Periplocin Overcomes Bortezomib Resistance by Suppressing the Growth and Down-Regulation of Cell Adhesion Molecules in Multiple Myeloma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Cells and Cell Culture
2.3. Patient Samples and CD138+ Cells Isolation
2.4. Cell Viability
2.5. Colony Forming Assay
2.6. Flow Cytometry Analysis
2.7. Migration Assay
2.8. Transcriptome Sequencing
2.9. Quantitative Real-Time PCR
2.10. Proteins Extraction and Western Blot
2.11. Xenograft Mouse Model
2.12. Statistical Analysis
3. Results
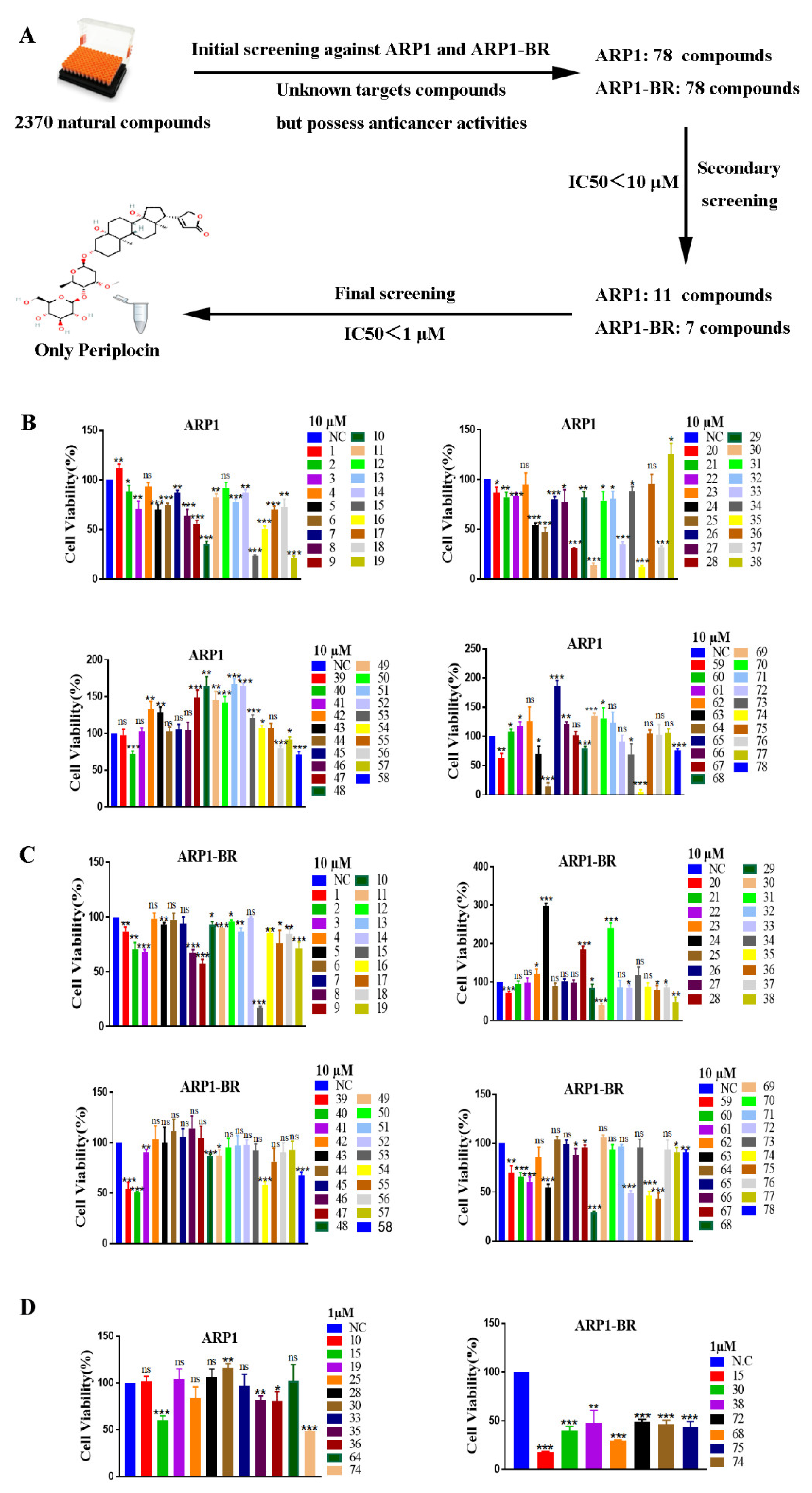
3.1. Periplocin Is an Effective Anti-MM Natural Compound
3.2. Periplocin Inhibits Proliferation and Induces Apoptosis in MM
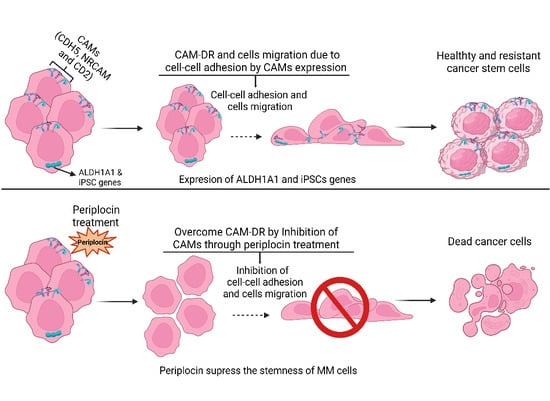
3.3. PeriplocinSuppresses Stemness and Cells Migration of MM
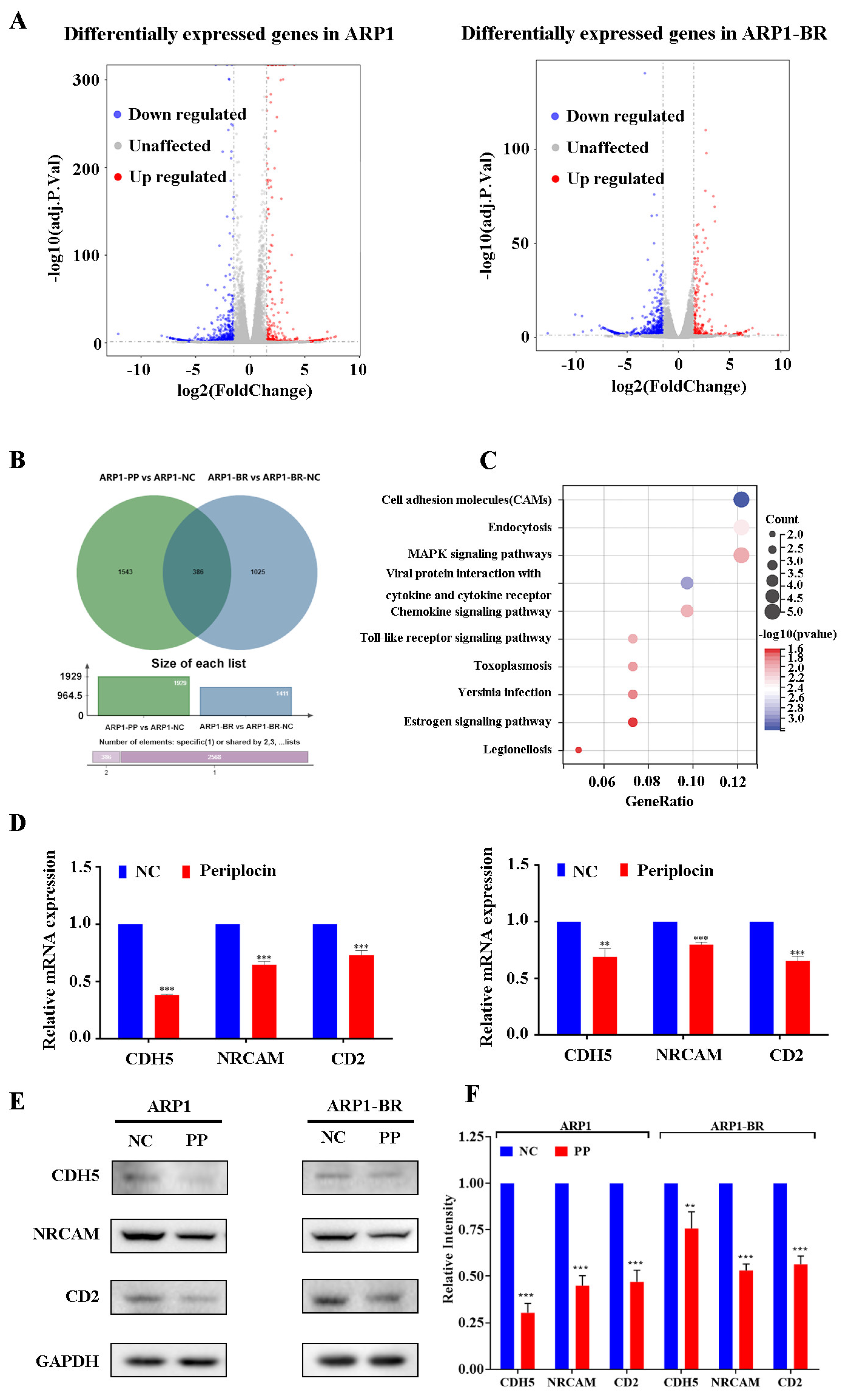
3.4. PeriplocinDownregulates Cell Adhesion Molecules
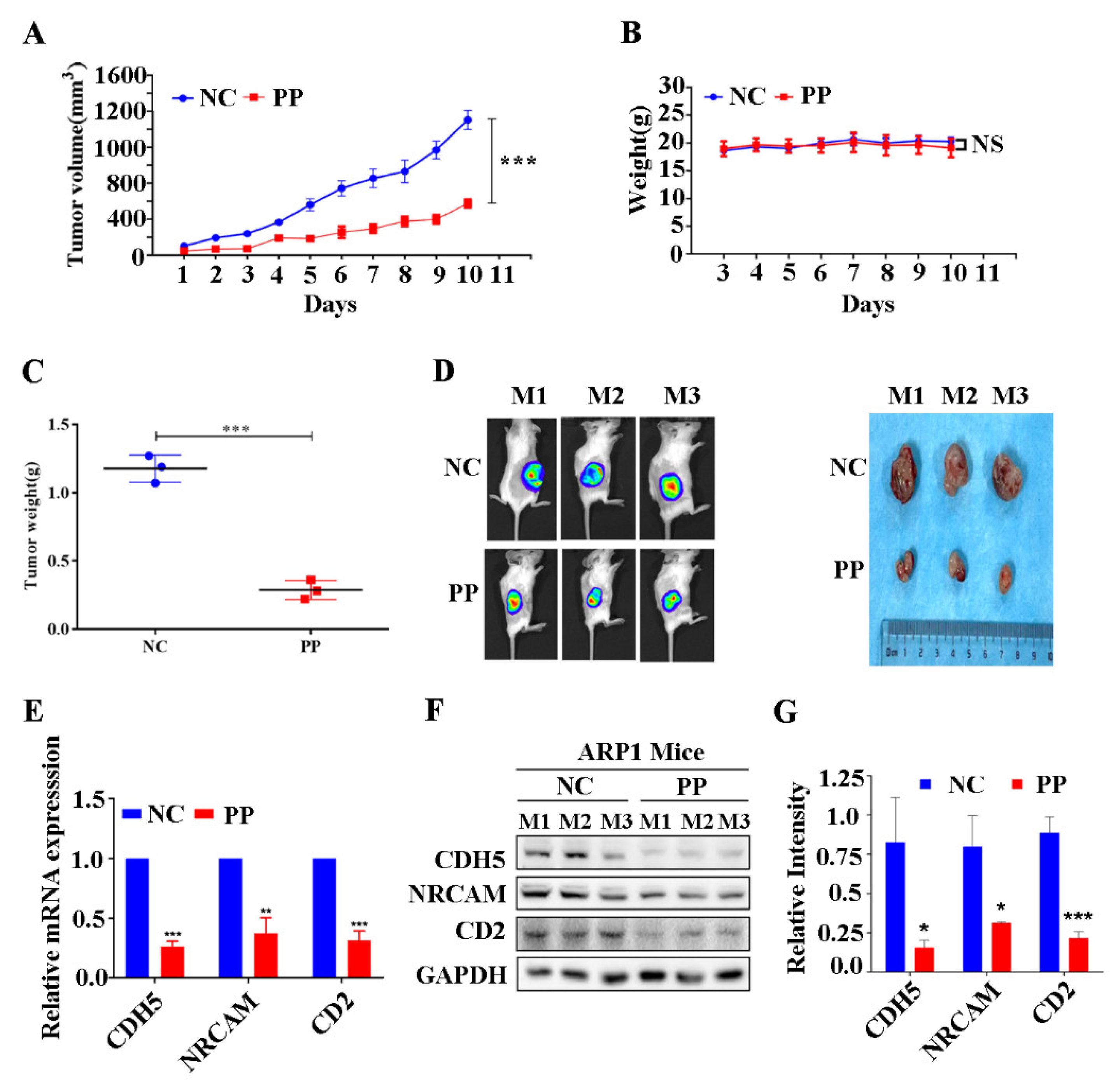
3.5. Periplocin Inhibits Tumor Growth of BTZ-Sensitive Xenograft Mouse Model of MM and Suppresses CAMs In Vivo
3.6. Periplocin Inhibits Tumor Growth of BTZ-Resistant Xenograft Mouse Model of MM and Suppresses CAMs In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dutta, A.K.; Alberge, J.B.; Sklavenitis-Pistofidis, R.; Lightbody, E.D.; Getz, G.; Ghobrial, I.M. Single-cell profiling of tumour evolution in multiple myeloma—Opportunities for precision medicine. Nat. Rev. Clin. Oncol. 2022, 19, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Attal, M.; Richardson, P.G.; Rajkumar, S.V.; San-Miguel, J.; Beksac, M.; Spicka, I.; Leleu, X.; Schjesvold, F.; Moreau, P.; Dimopoulos, M.A.; et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): Follow-up analysis of a randomized, phase 3 study. Lancet Oncol. 2022, 23, 416–427. [Google Scholar]
- Rajkumar, S.V.; Kyle, R.A. Progress in Myeloma—A Monoclonal Breakthrough. N. Engl. J. Med. 2016, 375, 1390–1392. [Google Scholar] [CrossRef]
- Goel, U.; Usmani, S.; Kumar, S. Current approaches to the management of newly diagnosed multiple myeloma. Am. J.Hematol. 2022, 97, S3–S25. [Google Scholar] [CrossRef]
- Adams, J.; Palombella, V.J.; Sausville, E.A.; Johnson, J.; Destree, A.; Lazarus, D.D.; Maas, J.; Pien, C.S.; Prakash, S.; Elliott, P.J. Proteasome inhibitors: A novel class of potent and effective antitumor agents. Cancer Res. 1999, 59, 2615–2622. [Google Scholar] [PubMed]
- Richardson, P.G.; Barlogie, B.; Berenson, J.; Singhal, S.; Jagannath, S.; Irwin, D.; Rajkumar, S.V.; Srkalovic, G.; Alsina, M.; Alexanian, R.; et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N. Engl. J. Med. 2003, 348, 2609–2617. [Google Scholar] [CrossRef]
- Moreau, P.; de Wit, E. Recent progress in relapsed multiple myeloma therapy: Implications for treatment decisions. Br. J.Haematol. 2017, 179, 198–218. [Google Scholar] [CrossRef] [PubMed]
- Nikesitch, N.; Rebeiro, P.; Ho, L.L.; Pothula, S.; Wang, X.M.; Khong, T.; Quek, H.; Spencer, A.; Lee, C.S.; Roberts, T.L.; et al. The Role of Chaperone-Mediated Autophagy in Bortezomib Resistant Multiple Myeloma. Cells 2021, 10, 3464. [Google Scholar] [CrossRef]
- Cohen, Y.C.; Zada, M.; Wang, S.Y.; Bornstein, C.; David, E.; Moshe, A.; Li, B.; Shlomi-Loubaton, S.; Gatt, M.E.; Gur, C.; et al. Identification of resistance pathways and therapeutic targets in relapsed multiple myeloma patients through single-cell sequencing. Nat. Med. 2021, 27, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Roy, M.; Liang, L.; Cao, W.; Hu, B.; Li, Y.; Xiao, X.; Wang, H.; Ye, M.; Sun, S.; et al. Deubiquitylase USP12 induces pro-survival autophagy and bortezomib resistance in multiple myeloma by stabilizing HMGB1. Oncogene 2022, 41, 1298–1308. [Google Scholar] [CrossRef]
- Wang, C.; Li, L.; Li, M.; Wang, W.; Jiang, Z. FTO promotes Bortezomib resistance via m6A-dependent destabilization of SOD2 expression in multiple myeloma. Cancer Gene Ther. 2022, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.A.; Lo Celso, C.; Purton, L.E.; Frisch, B.J. From the niche to malignant hematopoiesis and back: Reciprocal interactions between leukemia and the bone marrow microenvironment. JBMR Plus 2021, 5, e10516. [Google Scholar] [CrossRef] [PubMed]
- Bruce, A.; Evans, R.; Mezan, R.; Shi, L.; Moses, B.S.; Martin, K.H.; Gibson, L.F.; Yang, Y. Three-Dimensional Microfluidic Tri-Culture Model of the Bone Marrow Microenvironment for Study of Acute Lymphoblastic Leukemia. PLoS ONE 2015, 10, e0140506. [Google Scholar] [CrossRef]
- BouZerdan, M.; Nasr, L.; Kassab, J.; Saba, L.; Ghossein, M.; Yaghi, M.; Dominguez, B.; Chaulagain, C.P. Adhesion molecules in multiple myeloma oncogenesis and targeted therapy. Int. J. Hematol. Oncol. 2022, 11, Ijh39. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, L.; Desantis, V.; Solimando, A.G.; Ruggieri, S.; Annese, T.; Nico, B.; Fumarulo, R.; Vacca, A.; Frassanito, M.A. Microenvironment drug resistance in multiple myeloma: Emerging new players. Oncotarget 2016, 7, 60698–60711. [Google Scholar] [CrossRef]
- Hideshima, T.; Anderson, K.C. Signaling Pathway Mediating Myeloma Cell Growth and Survival. Cancers 2021, 13, 216. [Google Scholar] [CrossRef]
- Damiano, J.S.; Cress, A.E.; Hazlehurst, L.A.; Shtil, A.A.; Dalton, W.S. Cell adhesion mediated drug resistance (CAM-DR): Role of integrins and resistance to apoptosis in human myeloma cell lines. Blood 1999, 93, 1658–1667. [Google Scholar] [CrossRef]
- Preedy, V.R. Feature, Structure and Classifi cation of Adhesion Molecules: An Overview. Adhes. Mol. 2016, 1–20. [Google Scholar]
- Farahani, E.; Patra, H.K.; Jangamreddy, J.R.; Rashedi, I.; Kawalec, M.; Rao Pariti, R.K.; Batakis, P.; Wiechec, E. Cell adhesion molecules and their relation to (cancer) cell stemness. Carcinogenesis 2014, 35, 747–759. [Google Scholar] [CrossRef]
- Katz, B.Z. Adhesion molecules—The lifelines of multiple myeloma cells. Semin. Cancer Biol. 2010, 20, 186–195. [Google Scholar] [CrossRef]
- Vacca, A.; Loreto, M.D.; Ribatti, D.; Stefano, R.D.; Gadaleta-Caldarola, G.; Iodice, G.; Caloro, D.; Dammacco, F. Bone marrow of patients with active multiple myeloma: Angiogenesis and plasma cell adhesion molecules LFA-1, VLA-4, LAM-1, and CD44. Am. J. Hematol. 1995, 50, 9–14. [Google Scholar] [CrossRef]
- Schmidmaier, R.; Mörsdorf, K.; Baumann, P.; Emmerich, B.; Meinhardt, G. Evidence for cell adhesion-mediated drug resistance of multiple myeloma cells in vivo. Int. J. Biol. Markers 2006, 21, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Hawley, R.G.; Kodaka, M.; Okuno, H. Significance of VLA-4-VCAM-1 interaction and CD44 for transendothelial invasion in a bone marrow metastatic myeloma model. Clin. Exp. Metastasis 1999, 17, 623–629. [Google Scholar] [CrossRef]
- Cook, G.; Dumbar, M.; Franklin, I.M. The role of adhesion molecules in multiple myeloma. Leuk. Lymphoma 1992, 8, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Sevilla-Movilla, S.; Arellano-Sánchez, N.; Martínez-Moreno, M.; Gajate, C.; Sánchez-Vencells, A.; Valcárcel, L.V.; Agirre, X.; Valeri, A.; Martínez-López, J.; Prósper, F.; et al. Upregulated expression and function of the α4β1 integrin in multiple myeloma cells resistant to bortezomib. J. Pathol. 2020, 252, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Waldschmidt, J.M.; Simon, A.; Wider, D.; Müller, S.J.; Follo, M.; Ihorst, G.; Decker, S.; Lorenz, J.; Chatterjee, M.; Azab, A.K.; et al. CXCL12 and CXCR7 are relevant targets to reverse cell adhesion-mediated drug resistance in multiple myeloma. Br. J. Haematol. 2017, 179, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Jöhrer, K.; Ҫiҫek, S.S. Multiple Myeloma Inhibitory Activity of Plant Natural Products. Cancers 2021, 13, 2678. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Lee, J.H.; Chai, E.Z.P.; Kanchi, M.M.; Kar, S.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Ramar, P.S.; Looi, C.Y.; et al. Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds. Semin. Cancer Biol. 2016, 40–41, 35–47. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Zhou, K.; He, J.; Cao, J.; An, M.; Chang, Y.X. A Review on Phytochemistry and Pharmacology of Cortex Periplocae. Molecules 2016, 21, 1702. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Sun, L.; Li, Y.; Chen, B.; Wang, C. Periplocin inhibits the growth of pancreatic cancer by inducing apoptosis via AMPK-mTOR signaling. Cancer Med. 2021, 10, 325–336. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, G.; Zhang, Y.; Liu, G.; Shan, B. Periplocin of Cortex Periplocae inhibits Stat3 signaling and induces apoptosis in human hepatocellular carcinoma cell line SMMC-7721. J. Third Mil. Med. Univ. 2008, 30, 1448–1451. [Google Scholar]
- Lu, Z.J.; Zhou, Y.; Song, Q.; Qin, Z.; Zhang, H.; Zhou, Y.J.; Gou, L.T.; Yang, J.L.; Luo, F. Periplocin inhibits the growth of lung cancer in vitro and in vivo by blocking AKT/ERK signaling pathways. Cell. Physiol.Biochem. 2010, 26, 609–618. [Google Scholar] [CrossRef]
- Zhang, X.; Nan, H.; Guo, J.; Yang, S.; Liu, J. Periplocin induces apoptosis and inhibits inflammation in rheumatoid arthritis fibroblast-like synoviocytes via nuclear factor kappa B pathway. IUBMB Life 2020, 72, 1951–1959. [Google Scholar] [PubMed]
- Bao, C.; Liu, Y.; Sun, X.; Xing, C.; Zeng, L.; Sun, G. Periplocaforrestii saponin ameliorates CIA via suppressing proinflammatory cytokines and nuclear factor kappa-B pathways. PLoS ONE 2017, 12, e0176672. [Google Scholar] [CrossRef]
- Zhao, L.; Shan, B.; Du, Y.; Wang, M.; Liu, L.; Ren, F.Z. Periplocin from Cortex periplocae inhibits cell growth and down-regulates survivin and c-myc expression in colon cancer in vitro and in vivo via beta-catenin/TCF signaling. Oncol. Rep. 2010, 24, 375–383. [Google Scholar] [PubMed]
- Bae, E.S.; Byun, W.S.; Ock, C.W.; Kim, W.K.; Park, H.J.; Lee, S.K. Periplocin exerts antitumor activity by regulating Nrf2-mediated signaling pathway in gemcitabine-resistant pancreatic cancer cells. Biomed. Pharmacother 2023, 157, 114039. [Google Scholar] [PubMed]
- Tanaka, K.; Helena, A.Y.; Yang, S.; Han, S.; Selcuklu, S.D.; Kim, K.; Ramani, S.; Ganesan, Y.T.; Moyer, A.; Sinha, S.; et al. Targeting Aurora B kinase prevents and overcomes resistance to EGFR inhibitors in lung cancer by enhancing BIM-and PUMA-mediated apoptosis. Cancer Cell 2021, 39, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qi, Y.; Yu, J.; Hao, Y.; He, B.; Zhang, M.; Dai, Z.; Jiang, T.; Li, S.; Huang, F.; et al. Nuclear Aurora kinase A switches m6A reader YTHDC1 to enhance an oncogenic RNA splicing of tumor suppressor RBM4. Signal Transduct. Target. Ther. 2022, 7, 97. [Google Scholar] [CrossRef]
- Yi, H.; Liang, L.; Wang, H.; Luo, S.; Hu, L.; Wang, Y.; Shen, X.; Xiao, L.; Zhang, Y.; Peng, H.; et al. Albendazole inhibits NF-κB signaling pathway to overcome tumor stemness and bortezomib resistance in multiple myeloma. Cancer Lett. 2021, 520, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xiao, X.; Li, Z.; Luo, S.; Hu, L.; Yi, H.; Xiang, R.; Zhu, Y.; Wang, Y.; Zhu, L.; et al. Polyphyllin VII, a novel moesin inhibitor, suppresses cell growth and overcomes bortezomib resistance in multiple myeloma. Cancer Lett. 2022, 537, 215647. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, H.; Chen, P.; Shen, X.; Zhang, B.; Liu, J.; Peng, H.; Xiao, X. Identification and Characterization of Multiple Myeloma Stem Cell-Like Cells. Cancers 2021, 13, 3523. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.D.; Chen, C.P.; Bahna, F.; Honig, B.; Shapiro, L. Cadherin-mediated cell-cell adhesion: Sticking together as a family. Curr. Opin. Struct. Biol. 2003, 13, 690–698. [Google Scholar] [CrossRef]
- McEver, R.P.; Zhu, C. Rolling cell adhesion. Annu. Rev. Cell Dev. Biol. 2010, 26, 363–396. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, R.Z.; Stinchcombe, T.E.; Mitchell, B.S.; Shea, T.C.; Baldwin, A.S.; Stahl, S.; Adams, J.; Esseltine, D.L.; Elliott, P.J.; Pien, C.S.; et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J. Clin. Oncol. 2002, 20, 4420–4427. [Google Scholar] [CrossRef]
- Abdi, J.; Chen, G.; Chang, H. Drug resistance in multiple myeloma: Latest findings and new concepts on molecular mechanisms. Oncotarget 2013, 4, 2186–2207. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.C. New insights into therapeutic targets in myeloma. Hematol. Am. Soc.Hematol. Educ. Program. 2011, 2011, 184–190. [Google Scholar] [CrossRef]
- Gonzalez-Santamarta, M.; Quinet, G.; Reyes-Garau, D.; Sola, B.; Roué, G.; Rodriguez, M.S. Resistance to the Proteasome Inhibitors: Lessons from Multiple Myeloma and Mantle Cell Lymphoma. Adv. Exp. Med. Biol. 2020, 1233, 153–174. [Google Scholar]
- Glavey, S.V.; Naba, A.; Manier, S.; Clauser, K.; Tahri, S.; Park, J.; Reagan, M.R.; Moschetta, M.; Mishima, Y.; Gambella, M.; et al. Proteomic characterization of human multiple myeloma bone marrow extracellular matrix. Leukemia 2017, 31, 2426–2434. [Google Scholar] [CrossRef]
- Li, L.; Zhao, L.M.; Dai, S.L.; Cui, W.X.; Lv, H.L.; Chen, L.; Shan, B.E. Periplocin Extracted from Cortex Periplocae Induced Apoptosis of Gastric Cancer Cells via the ERK1/2-EGR1 Pathway. Cell. Physiol.Biochem. 2016, 38, 1939–1951. [Google Scholar] [CrossRef]
- Zhao, L.M.; Li, L.; Huang, Y.; Han, L.J.; Li, D.; Huo, B.J.; Dai, S.L.; Xu, L.Y.; Zhan, Q.; Shan, B.E. Antitumor Effect of Periplocin in TRAIL-Resistant gastric cancer cells via upregulation of death receptor through activating ERK1/2-EGR1 pathway. Mol. Carcinog. 2019, 58, 1033–1045. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Zhang, Y.; Zheng, B.; Wang, X.; Chen, J.; Chen, B.; Xie, G.; Yang, L. Periplocin Induces Apoptosis of Pancreatic Cancer Cells through Autophagy via the AMPK/mTOR Pathway. J. Oncol. 2022, 2022, 8055004. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, G.; Zhao, L.; Dai, S.; Han, J.; Hu, X.; Zhou, C.; Wang, F.; Ma, H.; Li, B.; et al. Periplocymarin Induced Colorectal Cancer Cells Apoptosis Via Impairing PI3K/AKT Pathway. Front. Oncol. 2021, 11, 753598. [Google Scholar] [CrossRef]
- Wang, W.; Fan, Y.; Huang, X.; Li, L.; Wang, S.; Xue, Z.; Ouyang, H.; He, J. Metabolomics study on the periplocin-induced cardiotoxicity and the compatibility of periplocin and Panax notoginseng saponins in reducing cardiotoxicity in rats by GC-MS. J. Sep. Sci. 2021, 44, 2785–2797. [Google Scholar] [CrossRef]
- Xu, L.Q.; Lu, H.Z.; Zhang, Y.L. Clinical observation on treatment of 147 cases of chronic congestive heart-failure by beiwujiapi mixture. Yunnan J. Tradit. Chin. Med. Mater. Med. 1989, 19, 29–31. [Google Scholar]
- Liang, S.; Deng, F.; Xing, H.; Wen, H.; Shi, X.; Martey, O.N.; Koomson, E.; He, X. P-glycoprotein-and organic anion-transporting polypeptide-mediated transport of periplocin may lead to drug–herb/drug–drug interactions. Drug Des. Devel.Ther. 2014, 8, 475–483. [Google Scholar]
- Han, L.; Dai, S.; Li, Z.; Zhang, C.; Wei, S.; Zhao, R.; Zhang, H.; Zhao, L.; Shan, B. Combination of the natural compound Periplocin and TRAIL induce esophageal squamous cell carcinoma apoptosis in vitro and in vivo: Implication in anticancer therapy. J. Exp. Clin. Cancer Res. 2019, 38, 501. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, J.; Tolomelli, G.; Xu, H.; Xia, J.; Wang, H.; Zhou, W.; Zhou, Y.; Das, S.; Gu, Z.; et al. RARα2 expression confers myeloma stem cell features. Am. J. Hematol. 2013, 122, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Prager, B.C.; Xie, Q.; Bao, S.; Rich, J.N. Cancer stem cells: The architects of the tumor ecosystem. Cell Stem Cell 2019, 24, 41–53. [Google Scholar] [CrossRef]
- Liu, S.; Ginestier, C.; Ou, S.J.; Clouthier, S.G.; Patel, S.H.; Monville, F.; Korkaya, H.; Heath, A.; Dutcher, J.; Kleer, C.G.; et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011, 71, 614–624. [Google Scholar] [CrossRef]
- Liu, S.; Ginestier, C.; Ou, S.J.; Clouthier, S.G.; Patel, S.H.; Monville, F.; Korkaya, H.; Heath, A.; Dutcher, J.; Kleer, C.G.; et al. ALDH1 activity identifies tumor-initiating cells and links to chromosomal instability signatures in multiple myeloma. Leukemia 2014, 28, 1155–1158. [Google Scholar]
- Wai Wong, C.; Dye, D.E.; Coombe, D.R. The role of immunoglobulin superfamily cell adhesion molecules in cancer metastasis. Int. J. Cell Biol. 2012, 2012, 340296. [Google Scholar] [CrossRef]
- Kawauchi, T. Cell adhesion and its endocytic regulation in cell migration during neural development and cancer metastasis. Int. J. Mol. Sci. 2012, 13, 4564–4590. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, B.; Boroumandieh, S.; Majidzadeh, A.K.; Salehi, M.; Jafari, F.; Farahmand, L. The role of activated leukocyte cell adhesion molecule (ALCAM) in cancer progression, invasion, metastasis, and recurrence: A novel cancer stem cell marker and tumor-specific prognostic marker. Exp. Mol. Pathol. 2020, 115, 104443. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zuo, X.; Wei, D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. Stem Cells Transl. Med. 2015, 4, 1033–1043. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, A.; Wang, H.; Wang, Y.; Li, Z.; Yang, C.; Ma, Z.; Xiao, X.; Liu, J. Periplocin Overcomes Bortezomib Resistance by Suppressing the Growth and Down-Regulation of Cell Adhesion Molecules in Multiple Myeloma. Cancers 2023, 15, 1526. https://doi.org/10.3390/cancers15051526
Aziz A, Wang H, Wang Y, Li Z, Yang C, Ma Z, Xiao X, Liu J. Periplocin Overcomes Bortezomib Resistance by Suppressing the Growth and Down-Regulation of Cell Adhesion Molecules in Multiple Myeloma. Cancers. 2023; 15(5):1526. https://doi.org/10.3390/cancers15051526
Chicago/Turabian StyleAziz, Abdul, Haiqin Wang, Yanpeng Wang, Zhenzhen Li, Chaoying Yang, Zekang Ma, Xiaojuan Xiao, and Jing Liu. 2023. "Periplocin Overcomes Bortezomib Resistance by Suppressing the Growth and Down-Regulation of Cell Adhesion Molecules in Multiple Myeloma" Cancers 15, no. 5: 1526. https://doi.org/10.3390/cancers15051526
APA StyleAziz, A., Wang, H., Wang, Y., Li, Z., Yang, C., Ma, Z., Xiao, X., & Liu, J. (2023). Periplocin Overcomes Bortezomib Resistance by Suppressing the Growth and Down-Regulation of Cell Adhesion Molecules in Multiple Myeloma. Cancers, 15(5), 1526. https://doi.org/10.3390/cancers15051526