The Influence of Ethnicity on Survival from Malignant Primary Brain Tumours in England: A Population-Based Cohort Study
Abstract
Simple Summary
Abstract
1. Background
2. Data and Methods
2.1. Study Population
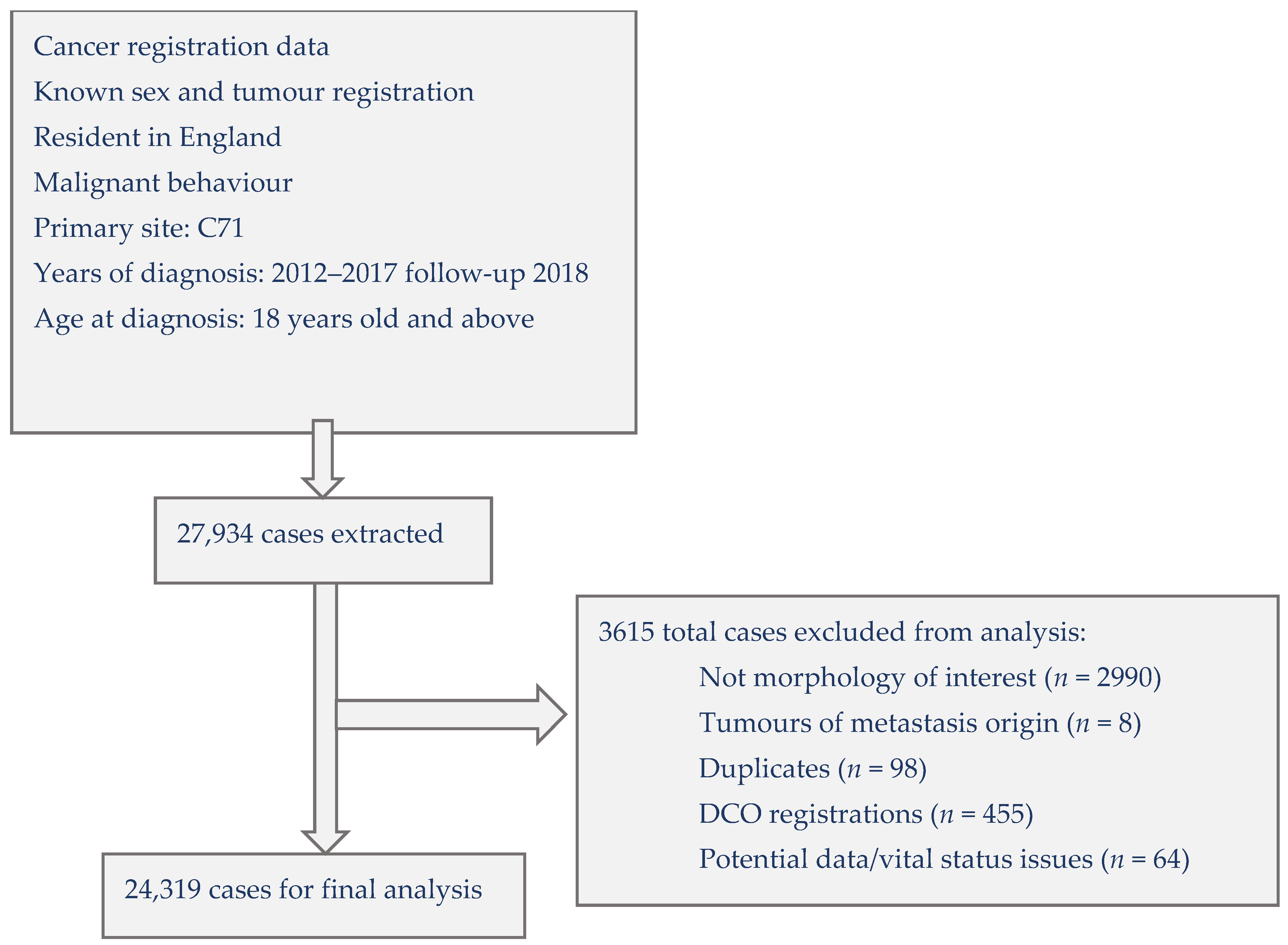
2.2. Selection of Cases
2.3. Data Analysis
2.4. Ethical Approval
3. Results
4. Discussion
4.1. Main Findings
4.2. Comparisons to Other Findings
4.3. Interpretations and Implications
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Research UK. Brain, Other CNS and Intracranial Tumours Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/brain-other-cns-and-intracranial-tumours?_ga=2.172768099.1375741702.1600433248-1594617780.1600433248 (accessed on 1 April 2016).
- ONS. Ethnicity Statistics for England. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/ethnicity (accessed on 1 July 2019).
- Wanis, H.A.; Møller, H.; Ashkan, K.; Davies, E.A. The incidence of major subtypes of primary brain tumors in adults in England 1995–2017. Neuro-Oncology 2021, 23, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Maile, E.J.; Barnes, I.; Finlayson, A.E.; Sayeed, S.; Ali, R. Nervous System and Intracranial Tumour Incidence by Ethnicity in England, 2001–2007: A Descriptive Epidemiological Study. PLoS ONE 2016, 11, e0154347. [Google Scholar] [CrossRef] [PubMed]
- Delon, C.; Brown, K.F.; Payne, N.W.S.; Kotrotsios, Y.; Vernon, S.; Shelton, J. Differences in cancer incidence by broad ethnic group in England, 2013–2017. Br. J. Cancer 2022, 126, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.; Forman, D. Cancer Incidence and Survival by Major Ethnic Group, England, 2002–2006; Cancer Research UK & NCIN: London, UK, 2009. [Google Scholar]
- Ratneswaren, T.; Jack, R.M.; Tataru, D.; Davies, E.A. The survival of patients with high grade glioma from different ethnic groups in South East England. J. Neurooncol. 2014, 120, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Elliss-Brookes, L.; McPhail, S.; Ives, A.; Greenslade, M.; Shelton, J.; Hiom, S.; Richards, M. Routes to diagnosis for cancer-determining the patient journey using multiple routine data sets. Br. J. Cancer 2012, 107, 1220–1226. [Google Scholar] [CrossRef]
- Jemal, A.; Murray, T.; Samuels, A.; Ghafoor, A.; Ward, E.; Thun, M.J. Cancer statistics, 2003. CA Cancer J. Clin. 2003, 53, 5–26. [Google Scholar] [CrossRef]
- Patel, N.P.; Lyon, K.A.; Huang, J.H. The effect of race on the prognosis of the glioblastoma patient: A brief review. Neurol. Res. 2019, 41, 967–971. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro-Oncology 2020, 22, iv1–iv96. [Google Scholar] [CrossRef]
- Gabriel, A.; Batey, J.; Capogreco, J.; Kimball, D.; Walters, A.; Tubbs, R.S.; Loukas, M. Adult brain cancer in the U.S. black population: A Surveillance, Epidemiology, and End Results (SEER) analysis of incidence, survival, and trends. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2014, 20, 1510–1517. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cote, D.J.; Ascha, M.; Kruchko, C.; Barnholtz-Sloan, J.S. Adult Glioma Incidence and Survival by Race or Ethnicity in the United States From 2000 to 2014. JAMA Oncol. 2018, 4, 1254–1262. [Google Scholar] [CrossRef]
- Barnholtz-Sloan, J.S.; Maldonado, J.L.; Williams, V.L.; Curry, W.T.; Rodkey, E.A.; Barker, F.G., 2nd; Sloan, A.E. Racial/ethnic differences in survival among elderly patients with a primary glioblastoma. J. Neurooncol. 2007, 85, 171–180. [Google Scholar] [CrossRef]
- Barnholtz-Sloan, J.S.; Sloan, A.E.; Schwartz, A.G. Racial differences in survival after diagnosis with primary malignant brain tumor. Cancer 2003, 98, 603–609. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Fulop, J.; Liu, M.; Blanda, R.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro-Oncology 2015, 17 (Suppl. S4), iv1–iv62. [Google Scholar] [CrossRef]
- McCormack, R.M.; Zhu, P.; Dono, A.; Takayasu, T.; Bhatia, A.; Blanco, A.I.; Tandon, N.; Ostrom, Q.T.; Gonzales, A.; Moreno, S.; et al. Role of Ethnicity and Geographic Location on Glioblastoma IDH1/IDH2 Mutations. World Neurosurg. 2021, 149, e894–e912. [Google Scholar] [CrossRef]
- The King’s Fund. The Health of People from Ethnic Minority Groups in England. Available online: https://www.kingsfund.org.uk/publications/health-people-ethnic-minority-groups-england (accessed on 12 January 2021).
- Braganza, M.Z.; Kitahara, C.M.; Berrington de González, A.; Inskip, P.D.; Johnson, K.J.; Rajaraman, P. Ionizing radiation and the risk of brain and central nervous system tumors: A systematic review. Neuro-Oncology 2012, 14, 1316–1324. [Google Scholar] [CrossRef]
- Jack, R.H.; Davies, E.A.; Møller, H. Lung cancer incidence and survival in different ethnic groups in South East England. Br. J. Cancer 2011, 105, 1049–1053. [Google Scholar] [CrossRef]
- Walter, F.M.; Penfold, C.; Joannides, A.; Saji, S.; Johnson, M.; Watts, C.; Brodbelt, A.; Jenkinson, M.D.; Price, S.J.; Hamilton, W.; et al. Missed opportunities for diagnosing brain tumours in primary care: A qualitative study of patient experiences. Br. J. Gen. Pract. 2019, 69, e224–e235. [Google Scholar] [CrossRef]
- Lyratzopoulos, G.; Neal, R.D.; Barbiere, J.M.; Rubin, G.P.; Abel, G.A. Variation in number of general practitioner consultations before hospital referral for cancer: Findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol. 2012, 13, 353–365. [Google Scholar] [CrossRef]
- Mendonca, S.C.; Abel, G.A.; Lyratzopoulos, G. Pre-referral GP consultations in patients subsequently diagnosed with rarer cancers: A study of patient-reported data. Br. J. Gen. Pract. 2016, 66, e171–e181. [Google Scholar] [CrossRef]
- Abel, G.A.; Shelton, J.; Johnson, S.; Elliss-Brookes, L.; Lyratzopoulos, G. Cancer-specific variation in emergency presentation by sex, age and deprivation across 27 common and rarer cancers. Br. J. Cancer 2015, 112, S129–S136. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.J.; Scheurer, M.E.; Woehrer, A.; Wiemels, J. Evolving evidence on tumor and germline genetic classification of gliomas: Implications for etiology and survival studies. Clin. Neuropathol. 2016, 35, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ohgaki, H.; Kleihues, P. Genetic pathways to primary and secondary glioblastoma. Am. J. Pathol. 2007, 170, 1445–1453. [Google Scholar] [CrossRef]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef]
- Crimmins, E.M.; Thyagarajan, B.; Levine, M.E.; Weir, D.R.; Faul, J. Associations of Age, Sex, Race/Ethnicity, and Education With 13 Epigenetic Clocks in a Nationally Representative U.S. Sample: The Health and Retirement Study. J. Gerontol. Ser. A 2021, 76, 1117–1123. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Linet, M.S.; Brenner, A.V.; Wang, S.S.; Melin, B.S.; Wang, Z.; Inskip, P.D.; Freeman, L.E.B.; Braganza, M.Z.; Carreón, T.; et al. Personal history of diabetes, genetic susceptibility to diabetes, and risk of brain glioma: A pooled analysis of observational studies. Cancer Epidemiol. Biomark. Prev. 2014, 23, 47–54. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, Z.; Huang, P. Diabetes mellitus and the risk of glioma: A meta-analysis. Oncotarget 2016, 7, 4483–4489. [Google Scholar] [CrossRef]
- Whyte, M.B.; Hinton, W.; McGovern, A.; van Vlymen, J.; Ferreira, F.; Calderara, S.; Mount, J.; Munro, N.; de Lusignan, S. Disparities in glycaemic control, monitoring, and treatment of type 2 diabetes in England: A retrospective cohort analysis. PLoS Med. 2019, 16, e1002942. [Google Scholar] [CrossRef]
- Klinger, N.V.; Mittal, S. Therapeutic Potential of Curcumin for the Treatment of Brain Tumors. Oxidative Med. Cell. Longev. 2016, 2016, 9324085. [Google Scholar] [CrossRef]
- Das, B.R.; Tangri, R.; Ahmad, F.; Roy, A.; Patole, K. Molecular investigation of isocitrate dehydrogenase gene (IDH) mutations in gliomas: First report of IDH2 mutations in Indian patients. Asian Pac. J. Cancer Prev. 2013, 14, 7261–7264. [Google Scholar] [CrossRef]
- Dasgupta, A.; Gupta, T.; Jalali, R. Indian data on central nervous tumors: A summary of published work. S. Asian J. Cancer 2016, 5, 147–153. [Google Scholar] [CrossRef]
- Deng, L.; Xiong, P.; Luo, Y.; Bu, X.; Qian, S.; Zhong, W.; Lv, S. Association between IDH1/2 mutations and brain glioma grade. Oncol. Lett. 2018, 16, 5405–5409. [Google Scholar] [CrossRef]
- Watanabe, T.; Nobusawa, S.; Kleihues, P.; Ohgaki, H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am. J. Pathol. 2009, 174, 1149–1153. [Google Scholar] [CrossRef]
| Cases | % | Deaths | Univariate | Mutually Adjusted | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | ||||||
| Age at diagnosis | |||||||
| <=34 | 1549 | 6.4 | 538 | 1.00 | 1.00 | ||
| 35–44 | 1662 | 6.8 | 802 | 1.44 | (1.22, 1.70) | 1.54 | (1.30, 1.82) |
| 45–54 | 3158 | 13.0 | 2278 | 2.84 | (2.46, 3.26) | 2.64 | (2.29, 3.04) |
| 55–64 | 4993 | 20.5 | 4285 | 4.89 | (4.28, 5.59) | 3.95 | (3.45, 4.53) |
| 65–74 | 6667 | 27.4 | 6184 | 8.11 | (7.11, 9.26) | 4.95 | (4.32, 5.67) |
| 75–84 | 4738 | 19.5 | 4635 | 15.69 | (13.75, 17.91) | 5.31 | (4.63, 6.09) |
| >=85 | 1552 | 6.4 | 1545 | 26.21 | (22.81, 30.12) | 6.37 | (5.52, 7.36) |
| Trend | χ2 (1) = 7273.09; p < 0.001 | χ2 (1) = 1279.79; p < 0.001 | |||||
| Sex | |||||||
| Male | 14,094 | 58.0 | 11,828 | 1.00 | 1.00 | ||
| Female | 10,225 | 42.1 | 8439 | 1.07 | (1.03, 1.10) | 0.96 | (0.92, 0.99) |
| Heterogeneity | χ2 (1) = 15.63; p < 0.001 | χ2 (1) = 7.60; p < 0.001 | |||||
| Area of residence | |||||||
| East Midlands | 2268 | 9.3 | 1913 | 1.24 | (1.16, 1.34) | 1.24 | (1.15, 1.34) |
| East of England | 2837 | 11.7 | 2404 | 1.41 | (1.31, 1.51) | 1.18 | (1.10, 1.27) |
| London | 2573 | 10.6 | 1970 | 1.00 | 1.00 | ||
| North East | 1414 | 5.8 | 1212 | 1.35 | (1.24, 1.47) | 1.22 | (1.12, 1.33) |
| North West | 3318 | 13.6 | 2747 | 1.21 | (1.13, 1.30) | 1.36 | (1.27, 1.46) |
| South East | 4094 | 16.8 | 3415 | 1.27 | (1.19, 1.36) | 1.10 | (1.03, 1.18) |
| South West | 2895 | 11.9 | 2483 | 1.38 | (1.29, 1.48) | 1.15 | (1.07, 1.24) |
| West Midlands | 2409 | 9.9 | 2041 | 1.31 | (1.22, 1.41) | 1.34 | (1.24, 1.44) |
| Yorkshire and The Humber | 2511 | 10.3 | 2082 | 1.31 | (1.22, 1.41) | 1.17 | (1.09, 1.26) |
| Heterogeneity | χ2 (8) = 126.13; p < 0.001 | χ2 (8) = 110.88; p < 0.001 | |||||
| Socio-economic deprivation | |||||||
| 1 least deprived | 5615 | 23.1 | 4705 | 1.00 | 1.00 | ||
| 2 | 5607 | 23.1 | 4724 | 1.07 | (1.03, 1.13) | 1.04 | (0.99, 1.09) |
| 3 | 4985 | 20.5 | 4169 | 1.09 | (1.04, 1.14) | 1.06 | (1.01, 1.11) |
| 4 | 4308 | 17.7 | 3548 | 1.09 | (1.03, 1.14) | 1.08 | (1.03, 1.14) |
| 5 most deprived | 3804 | 15.6 | 3121 | 1.09 | (1.04, 1.15) | 1.17 | (1.11, 1.24) |
| Trend | χ2 (1) = 11.53; p < 0.001 | χ2 (1) = 31.88; p < 0.001 | |||||
| Charlson co-morbidity score | |||||||
| 0 | 20,400 | 83.9 | 16,660 | 1.00 | 1.00 | ||
| 1 | 2108 | 8.7 | 1901 | 1.60 | (1.52, 1.69) | 1.07 | (1.01, 1.12) |
| 2+ | 1811 | 7.5 | 1706 | 2.19 | (2.07, 2.31) | 1.11 | (1.05, 1.17) |
| Trend | χ2 (1) = 1031.64; p < 0.001 | χ2 (1) = 16.55; p < 0.001 | |||||
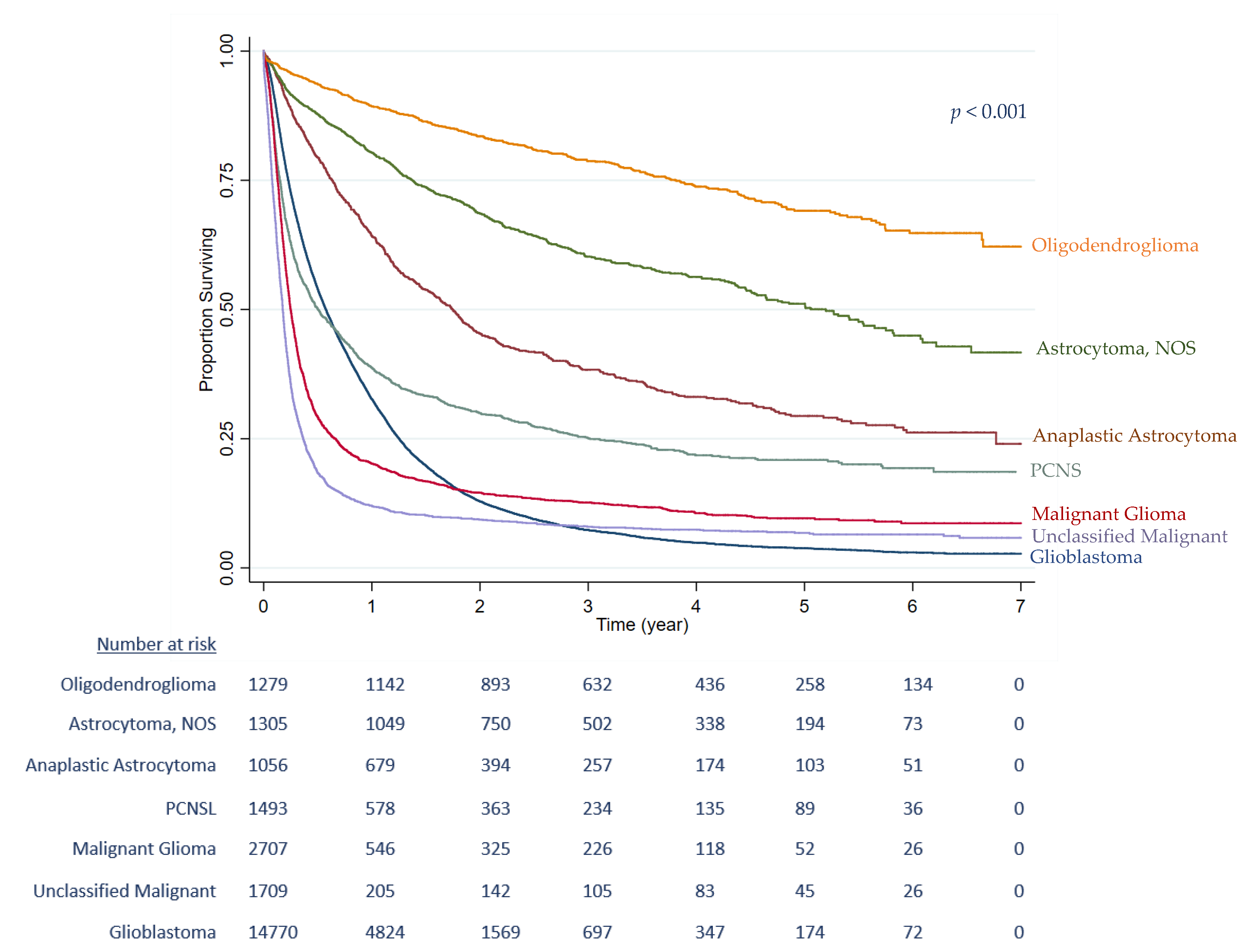
| Morphology | |||||||
| Glioblastoma | 14,768 | 60.7 | 13,619 | 1.00 | 1.00 | ||
| Astrocytoma, Anaplastic | 1058 | 4.4 | 675 | 0.40 | (0.36, 0.44) | 0.58 | (0.53, 0.65) |
| Astrocytoma, NOS | 1306 | 5.4 | 557 | 0.20 | (0.18, 0.23) | 0.20 | (0.18, 0.23) |
| Oligodendroglioma | 1279 | 5.3 | 321 | 0.11 | (0.09, 0.13) | 0.12 | (0.10, 0.15) |
| PCNSL | 1492 | 6.1 | 1124 | 0.97 | (0.91, 1.04) | 0.67 | (0.62, 0.72) |
| Malignant Glioma | 2707 | 11.1 | 2386 | 1.68 | (1.60, 1.76) | 0.67 | (0.63, 0.70) |
| Unclassified Malignant | 1709 | 7.0 | 1585 | 2.48 | (2.34, 2.61) | 0.79 | (0.75, 0.84) |
| Heterogeneity | χ2 (6) = 3293.59; p < 0.001 | χ2 (6) = 1345.58; p < 0.001 | |||||
| Treatment | |||||||
| Radiotherapy only | 2176 | 9.0 | 1964 | 1.00 | 1.00 | ||
| Chemotherapy only | 916 | 3.8 | 642 | 0.65 | (0.59, 0.72) | 0.80 | (0.72, 0.90) |
| Surgical resection only | 2317 | 9.5 | 1563 | 0.86 | (0.80, 0.93) | 1.56 | (1.45, 1.68) |
| Surgical resection + Radiotherapy + Chemotherapy | 5585 | 23.0 | 4200 | 0.24 | (0.22, 0.26) | 0.23 | (0.21, 0.25) |
| Radiotherapy + Chemotherapy | 1994 | 8.2 | 1588 | 0.40 | (0.37, 0.44) | 0.44 | (0.41, 0.48) |
| Surgical resection + Radiotherapy | 2525 | 10.4 | 2127 | 0.63 | (0.58, 0.67) | 0.57 | (0.53, 0.61) |
| Surgical resection + Chemotherapy | 323 | 1.3 | 227 | 0.46 | (0.39, 0.54) | 0.72 | (0.60, 0.85) |
| No treatment | 8483 | 34.9 | 7956 | 2.51 | (2.38, 2.65) | 2.47 | (2.33, 2.62) |
| Heterogeneity | χ2 (7) = 9705.48; p < 0.001 | χ2 (7) = 6350.45; p < 0.001 | |||||
| Route to Diagnosis | |||||||
| Emergency presentation | 12,926 | 53.2 | 11,622 | 1.00 | 1.00 | ||
| GP referral | 4833 | 19.9 | 3668 | 0.55 | (0.52, 0.57) | 0.73 | (0.70, 0.76) |
| Inpatient elective | 743 | 3.1 | 595 | 0.49 | (0.44, 0.54) | 0.87 | (0.78, 0.96) |
| Other outpatient | 4791 | 19.7 | 3569 | 0.46 | (0.44, 0.48) | 0.80 | (0.77, 0.84) |
| Two Week Wait (TWW) Urgent referral | 428 | 1.8 | 355 | 0.62 | (0.55, 0.70) | 0.82 | (0.72, 0.93) |
| Unknown | 598 | 2.5 | 458 | 0.69 | (0.62, 0.77) | 0.60 | (0.54, 0.67) |
| Heterogeneity (excluding unknown) | χ2 (4) = 1646.89; p < 0.001 | χ2 (4) = 230.11; p < 0.001 | |||||
| Univariate | Mutually Adjusted | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ethnic Group | Number of Patients | % | Number of Deaths | Median Age | Median Survival/Months | (95% CI) | HR (95% CI) | HR (95% CI) | ||
| White British | 20,795 | 85.5 | 17,653 | 66.0 | 17.0 | (16.6, 17.5) | 1.00 | (Ref) | 1.00 | (Ref) |
| Bangladeshi | 30 | 0.1 | 22 | 61.5 | 18.0 | (8.5, 85.5) | 0.98 | (0.62, 1.53) | 1.19 | (0.76, 1.88) |
| Indian | 321 | 1.3 | 231 | 59.0 | 27.7 | (23.4, 36.0) | 0.72 | (0.62, 0.84) | 0.89 | (0.76, 1.03) |
| Pakistani | 186 | 0.8 | 130 | 56.0 | 26.0 | (19.1, 34.5) | 0.75 | (0.61, 0.91) | 0.95 | (0.77, 1.16) |
| Chinese | 37 | 0.2 | 26 | 57.0 | 25.3 | (11.7, 50.7) | 0.85 | (0.56, 1.30) | 1.16 | (0.76, 1.76) |
| Black African | 84 | 0.4 | 59 | 53.5 | 29.8 | (20.8, 47.7) | 0.67 | (0.50, 0.91) | 0.98 | (0.72, 1.33) |
| Black Caribbean | 94 | 0.4 | 78 | 60.5 | 19.6 | (13.2, 33.8) | 0.85 | (0.65, 1.11) | 0.86 | (0.66, 1.12) |
| Any Other White | 1018 | 4.2 | 747 | 61.0 | 26.7 | (24.0, 31.5) | 0.73 | (0.67, 0.80) | 0.88 | (0.81, 0.96) |
| Other Ethnic Groups | 674 | 2.8 | 445 | 53.0 | 40.5 | (36.5, 47.4) | 0.53 | (0.47, 0.60) | 0.77 | (0.68, 0.87) |
| Unknown/Not Stated | 1080 | 4.4 | 876 | 68.0 | 10.2 | (9.4, 11.8) | 1.25 | (1.16, 1.35) | 0.82 | (0.76, 0.88) |
| Heterogeneity (excluding unknown) | χ2 (8) = 182.58; p < 0.001 | χ2 (8) = 29.78; p < 0.001 | ||||||||
| Adjusted for Age, Sex | and Socioeconomic Deprivation, Co-morbidity | and Morphology | and Route to Diagnosis | and Treatment Received | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethnic Group | HR | (95% CI) | p-Value | HR | (95% CI) | p-Value | HR | (95% CI) | p-Value | HR | (95% CI) | p-Value | HR | (95% CI) | p-Value |
| White British | 1.00 | (Ref) | 1.00 | (Ref) | 1.00 | (Ref) | 1.00 | (Ref) | 1.00 | (Ref) | |||||
| Bangladeshi | 1.47 | (0.94, 2.30) | 0.094 | 1.24 | (0.79, 1.95) | 0.341 | 1.15 | (0.74, 1.81) | 0.531 | 1.15 | (0.73, 1.80) | 0.546 | 1.05 | (0.67, 1.65) | 0.824 |
| Indian | 0.92 | (0.79, 1.07) | 0.290 | 0.89 | (0.76, 1.04) | 0.142 | 0.91 | (0.78, 1.06) | 0.213 | 0.90 | (0.77, 1.05) | 0.178 | 0.84 | (0.72, 0.98) | 0.025 |
| Pakistani | 1.16 | (0.95, 1.42) | 0.153 | 1.00 | (0.82, 1.23) | 0.989 | 1.04 | (0.85, 1.27) | 0.707 | 1.00 | (0.82, 1.23) | 0.984 | 0.96 | (0.78, 1.17) | 0.658 |
| Chinese | 1.29 | (0.85, 1.96) | 0.231 | 1.24 | (0.82, 1.88) | 0.315 | 1.22 | (0.80, 1.85) | 0.356 | 1.23 | (0.81, 1.86) | 0.342 | 1.09 | (0.72, 1.66) | 0.690 |
| Black African | 1.08 | (0.80, 1.46) | 0.611 | 0.98 | (0.72, 1.33) | 0.888 | 0.91 | (0.67, 1.24) | 0.551 | 0.90 | (0.66, 1.22) | 0.496 | 0.87 | (0.64, 1.17) | 0.355 |
| Black Caribbean | 1.02 | (0.78, 1.33) | 0.868 | 0.90 | (0.69, 1.17) | 0.416 | 0.84 | (0.65, 1.10) | 0.207 | 0.82 | (0.63, 1.07) | 0.153 | 0.81 | (0.62, 1.06) | 0.126 |
| Any Other White | 0.91 | (0.83, 0.99) | 0.034 | 0.90 | (0.82, 0.98) | 0.014 | 0.90 | (0.83, 0.98) | 0.019 | 0.89 | (0.81, 0.97) | 0.007 | 0.83 | (0.76, 0.91) | <0.001 |
| Other Ethnic Groups | 0.84 | (0.74, 0.94) | 0.003 | 0.80 | (0.71, 0.90) | <0.001 | 0.80 | (0.71, 0.90) | <0.001 | 0.79 | (0.70, 0.89) | <0.001 | 0.70 | (0.62, 0.79) | <0.001 |
| Unknown/Not Stated | 1.14 | (1.06, 1.23) | <0.001 | 1.17 | (1.09, 1.26) | <0.001 | 1.11 | (1.03, 1.19) | 0.008 | 1.10 | (1.02, 1.18) | 0.016 | 0.81 | (0.75, 0.88) | <0.001 |
| Heterogeneity (excluding unknown) | χ2 (8) = 20.85; p < 0.001 | χ2 (8) = 23.81; p < 0.001 | χ2 (8) = 23.76; p < 0.001 | χ2 (8) = 25.32; p < 0.001 | χ2 (8) = 56.43; p < 0.001 | ||||||||||
| Pathologically Confirmed Glioblastoma Diagnosis 1 | Diagnosed through a Hospital Stay That Included an Emergency Admission 2 | Optimal Treatment 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethnic Group | % Patients | OR | (95% CI) | p Value | % Patients | OR | (95% CI) | p Value | % Patients | OR | (95% CI) | p Value |
| White British | 56.0 | 1.00 | 53.2 | 1.00 | 24.8 | 1.00 | ||||||
| Bangladeshi | 56.7 | 1.77 | (0.79, 3.99) | 0.168 | 50.0 | 0.80 | (0.38, 1.69) | 0.559 | 13.3 | 0.42 | (0.13, 1.31) | 0.136 |
| Indian | 54.1 | 1.14 | (0.86, 1.52) | 0.360 | 53.9 | 1.19 | (0.94, 1.50) | 0.148 | 24.9 | 0.81 | (0.61, 1.08) | 0.150 |
| Pakistani | 40.6 | 0.74 | (0.51, 1.08) | 0.121 | 59.1 | 1.34 | (0.98, 1.83) | 0.068 | 18.3 | 0.66 | (0.44, 1.00) | 0.049 |
| Chinese | 48.6 | 1.22 | (0.54, 2.77) | 0.638 | 48.6 | 0.90 | (0.45, 1.79) | 0.765 | 18.9 | 0.53 | (0.22, 1.28) | 0.156 |
| Black African | 48.7 | 1.10 | (0.63, 1.92) | 0.741 | 54.8 | 1.18 | (0.75, 1.84) | 0.474 | 20.2 | 0.68 | (0.38, 1.23) | 0.203 |
| Black Caribbean | 51.8 | 0.96 | (0.56, 1.65) | 0.893 | 56.4 | 1.06 | (0.69, 1.63) | 0.799 | 27.7 | 1.27 | (0.75, 2.14) | 0.377 |
| Any Other White | 54.1 | 1.14 | (0.96, 1.34) | 0.124 | 54.6 | 1.16 | (1.02, 1.33) | 0.029 | 26.6 | 0.95 | (0.81, 1.12) | 0.545 |
| Other Ethnic Groups | 54.2 | 1.28 | (1.04, 1.56) | 0.017 | 51.0 | 1.10 | (0.94, 1.30) | 0.233 | 31.0 | 0.99 | (0.82, 1.19) | 0.911 |
| Unknown/Not Stated | 33.6 | 0.70 | (0.58, 0.84) | <0.001 | 50.1 | 0.61 | (0.53, 0.69) | <0.001 | 9.4 | 0.39 | (0.31, 0.49) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanis, H.A.; Møller, H.; Ashkan, K.; Davies, E.A. The Influence of Ethnicity on Survival from Malignant Primary Brain Tumours in England: A Population-Based Cohort Study. Cancers 2023, 15, 1464. https://doi.org/10.3390/cancers15051464
Wanis HA, Møller H, Ashkan K, Davies EA. The Influence of Ethnicity on Survival from Malignant Primary Brain Tumours in England: A Population-Based Cohort Study. Cancers. 2023; 15(5):1464. https://doi.org/10.3390/cancers15051464
Chicago/Turabian StyleWanis, Hiba A., Henrik Møller, Keyoumars Ashkan, and Elizabeth A. Davies. 2023. "The Influence of Ethnicity on Survival from Malignant Primary Brain Tumours in England: A Population-Based Cohort Study" Cancers 15, no. 5: 1464. https://doi.org/10.3390/cancers15051464
APA StyleWanis, H. A., Møller, H., Ashkan, K., & Davies, E. A. (2023). The Influence of Ethnicity on Survival from Malignant Primary Brain Tumours in England: A Population-Based Cohort Study. Cancers, 15(5), 1464. https://doi.org/10.3390/cancers15051464






