M.E.; Mieles, L.; et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transplant. 2010, 16, 262–278. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Mehta, N.; Yao, F.Y. Moving past “One Size (and Number) Fits All” in the selection of candidates with hepatocellular carcinoma for liver transplantation. Liver Transplant. 2013, 19, 1055–1058. [Google Scholar] [CrossRef]
- Nerenstone, S.R.; Ihde, D.C.; Friedman, M.A. Clinical trials in primary hepatocellular carcinoma: Current status and future directions. Cancer Treat. Rev. 1988, 15, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Verna, E.C.; Patel, Y.A.; Aggarwal, A.; Desai, A.P.; Frenette, C.; Pillai, A.A.; Salgia, R.; Seetharam, A.; Sharma, P.; Sherman, C.; et al. Liver transplantation for hepatocellular carcinoma: Management after the transplant. Am. J. Transplant. 2020, 20, 333–347. [Google Scholar] [CrossRef]
- Yao, F.Y.; Fidelman, N.; Lysy, P.A.; Smets, F.; Sibille, C.; Najimi, M.; Sokal, E.M. Reassessing the boundaries of liver transplantation for hepatocellular carcinoma: Where do we stand with tumor down-staging? Hepatology 2016, 63, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.D.; Waljee, A.K.; Singal, A.G. Downstaging hepatocellular carcinoma: A systematic review and pooled analysis. Liver Transplant. 2015, 21, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Chen, C.-L. Living donor liver transplantation for hepatocellular carcinoma achieves better outcomes. HepatoBiliary Surg. Nutr. 2016, 5, 415–421. [Google Scholar] [CrossRef]
- Chaiteerakij, R.; Zhang, X.; Addissie, B.D.; Mohamed, E.A.; Harmsen, W.S.; Theobald, P.J.; Peters, B.E.; Balsanek, J.G.; Ward, M.M.; Giama, N.H.; et al. Combinations of biomarkers and Milan criteria for predicting hepatocellular carcinoma recurrence after liver transplantation. Liver Transplant. 2015, 21, 599–606. [Google Scholar] [CrossRef]
- Lee, J.-H.; Cho, Y.; Kim, H.Y.; Cho, E.J.; Lee, D.H.; Yu, S.J.; Lee, J.W.; Yi, N.-J.; Lee, K.-W.; Kim, S.H.; et al. Serum Tumor Markers Provide Refined Prognostication in Selecting Liver Transplantation Candidate for Hepatocellular Carcinoma Patients Beyond the Milan Criteria. Ann. Surg. 2016, 263, 842–850. [Google Scholar] [CrossRef]
- Toso, C.; Mentha, G.; Kneteman, N.M.; Majno, P. The place of downstaging for hepatocellular carcinoma. J. Hepatol. 2010, 52, 930–936. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Gómez-Rodríguez, R.; Romero-Gutiérrez, M.; Artaza-Varasa, T.; González-Frutos, C.; Ciampi-Dopazo, J.J.; Cruz-Pérez, G.-D.; Sánchez-Ruano, J.J. The value of the Barcelona Clinic Liver Cancer and alpha-fetoprotein in the prognosis of hepatocellular carcinoma. Rev. Esp. Enferm. Dig. 2012, 104, 298–304. [Google Scholar] [CrossRef]
- Berry, K.; Ioannou, G.N. Serum alpha-fetoprotein level independently predicts posttransplant survival in patients with hepatocellular carcinoma. Liver Transplant. 2013, 19, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, A.R.; Young, R.S.; Marangoni, G.; Lodge, J.P.A.; Prasad, K.R. Systematic review: The prognostic role of alpha-fetoprotein following liver transplantation for hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2012, 35, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.; Mehta, N.; Sapisochin, G.; Roberts, J.P.; Yao, F.Y. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transplant. 2014, 20, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Hassanain, M.; Simoneau, E.; Tzimas, G.N.; Chaudhury, P.; Deschenes, M.; Valenti, D.; Ghali, P.; Wong, P.; Cabrera, T.; et al. Magnitude of Change in Alpha-Fetoprotein in Response to Transarterial Chemoembolization Predicts Survival in Patients Undergoing Liver Transplantation for Hepatocellular Carcinoma. Curr. Oncol. 2013, 20, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Duvoux, C.; Roudot–Thoraval, F.; Decaens, T.; Pessione, F.; Badran, H.; Piardi, T.; Francoz, C.; Compagnon, P.; Vanlemmens, C.; Dumortier, J.; et al. Liver Transplantation for Hepatocellular Carcinoma: A Model Including α-Fetoprotein Improves the Performance of Milan Criteria. Gastroenterology 2012, 143, 986–994 e983. [Google Scholar] [CrossRef]
- Lai, Q.; Avolio, A.W.; Graziadei, I.; Otto, G.; Rossi, M.; Tisone, G.; Goffette, P.; Vogel, W.; Pitton, M.B.; Lerut, J.; et al. Alpha-fetoprotein and modified response evaluation criteria in Solid Tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transplant. 2013, 19, 1108–1118. [Google Scholar] [CrossRef]
- Shen, J.-Y.; Li, C.; Wen, T.-F.; Yan, L.-N.; Li, B.; Wang, W.-T.; Yang, J.-Y.; Xu, M.-Q. Alpha fetoprotein changes predict hepatocellular carcinoma survival beyond the Milan criteria after hepatectomy. J. Surg. Res. 2017, 209, 102–111. [Google Scholar] [CrossRef]
- Jeong, Y.; Yoon, S.M.; Han, S.; Shim, J.H.; Kim, K.M.; Lim, Y.-S.; Lee, H.C.; Kim, S.Y.; Park, J.-H.; Lee, S.-W.; et al. Propensity Score Matching Analysis of Changes in Alpha-Fetoprotein Levels after Combined Radiotherapy and Transarterial Chemoembolization for Hepatocellular Carcinoma with Portal Vein Tumor Thrombus. PLoS ONE 2015, 10, e0135298. [Google Scholar] [CrossRef]
- Xu, X.-S.; Qu, K.; Liu, C.; Zhang, Y.-L.; Liu, J.; Song, Y.-Z.; Zhang, P.; Liu, S.-N.; Chang, H.-L. Highlights for α-fetoprotein in determining prognosis and treatment monitoring for hepatocellular carcinoma. World J. Gastroenterol. 2012, 18, 7242–7250. [Google Scholar] [CrossRef]
- Chen, C.-L.; Chen, Y.-S.; de Villa, V.H.; Wang, C.-C.; Lin, C.-L.; Goto, S.; Wang, S.-H.; Cheng, Y.-F.; Huang, T.-L.; Jawan, B.; et al. Minimal blood loss living donor hepatectomy12. Transplantation 2000, 69, 2580–2586. [Google Scholar] [CrossRef]
- de Villa, V.H.; Chen, C.-L.; Chen, Y.-S.; Wang, C.-C.; Lin, C.-C.; Cheng, Y.-F.; Huang, T.-L.; Jawan, B.; Eng, H.-L. Right Lobe Living Donor Liver Transplantation—Addressing the Middle Hepatic Vein Controversy. Ann. Surg. 2003, 238, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Merani, S.; Majno, P.; Kneteman, N.M.; Berney, T.; Morel, P.; Mentha, G.; Toso, C. The impact of waiting list alpha-fetoprotein changes on the outcome of liver transplant for hepatocellular carcinoma. J. Hepatol. 2011, 55, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Dodge, J.L.; Roberts, J.P.; Hirose, R.; Yao, F.Y. Alpha-Fetoprotein Decrease from >1000 to <500 ng/mL in Patients with Hepatocellular Carcinoma Leads to Improved Posttransplant Outcomes. Hepatology 2018, 69, 1193–1205. [Google Scholar] [CrossRef]
- Bharat, A.; Brown, D.B.; Crippin, J.S.; Gould, J.E.; Lowell, J.A.; Shenoy, S.; Desai, N.M.; Chapman, W.C. Pre-Liver Transplantation Locoregional Adjuvant Therapy for Hepatocellular Carcinoma as a Strategy to Improve Longterm Survival. J. Am. Coll. Surg. 2006, 203, 411–420. [Google Scholar] [CrossRef]
- Mizejewski, G.J. Does alpha-fetoprotein contribute to the mortality and morbidity of human hepatocellular carcinoma? A commentary. J. Hepatocell. Carcinoma 2016, 3, 37–40. [Google Scholar] [CrossRef]
- Chevret, S.; Trinchet, J.-C.; Mathieu, D.; Rached, A.A.; Beaugrand, M.; Chastang, C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hepatocellulaire. J. Hepatol. 1999, 31, 133–141. [Google Scholar] [CrossRef]
- Farinati, F.; Rinaldi, M.; Gianni, S.; Naccarato, R. How should patients with hepatocellular carcinoma be staged? Validation of a new prognostic system. Cancer 2000, 89, 2266–2273. [Google Scholar] [CrossRef]
- Mailey, B.; Artinyan, A.; Khalili, J.; Denitz, J.; Sanchez-Luege, N.; Sun, C.-L.; Bhatia, S.; Nissen, N.; Colquhoun, S.D.; Kim, J. Evaluation of Absolute Serum α-Fetoprotein Levels in Liver Transplant for Hepatocellular Cancer. Arch. Surg. 2011, 146, 26–33. [Google Scholar] [CrossRef]
- Wong, L.L.; Naugler, W.E.; Schwartz, J.; Scott, D.L.; Bhattacharya, R.; Reyes, J.; Orloff, S.L. Impact of locoregional therapy and alpha-fetoprotein on outcomes in transplantation for liver cancer: A UNOS Region 6 pooled analysis. Clin. Transplant. 2013, 27, E72–E79. [Google Scholar] [CrossRef]
- Han, K.; Tzimas, G.N.; Barkun, J.S.; Metrakos, P.; Tchervenkov, J.I.; Hilzenrat, N.; Wong, P.; Deschênes, M. Preoperative Alpha-Fetoprotein Slope is Predictive of Hepatocellular Carcinoma Recurrence after Liver Transplantation. Can. J. Gastroenterol. 2007, 21, 39–45. [Google Scholar] [CrossRef]
- Vibert, E.; Azoulay, D.; Hoti, E.; Iacopinelli, S.; Samuel, D.; Salloum, C.; Lemoine, A.; Bismuth, H.; Castaing, D.; Adam, R. Progression of Alphafetoprotein Before Liver Transplantation for Hepatocellular Carcinoma in Cirrhotic Patients: A Critical Factor. Am. J. Transplant. 2010, 10, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Halazun, K.J.; Rosenblatt, R.E.; Mehta, N.; Lai, Q.; Hajifathalian, K.; Gorgen, A.; Brar, G.; Sasaki, K.; Doyle, M.B.M.; Tabrizian, P.; et al. Dynamic α-Fetoprotein Response and Outcomes After Liver Transplant for Hepatocellular Carcinoma. JAMA Surg. 2021, 156, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.-Z.; Cheng, J.-W.; Yan, J.-Y.; Huang, A.; Wang, Y.-P.; Zhang, S.-Y.; Cao, Y.; Huang, X.-W.; Fan, J.; Zhou, J.; et al. Efficacy and safety of lenvatinib for preventing tumor recurrence after liver transplantation in hepatocellular carcinoma beyond the Milan criteria. Ann. Transl. Med. 2022, 10, 1091. [Google Scholar] [CrossRef] [PubMed]

| Clinicopathologic Characteristics | All Patients (n = 370) | Nonrecurrence (n = 323) | Recurrence (n = 47) | p |
|---|---|---|---|---|
| Age (years), median (IQR) | 55.4 (50.9–60.7) | 55.5 (50.2–60.7) | 54.7 (50.5–60.1) | 0.609 |
| Gender, male, n (%) | 295 (79.7) | 254 (78.6) | 41 (87.2) | 0.171 |
| BMI, median (IQR) | 25.2 (22.8–27.5) | 25.2 (22.8–27.5) | 25.3 (23.1–27.4) | 0.846 |
| Liver disease, n (%) | ||||
| HBV | 226 (61.1) | 193 (59.8) | 33 (70.2) | 0.169 |
| HCV | 135 (36.5) | 122 (37.8) | 13 (27.7) | 0.178 |
| Alcoholic | 24 (6.5) | 20 (6.2) | 4 (8.5) | 0.753 |
| Others | 6 (1.6) | 6 (1.9) | - | 0.346 |
| Child–Pugh class, n (%) | 0.963 | |||
| A | 204 (55.1) | 179 (55.4) | 25 (53.2) | |
| B | 23(33.2) | 105 (32.5 | 18 (38.3) | |
| C | 43(11.6) | 39 (12.1) | 4 (8.5) | |
| MELD, median (IQR) | 9.0 (7.0–12.0) | 9.0 (7.0–12.0) | 9.0 (7.0–12.0) | 0.996 |
| Pre-LRT-maximal AFP (ng/mL), median (IQR) | 23.5 (10.8–109.9) | 22.0 (10.7–106.0) | 30.0 (11.3–200.0) | 0.396 |
| Pre-LRT-maximal AFP > 20 ng/mL, n (%) | 193 (52.2) | 167 (51.7) | 26 (55.3) | 0.643 |
| Pretransplant AFP (ng/mL), median (IQR) | 10.5 (5.5–26.5) | 10.2 (5.5–22.9) | 12.7 (5.8–75.1) | 0.050 |
| Pretransplant AFP > 20 ng/mL, n (%) | 110 (29.7) | 87 (27.0) | 23 (47.6) | 0.002 |
| Pretransplant LRT number, median (IQR) | 2 (1–3) | 2 (1–3) | 3 (2–5) | <0.001 |
| Pretransplant LRT methods, n (%) | 0.003 | |||
| RFA only | 74 (20.0) | 72 (22.3) | 2 (4.3) | |
| TACE only | 128(34.6) | 114 (35.3) | 14 (29.8) | |
| PEI only | 9 (2.4) | 7 (2.2) | 2 (4.3) | |
| TACE and RFA | 97 (26.2) | 77 (23.8) | 20 (42.6) | |
| TACE and PEI | 28 (7.6) | 24 (7.4) | 4 (8.5) | |
| RFA and PEI | 8 (2.2) | 7 (2.2) | 1 (2.1) | |
| TACE, RFA, and PEI | 26 (7.0) | 22 (6.8) | 4 (8.5) | |
| Tumor number, median (IQR) | 2 (1–3) | 2 (1–3) | 2 (2–4) | 0.067 |
| Largest tumor size (cm), median (IQR) | 2.7 (2.0–3.5) | 2.6 (2.0–3.5) | 3.0 (2.5–5.0) | 0.001 |
| Total tumor size (cm), median (IQR) | 4.5 (2.8–7.0) | 4.3 (2.6–6.5) | 6.5 (3.8–11.0) | <0.001 |
| Beyond Milan criteria, n (%) | 168 (45.4) | 138 (42.7) | 30 (63.8) | 0.007 |
| Beyond UCSF criteria, n (%) | 122 (33.0) | 95 (29.4) | 27 (57.4) | <0.001 |
| Microvascular invasion, n (%) | 104 (28.1) | 73 (22.6) | 31 (66.0) | <0.001 |
| AJCC T stage, n (%) | <0.001 | |||
| T1 | 96 (25.9) | 94 (29.1) | 2 (4.3) | |
| T2 | 250 (67.6) | 213 (65.9) | 37 (78.7) | |
| T3 | 18 (4.9) | 13 (4.0) | 5 (10.6) | |
| T4 | 6 (1.6) | 3 (0.9) | 3 (6.4) | |
| Tumor necrosis, n (%) | 0.050 | |||
| No tumor necrosis | 31 (8.4) | 30 (9.3) | 1 (2.1) | |
| Partial tumor necrosis | 270 (73.0) | 224 (69.3) | 46 (97.9) | |
| Complete tumor necrosis | 69 (18.6) | 69 (21.4) | 0 (0) |
| Clinicopathologic Characteristics | Control (n = 177) | Complete AFP Response (n = 84) | Partial AFP Response (n = 67) | No AFP Response (n = 42) | p * | p ** |
|---|---|---|---|---|---|---|
| Age (years), median (IQR) | 55.5 (51.3–60.6) | 55.5 (49.5–60.7) | 54.3 (49.0–59.9) | 55.5 (51.6–60.8) | 0.703 | 0.599 |
| Male (%) | 146 (82.5) | 64 (76.2) | 54 (80.6) | 31 (73.8) | 0.256 | 0.683 |
| BMI, median (IQR) | 25.5 (22.7–27.7) | 24.8 (23.1–27.3) | 25.2 (22.5–27.5) | 25.1 (22.6–27.3) | 0.809 | 0.986 |
| Liver disease (%) | ||||||
| HBV | 111 (62.7) | 56 (66.7) | 34 (50.7) | 25 (59.5) | 0.226 | 0.141 |
| HCV | 55 (31.1) | 31 (36.9) | 29 (43.3) | 20 (47.6) | 0.120 | 0.480 |
| Alcoholic | 13 (7.3) | 5 (6.0) | 4 (6.0) | 2 (4.8) | 0.921 | 0.957 |
| Others | 5 (2.8) | 0 | 1 (1.5) | 0 | 0.183 | 0.389 |
| Child–Pugh class, n (%) | 0.576 | 0.370 | ||||
| A | 100 (56.5) | 45 (53.6) | 40 (59.7) | 19 (45.2) | ||
| B | 54 (30.5) | 33 (39.3) | 19 (28.4) | 17 (40.5) | ||
| C | 23 (13.0) | 6 (7.1) | 8 (11.9) | 6 (14.3) | ||
| MELD, median (IQR) | 9 (7–12) | 9.5 (7–12) | 10 (7–12) | 11 (8–13.3) | 0.132 | 0.206 |
| Maximal AFP (ng/mL), median (IQR) | 9.9 (6.0–14.6) | 71.2 (33.4–229.7) | 301.0 (97.3–743.0) | 72.7 (32.6–174.5) | <0.001 | <0.001 |
| Maximal AFP >400 ng/mL, n (%) | - | 15 (17.2%) | 28 (41.8%) | 6 (14.3%) | <0.001 | 0.001 |
| Pretransplant AFP (ng/mL), median (IQR) | 5.7 (3.5–9.7) | 10.2 (5.2–14.3) | 44.2 (28.9–87.0) | 72.7 (29.1–88.1) | <0.001 | <0.001 |
| Pretransplant AFP > 400, n (%) | - | - | 3 (4.5) | 6 (14.3) | <0.001 | 0.002 |
| pretransplant LRT number, median (IQR) | 2 (1–4) | 2 (1–3) | 2 (1–3) | 3 (1–4) | 0.327 | 0.239 |
| Pretransplant LRT methods, n (%) | 0.102 | 0.195 | ||||
| TACE only | 56 (31.6) | 25 (29.8) | 32 (47.8) | 15 (35.7) | ||
| RFA only | 36 (20.3) | 26 (31.0) | 8 (11.9) | 4 (9.5) | ||
| PEI only | 5 (2.8) | 3 (3.6) | 0 | 1 (2.4) | ||
| TACE and RFA | 44 (24.9) | 19 (22.6) | 21 (31.3) | 13 (31.0) | ||
| TACE and PEI | 17 (9.6) | 6 (7.1) | 3 (4.5) | 2 (4.8) | ||
| RFA and PEI | 4 (2.3) | 1 (1.2) | 1 (1.5) | 2 (4.8) | ||
| TACE, RFA, and PEI | 15 (8.5) | 4 (4.8) | 2 (3.0) | 5 (11.9) | ||
| Tumor number, median (IQR) | 2 (1–3) | 2.0 (1–3) | 2 (1–4) | 2.5 (2–5) | 0.204 | 0.111 |
| Largest tumor size (cm), median (IQR) | 2.6 (2.0–3.5) | 3.0 (2.1–3.8) | 2.7 (2.0–3.4) | 2.8 (2.0–3.6) | 0.678 | 0.769 |
| Total tumor size (cm), median (IQR) | 4.3 (2.7–6.3) | 4.5 (2.9–6.9) | 4.2 (2.7–8.0) | 6.1 (3.2–10.0) | 0.179 | 0.168 |
| Beyond Milan criteria, n (%) | 73 (41.2) | 38 (45.2) | 32 (47.8) | 25 (59.5) | 0.039 | 0.305 |
| Beyond UCSF criteria, n (%) | 56 (31.6) | 25 (29.8) | 23 (34.3) | 18 (42.9) | 0.485 | 0.344 |
| Microvascular invasion, n (%) | 42(23.7) | 18(21.4) | 22(32.8) | 22(52.4) | 0.001 | 0.002 |
| AJCC T stage, n (%) | 0.040 | 0.029 | ||||
| T1 | 53 (29.9) | 24 (28.6) | 16 (23.9) | 3 (7.1) | ||
| T2 | 112 (63.3) | 57 (67.9) | 45 (67.2) | 36 (85.7) | ||
| T3 | 8 (4.5) | 3 (3.6) | 5 (7.5) | 2 (4.8) | ||
| T4 | 4 (2.3) | 0 | 1 (1.5) | 1 (2.4) | ||
| Tumor necrosis, n (%) | 0.07 | 0.087 | ||||
| No tumor necrosis | 19 (10.7) | 5 (6.0) | 7 (10.4) | 4 (9.5) | ||
| Partial tumor necrosis | 119 (67.2) | 59 (70.2) | 55 (82.1) | 33 (78.6) | ||
| Complete tumor necrosis | 39 (22.0) | 20 (23.8) | 5 (7.5) | 5 (11.9) | ||
| Recurrence, n (%) | 21 (11.9) | 4 (4.8) | 8 (11.9) | 14 (33.3) | <0.001 | <0.001 |
| Recurrence, n (%) | 21 (11.9) | 4 (4.8) | 8 (11.9) | 14 (33.3) | <0.001 | <0.001 |
| Recurrence model | 0.776 | 0.612 | ||||
| Intra-hepatic metastasis | 10 (47.6) | 1 (25.0) | 3(37.5) | 7(50.0) | ||
| Extrahepatic metastasis | 11 (52.4) | 3 (75.0) | 5 (62.5) | 7 (50.0) | ||
| Early extrahepatic metastasis (<6 months), n (%) | 2 (1.1) | 0 (0.0) | 1 (1.5) | 1 (2.4) | - | - |
| Recurrence months, median (IQR) | 15.2 (7.8–29.60) | 24.6 (17.6–44.1) | 10.9 (6.4–23.1) | 13.9 (8.0–34.5) | 0.736 | 0.594 |
| RFS months, median (IQR) | 76.1 (49.1–112.3) | 95.2 (73.0–121.5) | 89.7 (56.5–118.2) | 69.8 (21.6–104.2) | 0.003 | 0.004 |
| Expired, n (%) | 38 (21.5) | 10 (11.9) | 10 (14.9) | 16 (38.1) | 0.004 | 0.001 |
| OS months, median (IQR) | 76.1 (50.7–112.7) | 95.2 (74.0–121.5) | 90.2 (59.5–118.2) | 75.6 (41.0–110.0) | 0.033 | 0.085 |
| Clinicopathologic Characteristics | HR (95% CI) Univariable | p | HR (95% CI) Multivariable | p |
|---|---|---|---|---|
| Age (years) | 0.996 (0.962–1.032) | 0.842 | ||
| Gender, male | 1.724 (0.732–4.061) | 0.213 | ||
| BMI | 0.968 (0.892–1.051) | 0.439 | ||
| Liver disease | ||||
| HBV | 1.478 (0.791–2.763) | 0.221 | ||
| HCV | 0.672 (0.355–1.273) | 0.223 | ||
| Alcoholic | 1.352 (0.485–3.769) | 0.564 | ||
| Others | 0.049 (0.00–1614.0) | 0.569 | ||
| Child–Pugh class | 0.671 | |||
| A | 1 | |||
| B | 1.196 (0.652–2.192) | 0.563 | ||
| C | 0.755 (0.263–2.169) | 0.601 | ||
| MELD | 1.021 (0.971–1.072) | 0.421 | ||
| Log10 maximal AFP (ng/mL) | 1.135 (0.800–1.610) | 0.478 | ||
| Log10 pretransplant AFP (ng/mL) | 1.743 (1.154–2.630) | 0.008 | ||
| Pretransplant LRT number | 1.108 (1.042–1.178) | 0.001 | 1.098 (1.017–1.186) | 0.016 |
| Pretransplant LRT methods | 0.091 | |||
| RFA only | ||||
| TACE only | 4.299 (0.977–18.92) | 0.054 | ||
| PEI only | 7.974 (1.123–56.62) | 0.038 | ||
| TACE and RFA | 8.660 (2.024–37.06) | 0.004 | ||
| TACE and PEI | 5.557 (1.018–30.342) | 0.048 | ||
| RFA and PEI | 4.582 (0.415–50.544) | 0.214 | ||
| TACE, RFA, and PEI | 6.025 (1.103–32.900) | 0.038 | ||
| Group (AFP response) | <0.001 | 0.004 | ||
| Normal AFP | 1 | 1 | ||
| Complete AFP response | 0.369 (0.126–1.074) | 0.067 | 0.289 (0.096–0.870) | 0.027 |
| Partial AFP response | 1.000 (0.443–2.258) | 1.000 | 0.951 (0.418–2.164) | 0.905 |
| No AFP response | 3.213 (1.633–6.322) | 0.001 | 2.272 (1.115–4.631) | 0.024 |
| Pathological tumor characteristics | ||||
| Tumor number | 1.142 (1.042–1.252) | 0.005 | ||
| Largest tumor size (cm) | 1.596 (1.322–1.927) | <0.001 | 1.515 (1.194–1.923) | 0.001 |
| Total tumor size (cm) | 1.137 (1.079–1.198) | <0.001 | ||
| Beyond Milan criteria | 2.176 (1.200–3.946) | 0.010 | ||
| Beyond UCSF criteria | 2.936 (1.646–5.236) | 0.001 | ||
| AJCC T stage | <0.001 | |||
| T1 | 1 | |||
| T2 | 7.462 (1.798–30.97) | 0.006 | ||
| T3 | 17.77 (3.445–91.61) | 0.001 | ||
| T4 | 38.28 (6.387–229.4) | <0.001 |
| Clinicopathologic Characteristics | HR (95% CI) for Univariable | p | HR (95% CI) for Multivariable | p |
|---|---|---|---|---|
| Age (years) | 1.000 (0.972–1.029) | 0.975 | ||
| Gender, male | 0.923 (0.523–1.628) | 0.782 | ||
| BMI | 0.976 (0.914–1.041) | 0.454 | ||
| Liver disease | ||||
| HBV | 0.895 (0.561–1.431) | 0.644 | ||
| HCV | 1.130 (0.706–1.809) | 0.611 | ||
| Alcoholic | 0.929 (0.339–2.548) | 0.886 | ||
| Others | 0.906 (0.126–6.525) | 0.922 | ||
| Child–Pugh class | 0.574 | |||
| A | 1 | |||
| B | 1.234 (0.753–2.024) | 0.404 | ||
| C | 1.364 (0.677–2.749) | 0.386 | ||
| MELD | 1.013 (0.974–1.055) | 0.517 | ||
| Log10 maximal AFP (ng/mL) | 1.012 (0.756–1.355) | 0.936 | ||
| Log10 pretransplant AFP (ng/mL) | 1.491 (1.053–2.112) | 0.024 | ||
| Pretransplant LRT number | 1.065 (1.004–1.131) | 0.038 | ||
| Pretransplant LRT methods | 0.220 | |||
| RFA only | 1 | |||
| TACE only | 1.737 (0.781–3.861) | 0.176 | ||
| PEI only | 0.861 (0.108–6.897) | 0.888 | ||
| TACE and RFA | 2.379 (1.068–5.296) | 0.034 | ||
| TACE and PEI | 3.166 (1.244–8.059) | 0.016 | ||
| RFA and PEI | 1.064 (0.133–8.510) | 0.953 | ||
| TACE, RFA, and PEI | 1.795 (0.587–5.488) | 0.305 | ||
| Group (AFP response) | 0.005 | 0.010 | ||
| Normal AFP | 1 | |||
| Complete AFP response | 0.496 (0.247–0.995) | 0.049 | 0.523 (0.260–1.052) | 0.069 |
| Partial AFP response | 0.665 (0.331–1.334) | 0.251 | 0.667 (0.331–1.341) | 0.255 |
| No AFP response | 1.873 (1.044–3.361) | 0.035 | 1.834 (1.000–3.365) | 0.050 |
| s | ||||
| Tumor number | 1.034 (0.939–1.139) | 0.497 | ||
| Largest tumor size (cm) | 1.193 (1.010–1.410) | 0.038 | ||
| Total tumor size (cm) | 1.047 (0.992–1.105) | 0.096 | ||
| Beyond Milan criteria | 1.072 (0.678–1.695) | 0.766 | ||
| Beyond UCSF criteria | 1.353 (0.845–2.169) | 0.208 | ||
| AJCC T stage | <0.001 | 0.001 | ||
| T1 | 1 | 1 | ||
| T2 | 1.390 (0.766–2.522) | 0.278 | 1.258 (0.685–0.307) | 0.459 |
| T3 | 3.745 (1.564–8.964) | 0.003 | 9.403 (2.816–31.40) | 0.001 |
| T4 | 6.984 (2.291–21.29) | 0.001 | - | 0.982 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, I.-H.; Hsu, C.-C.; Yong, C.-C.; Cheng, Y.-F.; Wang, C.-C.; Lin, C.-C.; Chen, C.-L. AFP Response to Locoregional Therapy Can Stratify the Risk of Tumor Recurrence in HCC Patients after Living Donor Liver Transplantation. Cancers 2023, 15, 1551. https://doi.org/10.3390/cancers15051551
Chen I-H, Hsu C-C, Yong C-C, Cheng Y-F, Wang C-C, Lin C-C, Chen C-L. AFP Response to Locoregional Therapy Can Stratify the Risk of Tumor Recurrence in HCC Patients after Living Donor Liver Transplantation. Cancers. 2023; 15(5):1551. https://doi.org/10.3390/cancers15051551
Chicago/Turabian StyleChen, I-Hsuan, Chien-Chin Hsu, Chee-Chien Yong, Yu-Fan Cheng, Chih-Chi Wang, Chih-Che Lin, and Chao-Long Chen. 2023. "AFP Response to Locoregional Therapy Can Stratify the Risk of Tumor Recurrence in HCC Patients after Living Donor Liver Transplantation" Cancers 15, no. 5: 1551. https://doi.org/10.3390/cancers15051551
APA StyleChen, I.-H., Hsu, C.-C., Yong, C.-C., Cheng, Y.-F., Wang, C.-C., Lin, C.-C., & Chen, C.-L. (2023). AFP Response to Locoregional Therapy Can Stratify the Risk of Tumor Recurrence in HCC Patients after Living Donor Liver Transplantation. Cancers, 15(5), 1551. https://doi.org/10.3390/cancers15051551






