Characterization of Primary Mucinous Ovarian Cancer by Diffusion-Weighted and Dynamic Contrast Enhancement MRI in Comparison with Serous Ovarian Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Protocol
2.2. MRI Protocol
2.3. Statistical Analysis
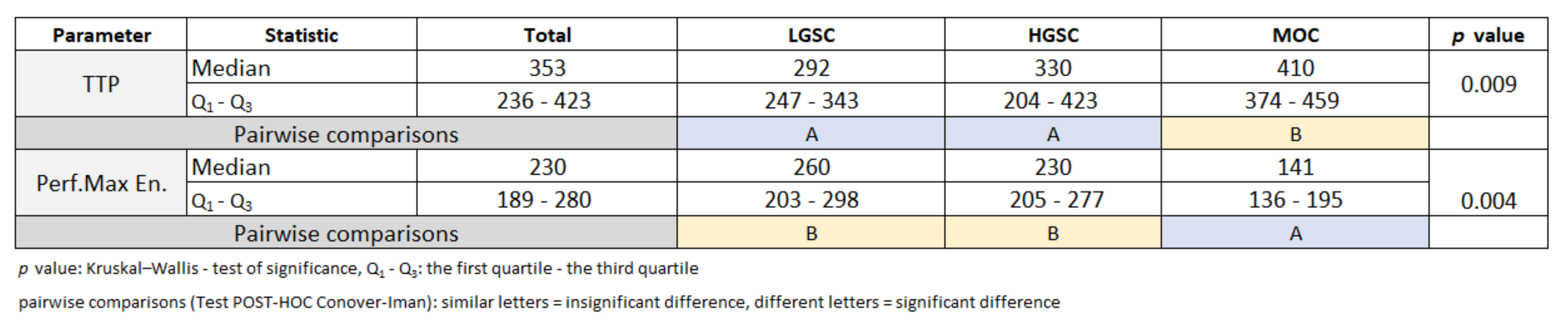
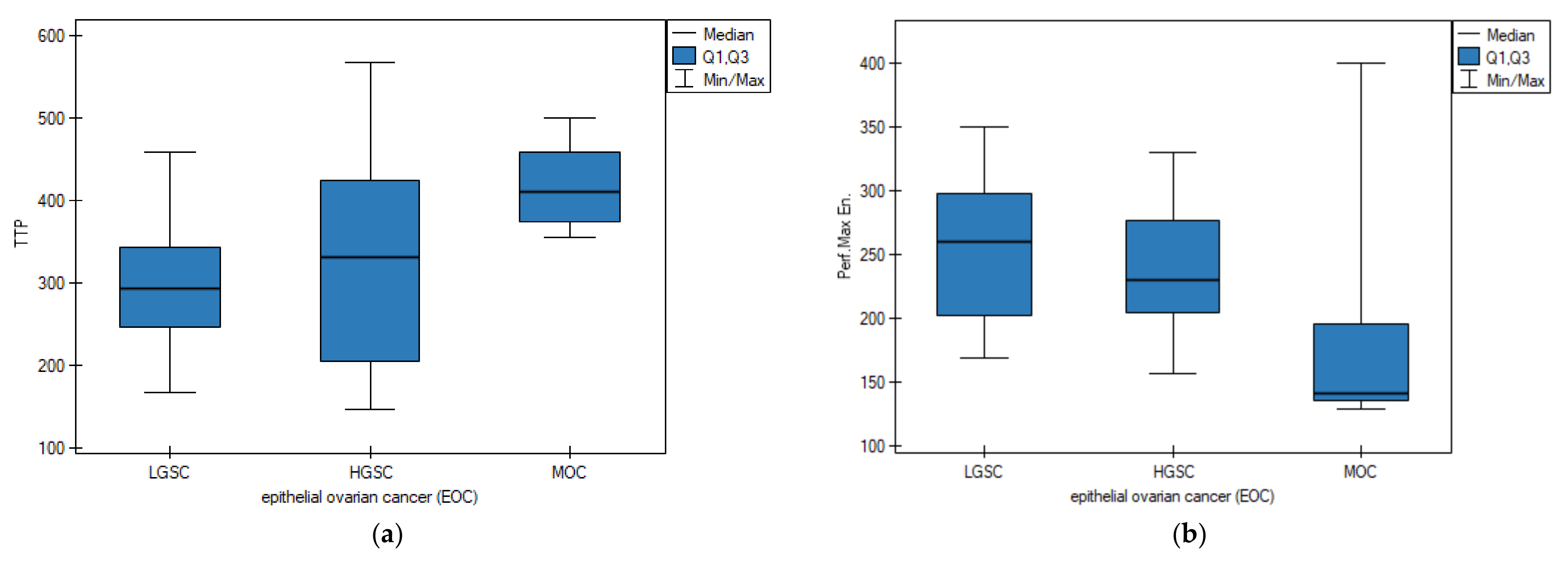
3. Results
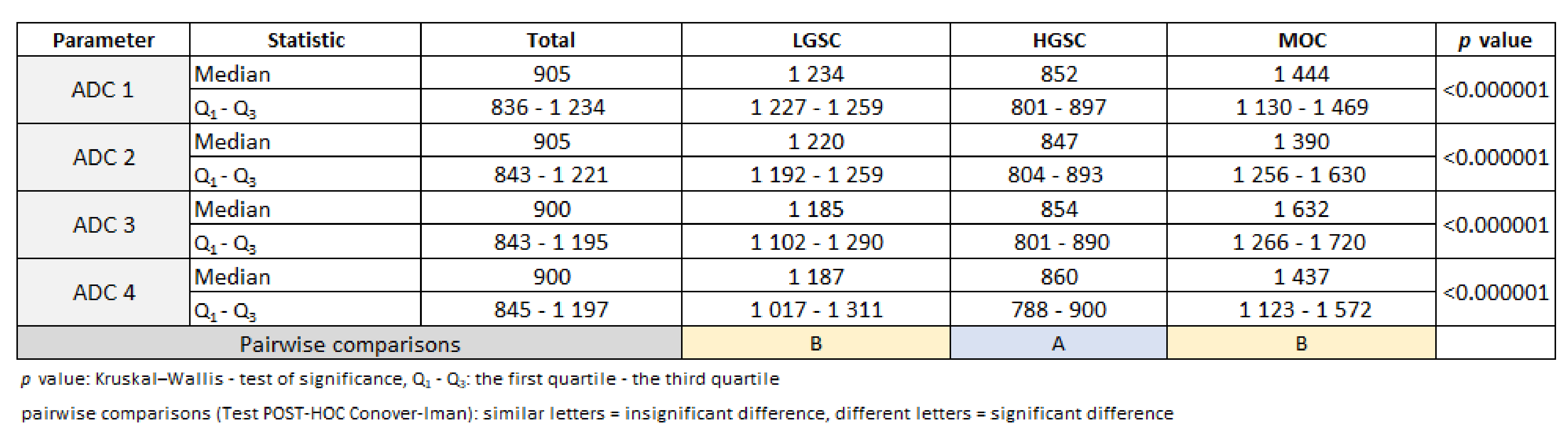
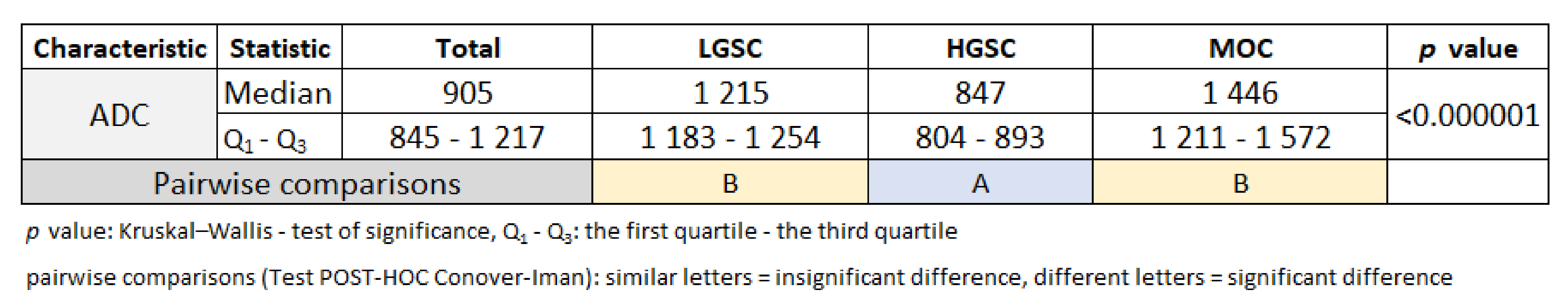
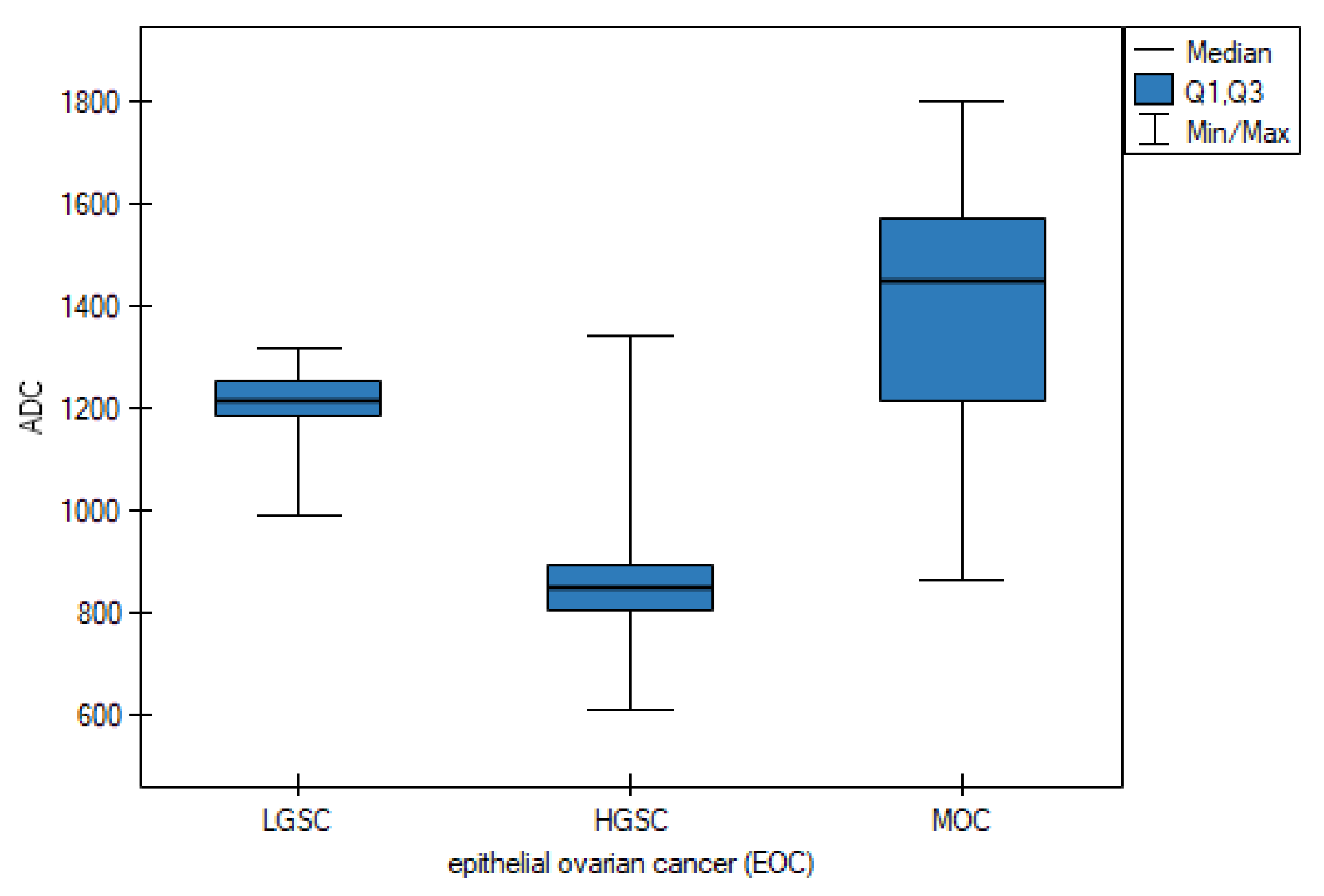
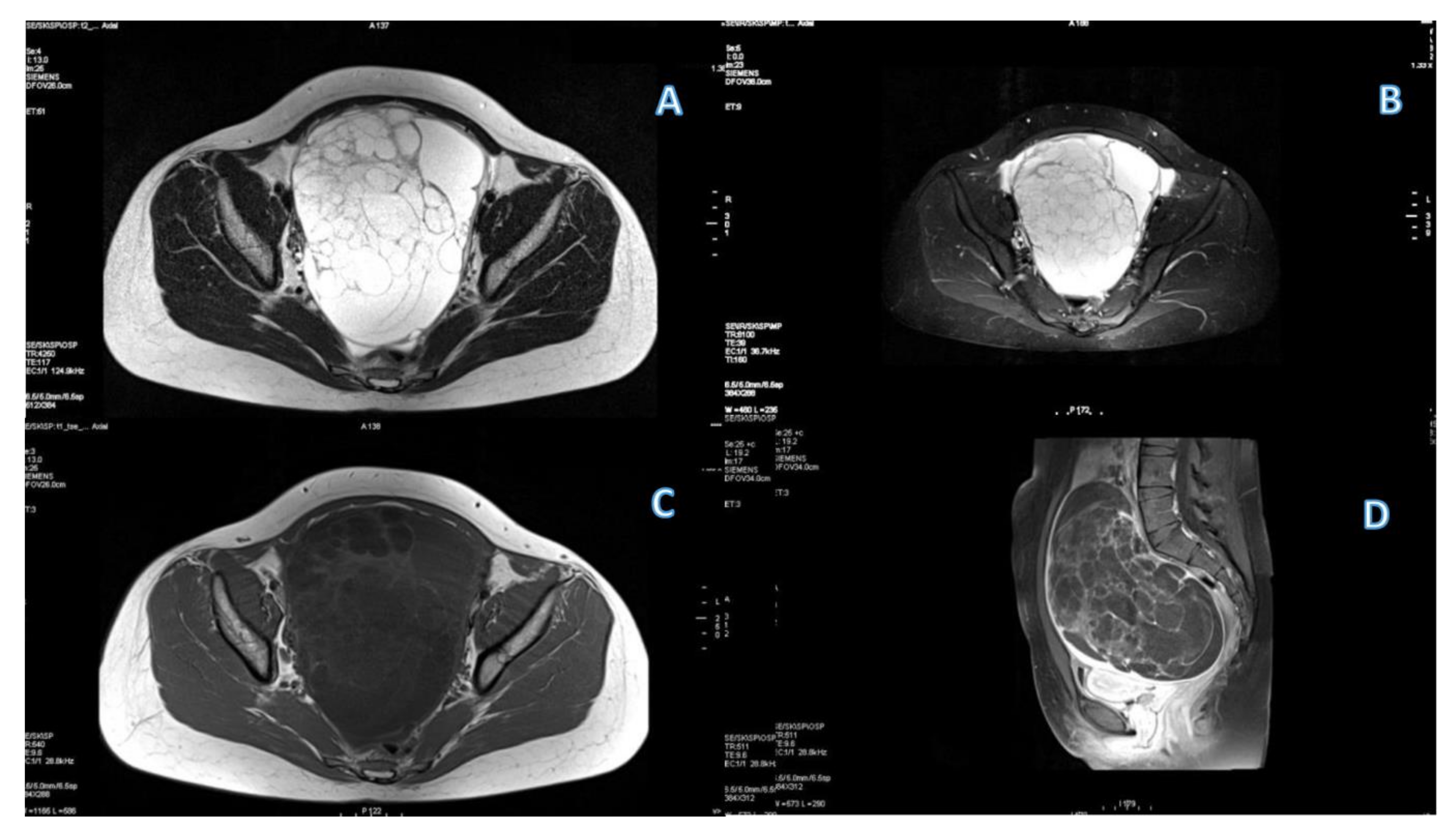
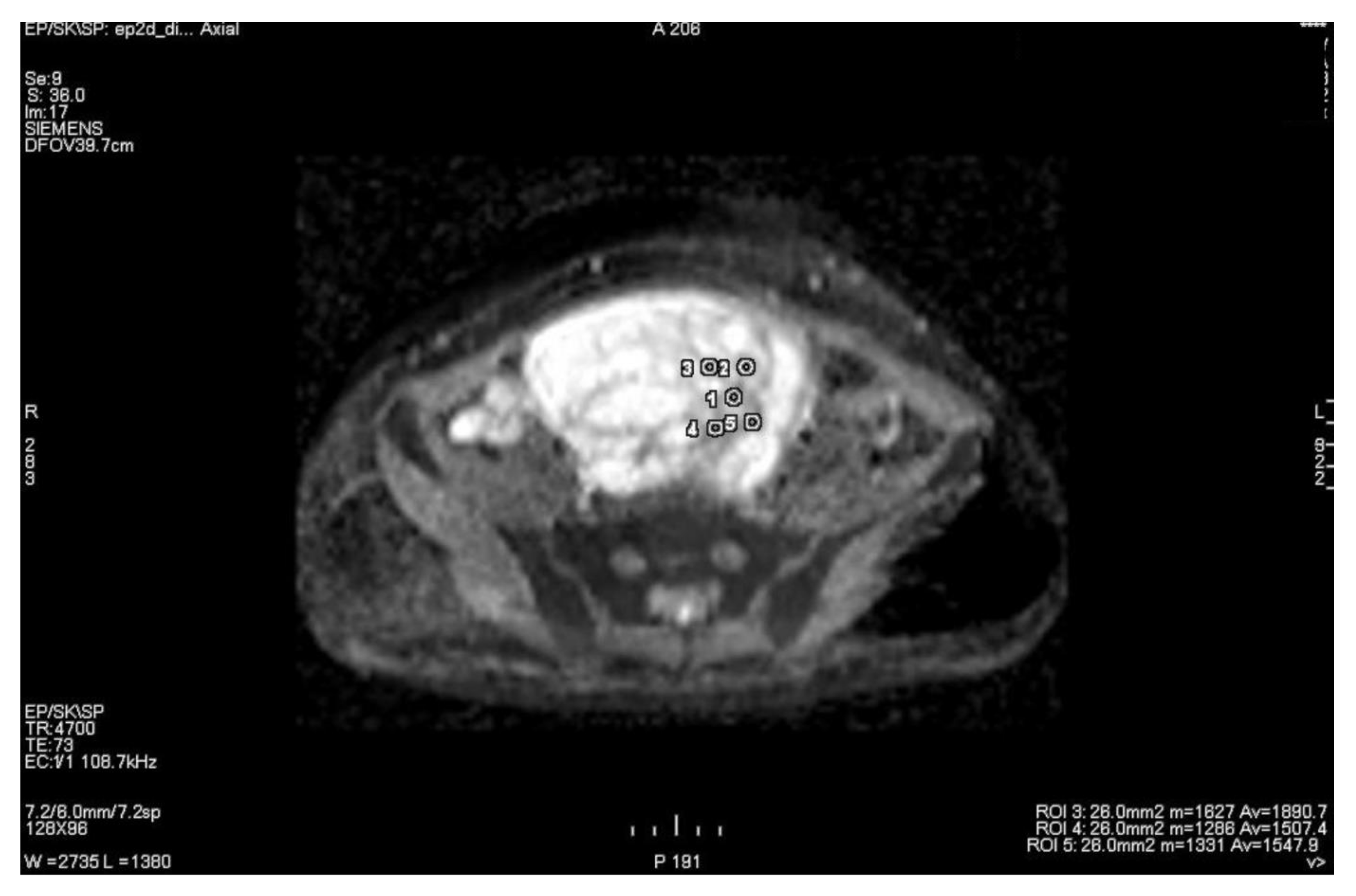
3.1. Primary Tumor DWI
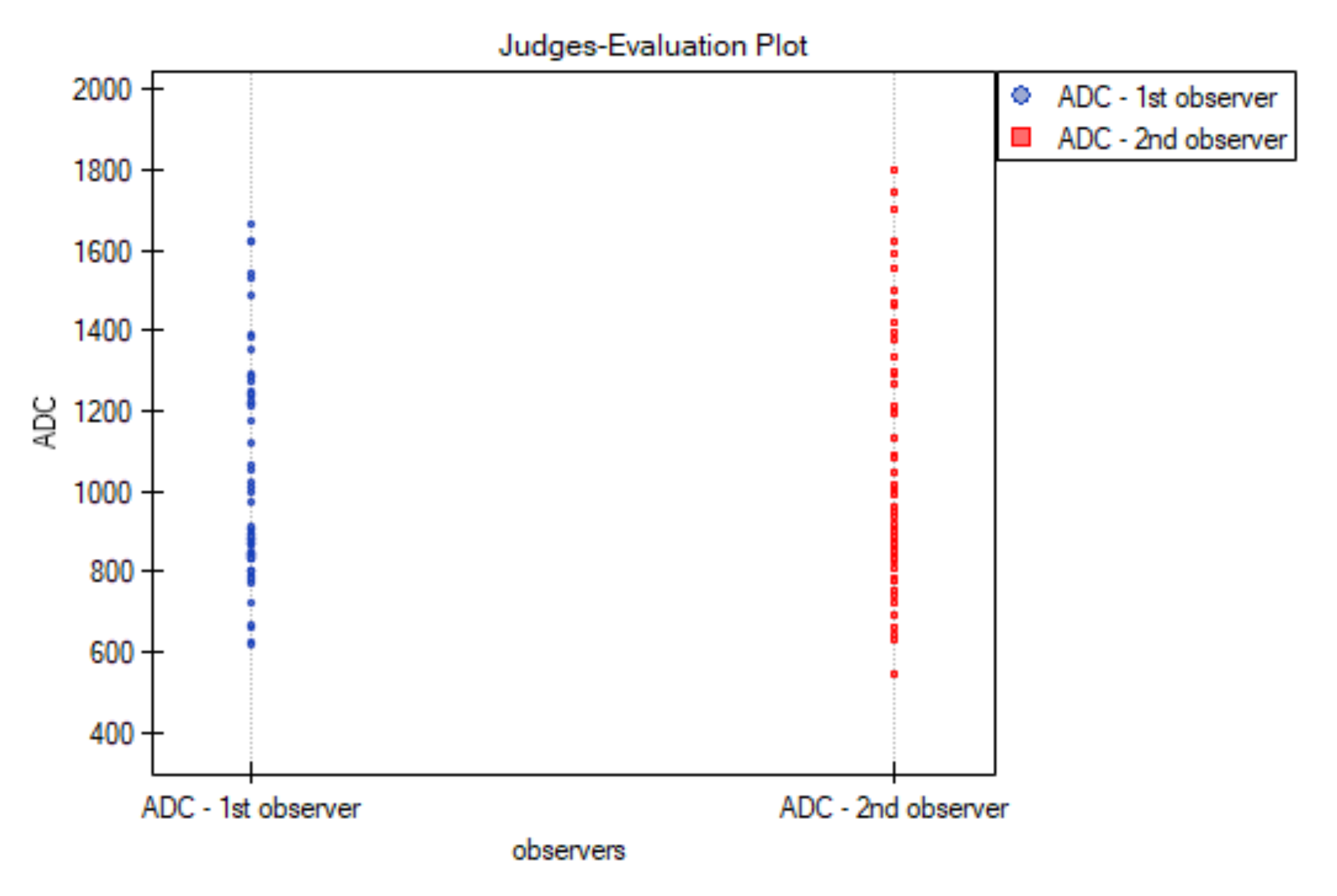
3.2. Inter-Observer Agreement
3.3. Primary Tumor DCE
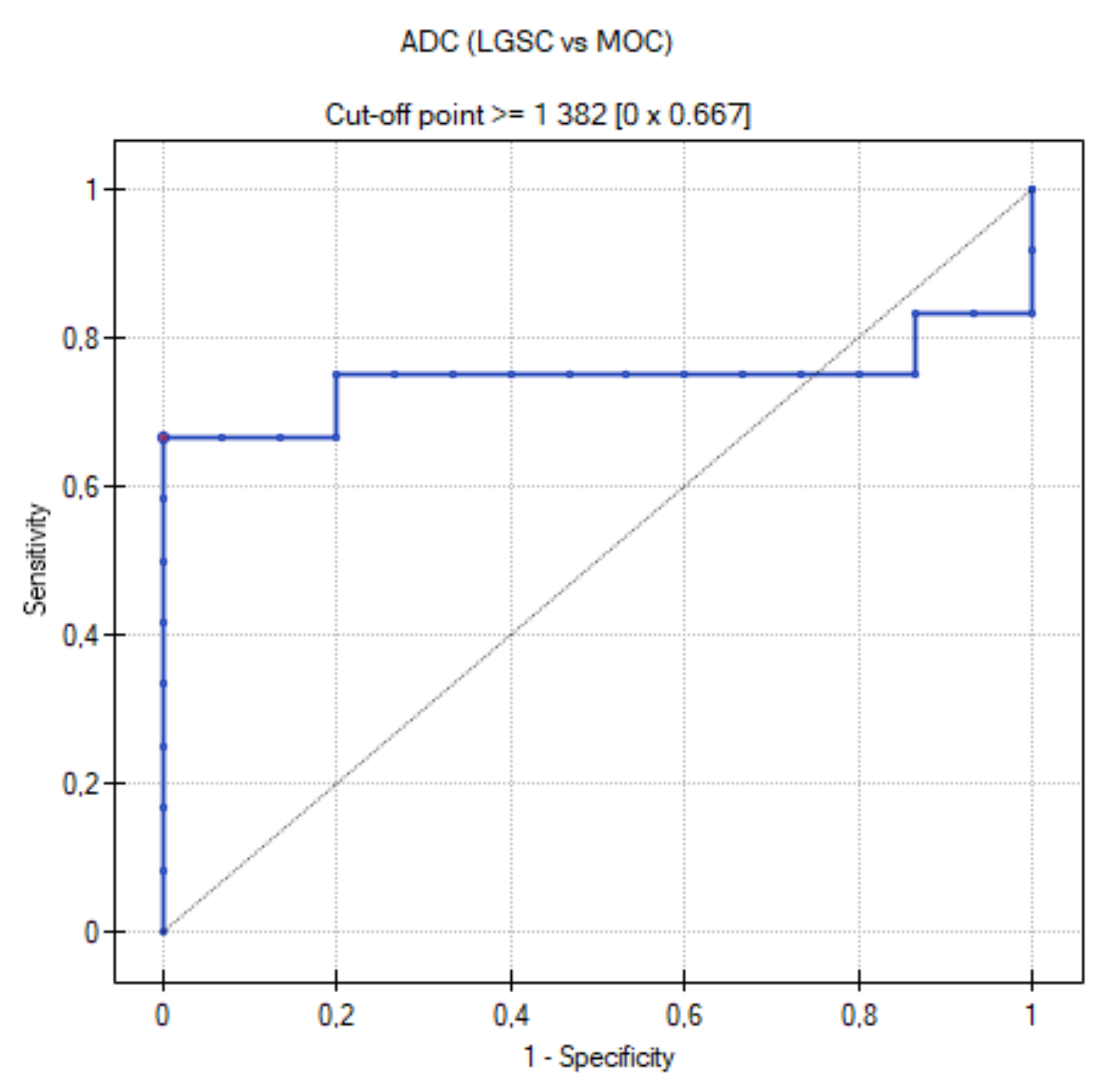
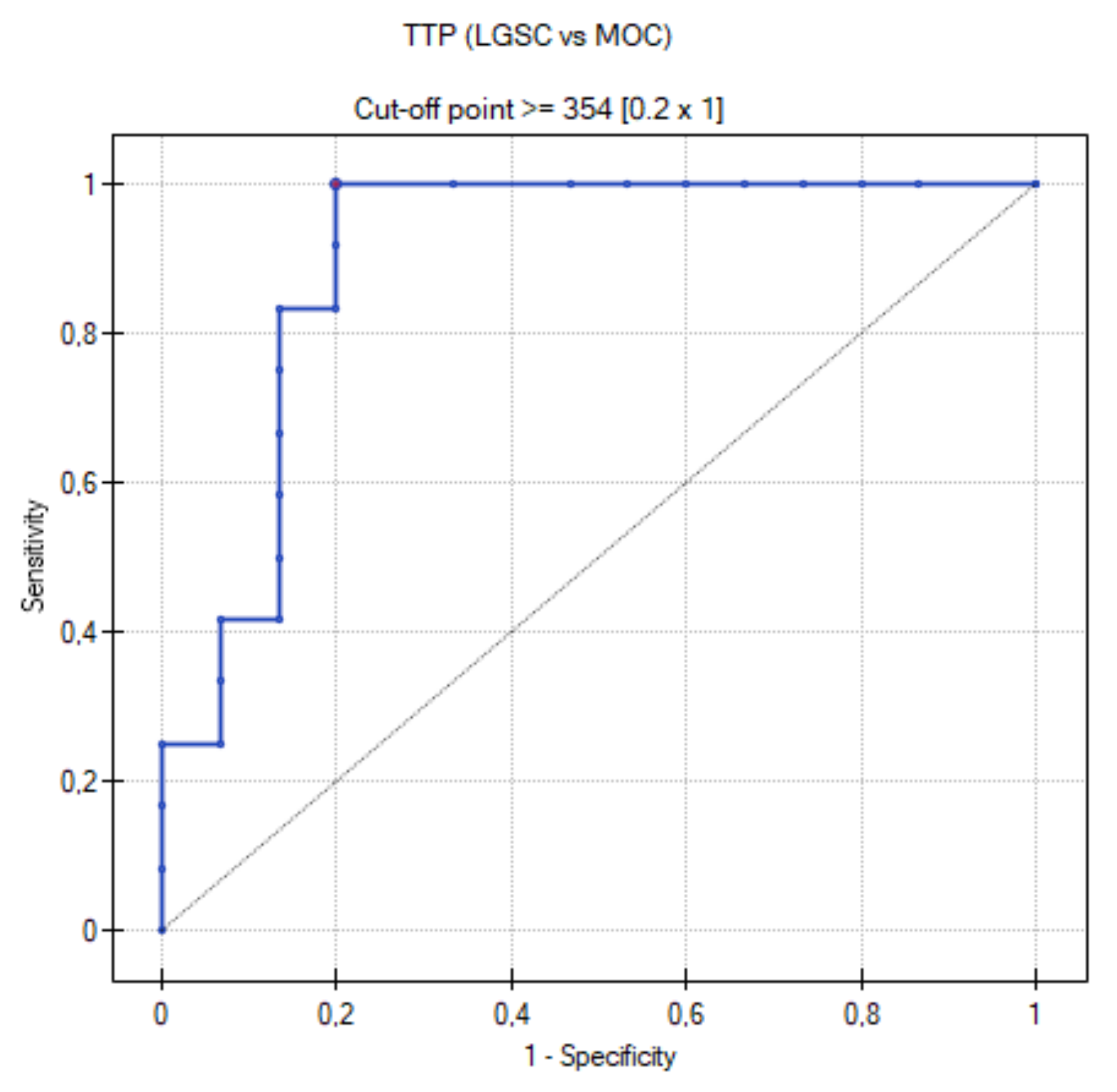
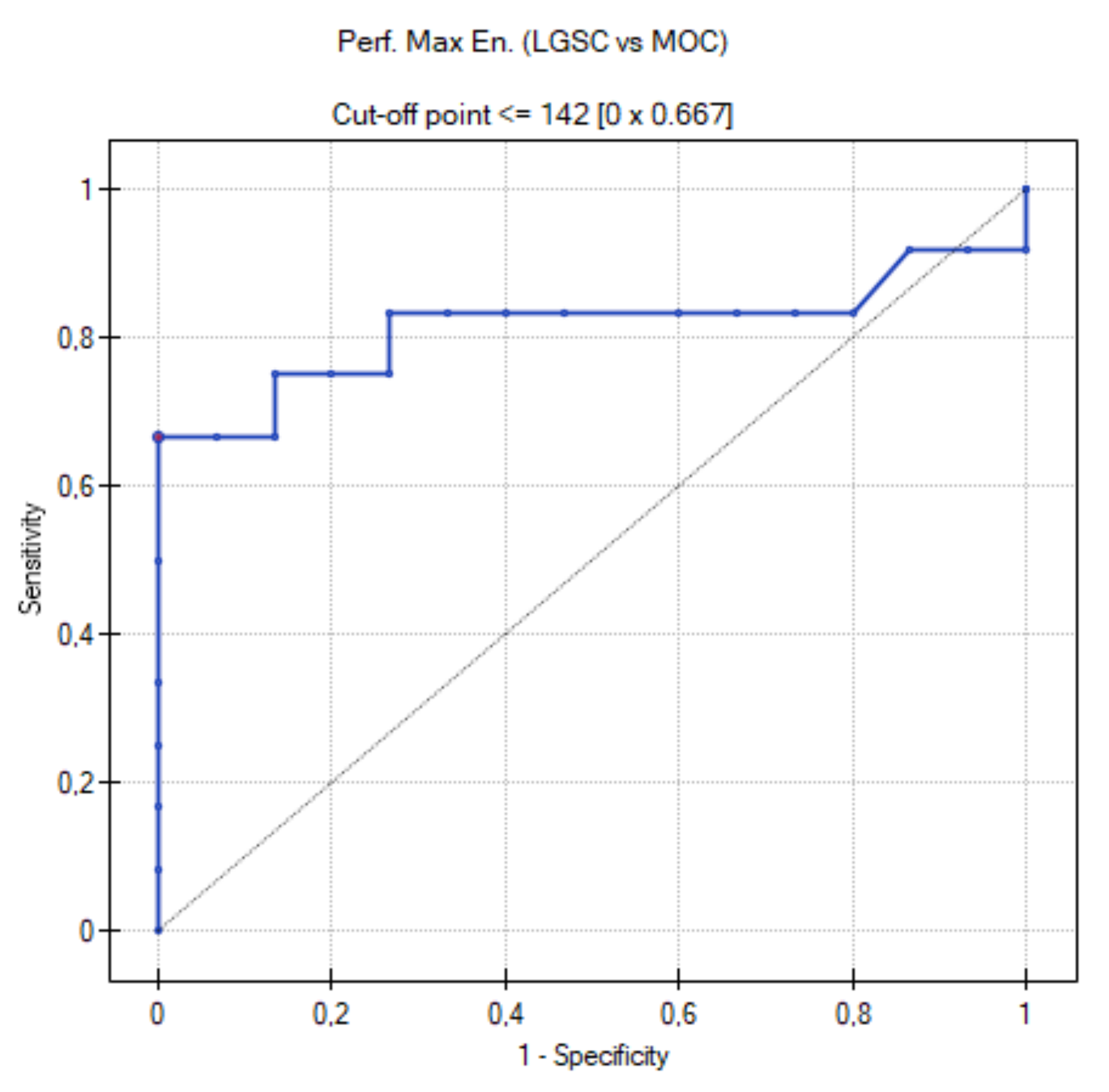
3.4. ROC Curve for LGSC vs. MOC
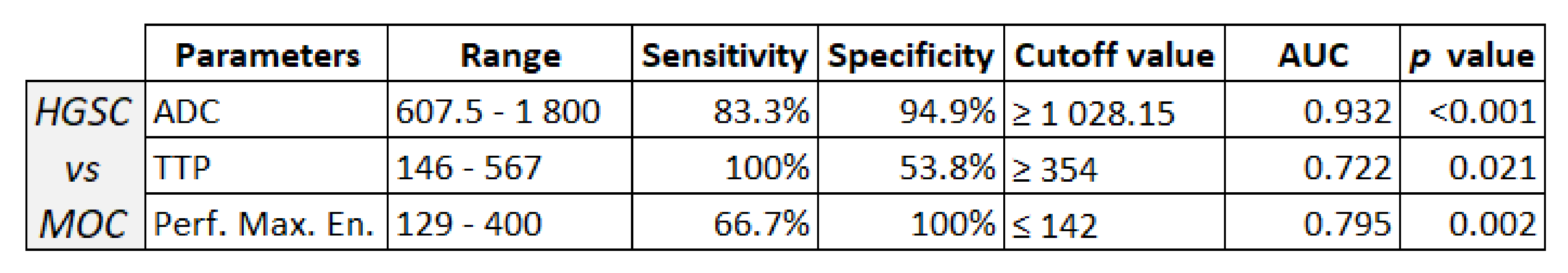
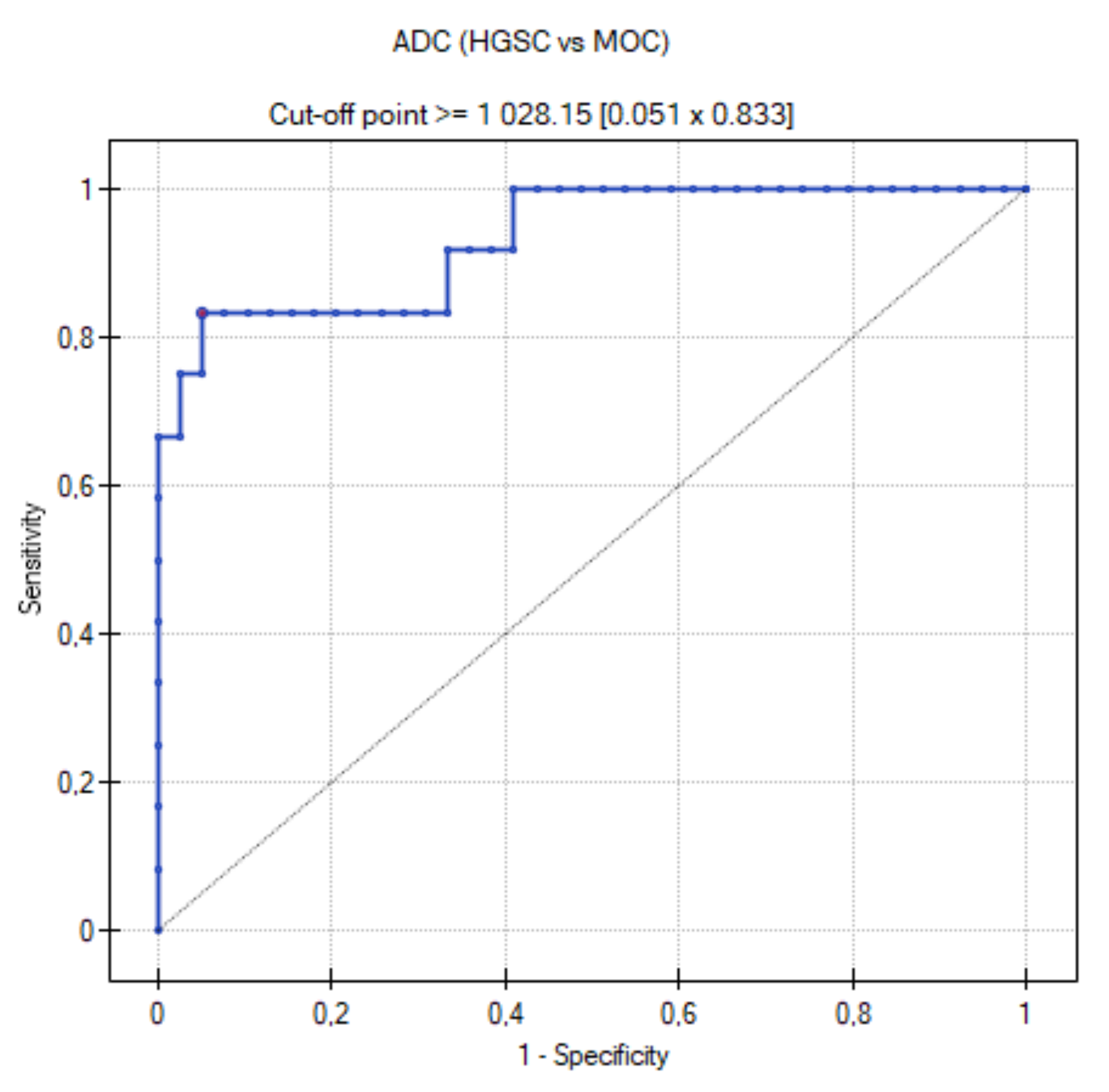
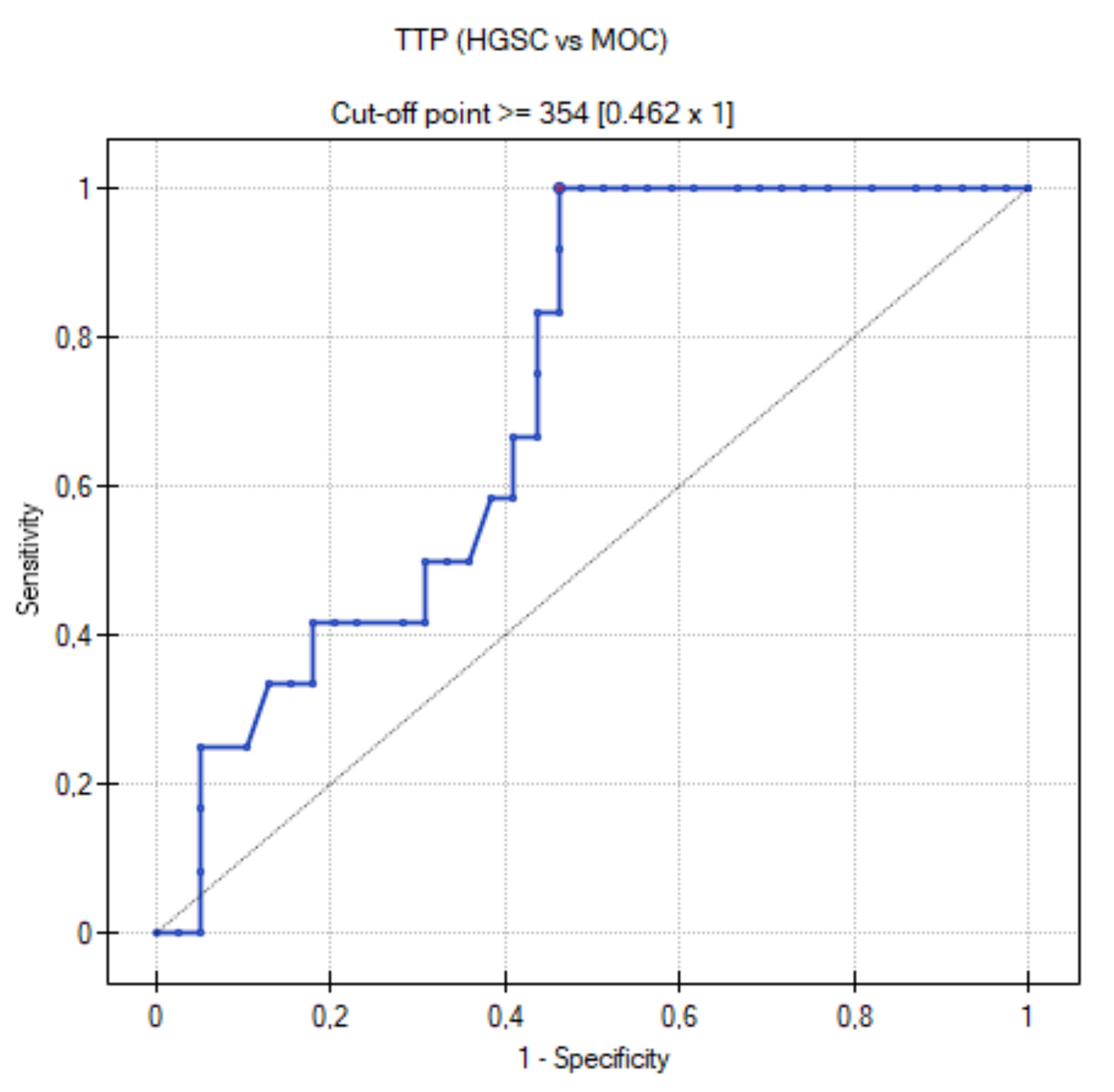
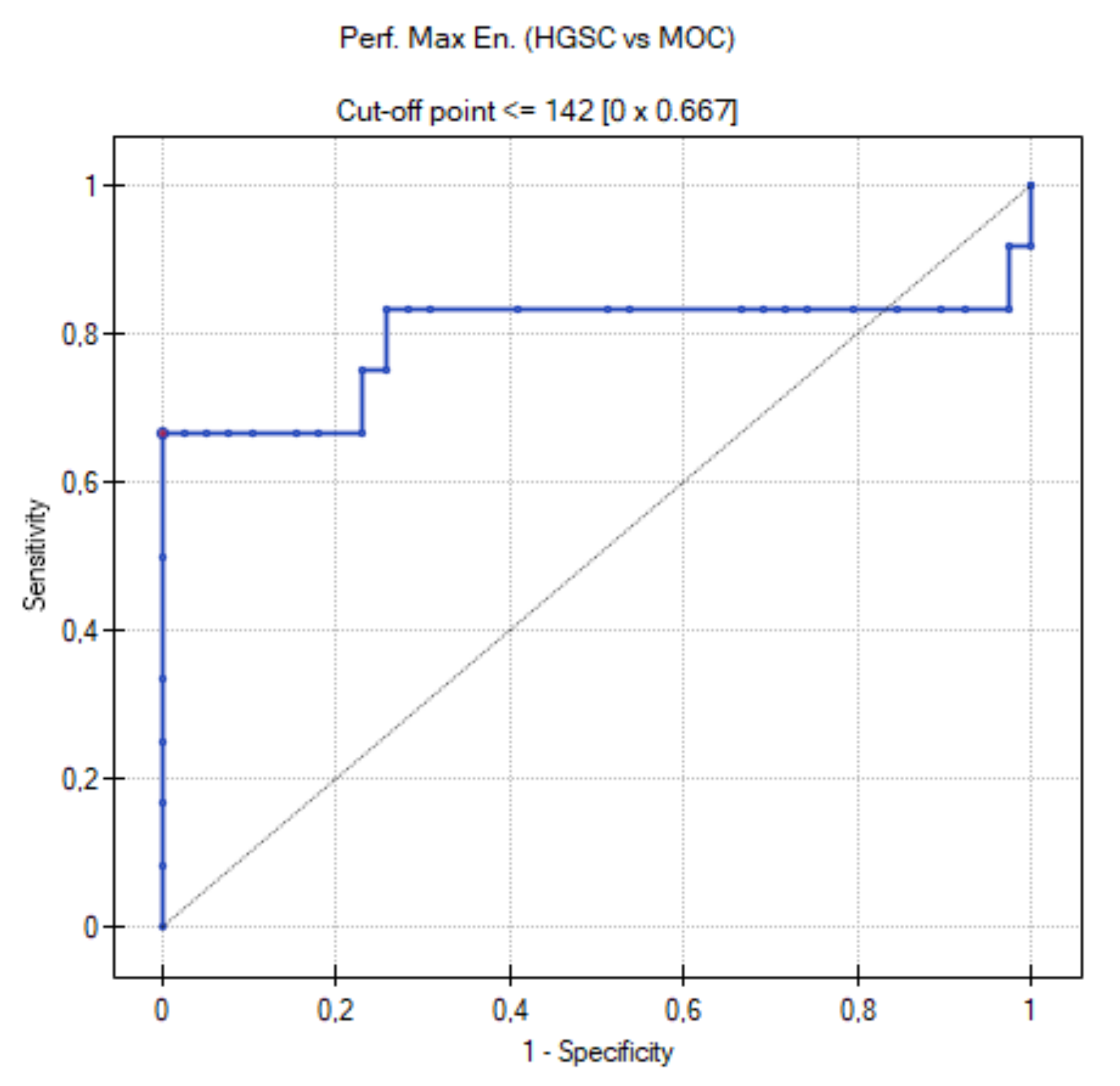
3.5. ROC Curve for HGSC vs. MOC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ledermann, J.A.; Raja, F.A.; Fotopoulou, C.; Gonzales-Martín, A.; Colombo, N.; Sessa, C.; ESMO Guidelines Working Group. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Lowe, K.A.; Chia, V.M.; Taylor, A.; O’Malley, C.; Kelsh, M.; Mohamed, M.; Mowat, F.S.; Goff, B. An international assessment of ovarian cancer incidence and mortality. Gynecol. Oncol. 2013, 130, 107–114. [Google Scholar] [CrossRef]
- Koshiyama, M.; Matsumura, N.; Konishi, I. Recent Concepts of Ovarian Carcinogenesis: Type I and Type II. BioMed Res. Int. 2014, 2014, 934261. [Google Scholar] [CrossRef] [PubMed]
- Babaier, A.; Ghatage, P. Mucinous Cancer of the Ovary: Overview and Current Status. Diagnostics 2020, 10, 52. [Google Scholar] [CrossRef]
- Sioulas, V.D.; Schiavone, M.B.; Kadouri, D.; Zivanovic, O.; Roche, K.L.; O’Cearbhaill, R.; Abu-Rustum, N.R.; Levine, D.A.; Sonoda, Y.; Gardner, G.J.; et al. Optimal primary management of bulky stage IIIC ovarian, fallopian tube and peritoneal carcinoma: Are the only options complete gross resection at primary debulking surgery or neoadjuvant chemotherapy? Gynecol. Oncol. 2017, 145, 15–20. [Google Scholar] [CrossRef]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.; van der Burg, M.E.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef]
- Seidman, J.D.; Kurman, R.J.; Ronnett, B.M. Primary and metastatic mucinous adenocarcinomas in the ovaries: Incidence in routine practice with a new approach to improve intraoperative diagnosis. Am. J. Surg. Pathol. 2003, 27, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Köbel, M.; Kalloger, S.E.; Huntsman, D.G.; Santos, J.L.; Swenerton, K.D.; Seidman, J.D.; Gilks, C.B. Differences in tumor type in low-stage versus high-stage ovarian carcinomas. Int. J. Gynecol. Pathol. 2010, 29, 203–211. [Google Scholar] [CrossRef]
- Kelemen, L.E.; Köbel, M. Mucinous carcinomas of the ovary and colorectum: Different organ, same dilemma. Lancet Oncol. 2011, 12, 1071–1080. [Google Scholar] [CrossRef]
- Kurman, R.J.; Carcangiu, M.L.; Herrington, S.; Young, R.H. World Health Organization Classification of Tumours of the Female Reproductive Organs, 4th ed.; IARC: Lyon, France, 2014. [Google Scholar]
- Prat, J.; D’Angelo, E.; Espinosa, I. Ovarian carcinomas: At least five different diseases with distinct histological features and molecular genetics. Hum. Pathol. 2018, 80, 11–27. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Kajiyama, H.; Mizuno, M.; Shibata, K.; Kawai, M.; Nagasaka, T.; Kikkawa, F. Clinicopathologic features of epithelial ovarian carcinoma in younger vs. older patients: Analysis in Japanese women. J. Gynecol. Oncol. 2014, 25, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Seidman, J.D.; Horkayne-Szakaly, I.; Haiba, M.; Boice, C.R.; Kurman, R.J.; Ronnett, B.M. The Histologic Type and Stage Distribution of Ovarian Carcinomas of Surface Epithelial Origin. Int. J. Gynecol. Pathol. 2004, 23, 41–44. [Google Scholar] [CrossRef]
- Morice, P.; Gouy, S.; Leary, A. Mucinous ovarian carcinoma. N. Engl. J. Med. 2019, 380, 1256–1266. [Google Scholar] [CrossRef]
- Forstner, R.; Sala, E.; Kinkel, K.; Spencer, J.A. ESUR guidelines: Ovarian cancer staging and follow-up. Eur. Radiol. 2010, 20, 2773–2780. [Google Scholar] [CrossRef] [PubMed]
- Michielsen, K.; Dresen, R.; Vanslembrouck, R.; De Keyzer, F.; Amant, F.; Mussen, E.; Leunen, K.; Berteloot, P.; Moerman, P.; Vergote, I.; et al. Diagnostic value of whole body diffusion-weighted MRI compared to computed tomography for pre-operative assessment of patients suspected for ovarian cancer. Eur. J. Cancer 2017, 83, 88–98. [Google Scholar] [CrossRef]
- Elsherif, S.B.; Faria, S.C.; Lall, C.; Iyer, R.; Bhosale, P.R. Ovarian Cancer Genetics and Implications for Imaging and Therapy. J. Comput. Assist. Tomogr. 2019, 43, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, A.; Anttila, M.; Rautiainen, S.; Arponen, O.; Kivelä, A.; Mäkinen, P.; Härmä, K.; Hämäläinen, K.; Kosma, V.M.; Ylä-Herttuala, S.; et al. Primary and metastaic ovarian cancer: Characterization by 3.0T diffusion-weighted MRI. Eur. Radiol. 2017, 27, 4002–4012. [Google Scholar] [CrossRef]
- Lindgren, A.; Anttila, M.; Arponen, O.; Rautiainen, S.; Könönen, M.; Vanninen, R.; Sallinen, H. Prognosti value of preoperative dynamic contrast-enhancement magnetic resonance imaging in epithelial ovarian cancer. Eur. J. Radiol. 2019, 115, 66–73. [Google Scholar] [CrossRef]
- Marko, J.; Marko, K.I.; Pachigolla, S.L.; Crothers, B.A.; Mattu, R.; Wolfman, D.J. Mucinous Neoplasms of the ovary: Rodiologic-pathologic correlation. RadioGraphics 2019, 39, 982–997. [Google Scholar] [CrossRef]
- Bassiouny, D.; Ismiil, N.; Dubé, V.; Han, G.; Cesari, M.; Lu, F.-I.; Slodkowska, E.; Parra-Herran, C.; Chiu, H.F.; Naeim, M.; et al. Comprehensive Clinicopathologic and Updated Immunohistochemical Characterization of Primary Ovarian Mucinous Carcinoma. Int. J. Surg. Pathol. 2018, 26, 306–317. [Google Scholar] [CrossRef]
- Mccluggage, W.G. Immunohistochemistry in the distinction between primary and metastatic ovarian mucinous neoplasms. J. Clin. Pathol. 2012, 65, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Portelance, L.; Corradini, S.; Erickson, B.; Lalondrelle, S.; Padgett, K.; van der Leij, F.; van Lier, A.; Jürgenliemk-Schulz, I. Online Magnetic Resonance-Guided Radiotherapy (oMRgRT) for Gynecological Cancers. Front. Oncol. 2021, 11, 628131. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.S.; Felix, A.; Cunha, T.M. Clues to the diagnosis of borderline ovarian tumours: An imaging guide. Eur. J. Radiol. 2021, 143, 109904. [Google Scholar] [CrossRef]
- Ohya, A.; Fujinaga, Y. Magnetic resonance imaging findings of cystic ovarian tumors: Major differential diagnoses in five types frequently encountered in daily clinical practice. Jpn. J. Radiol. 2022, 40, 1213–1234. [Google Scholar] [CrossRef]
- Taylor, E.C.; Irshaid, L.; Mathur, M. Multimodality Imaging Approach to Ovarian Neoplasms with Pathologic Correlation. RadioGraphics 2021, 41, 289–315. [Google Scholar] [CrossRef]
- Matsumoto, J.; Yoshida, K.; Inoue, D.; Yoneda, N.; Ikeda, H.; Mizumoto, Y.; Gabata, T. Solidified mucinous tumor of the ovary presenting characteristic MRI finding. Eur. J. Radiol. Open 2019, 6, 68–71. [Google Scholar] [CrossRef]
- Elsherif, S.B.; Bhosale, P.R.; Lall, C.; Menias, C.O.; Itani, M.; Butler, K.A.; Ganeshan, D. Current update on malignant epithelial ovarian tumors. Abdom. Radiol. 2021, 46, 2264–2280. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, L.; Indima, N.; Peng, K.; Li, Q.; Hua, T.; Tang, G. CT and MRI findings of type I and type II epithelial ovarian cancer. Eur. J. Radiol. 2017, 90, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Fagotti, A.; Avesani, G.; Trombadori Federico, A.; D’Indinosante, M.; Giudice, M.T.; Pelligra, S.; Lodoli, C.; Marchetti, C. Whole solid tumour volume histogram analysis of the apparent diffusion coefficient for differentiating high-grade from low-grade serous ovarian carcinoma: Correlation with Ki-67 proliferation status. Clin. Radiol. 2019, 74, 918–925. [Google Scholar]
- Derlatka, P.; Grabowska-Derlatka, L.; Halaburda-Rola, M.; Szeszkowski, W.; Czajkowski, K. The Value of Magnetic Resonance Diffusion-Weighted Imaging and Dynamic Contrast Enhancement in the Diagnosis and Prognosis of Treatment Response in Patients with Epithelial Serous Ovarian Cancer. Cancers 2022, 14, 2464. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-M.; Qiang, J.-W.; Ma, F.-H.; Zhao, S.-H. The value of dynamic contrast–enhanced MRI in characterizing complex ovarian tumors. J. Ovarian Res. 2017, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Kaga, T.; Kato, H.; Hatano, Y.; Kawaguchi, M.; Furui, T.; Morishige, K.I.; Matsuo, M. Can MRI features differentiate ovarian mucinous carcinoma from mucinous borderline tumor? Eur. J. Radiol. 2020, 132, 109281. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, X.; Ma, F.; Li, H.; Zhao, S.; Li, Y.; Qiang, J. MRI characteristics for differentiating mucinous borderline ovarian tumours from mucinous ovarian cancers. Clin. Radiol. 2021, 77, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [PubMed]
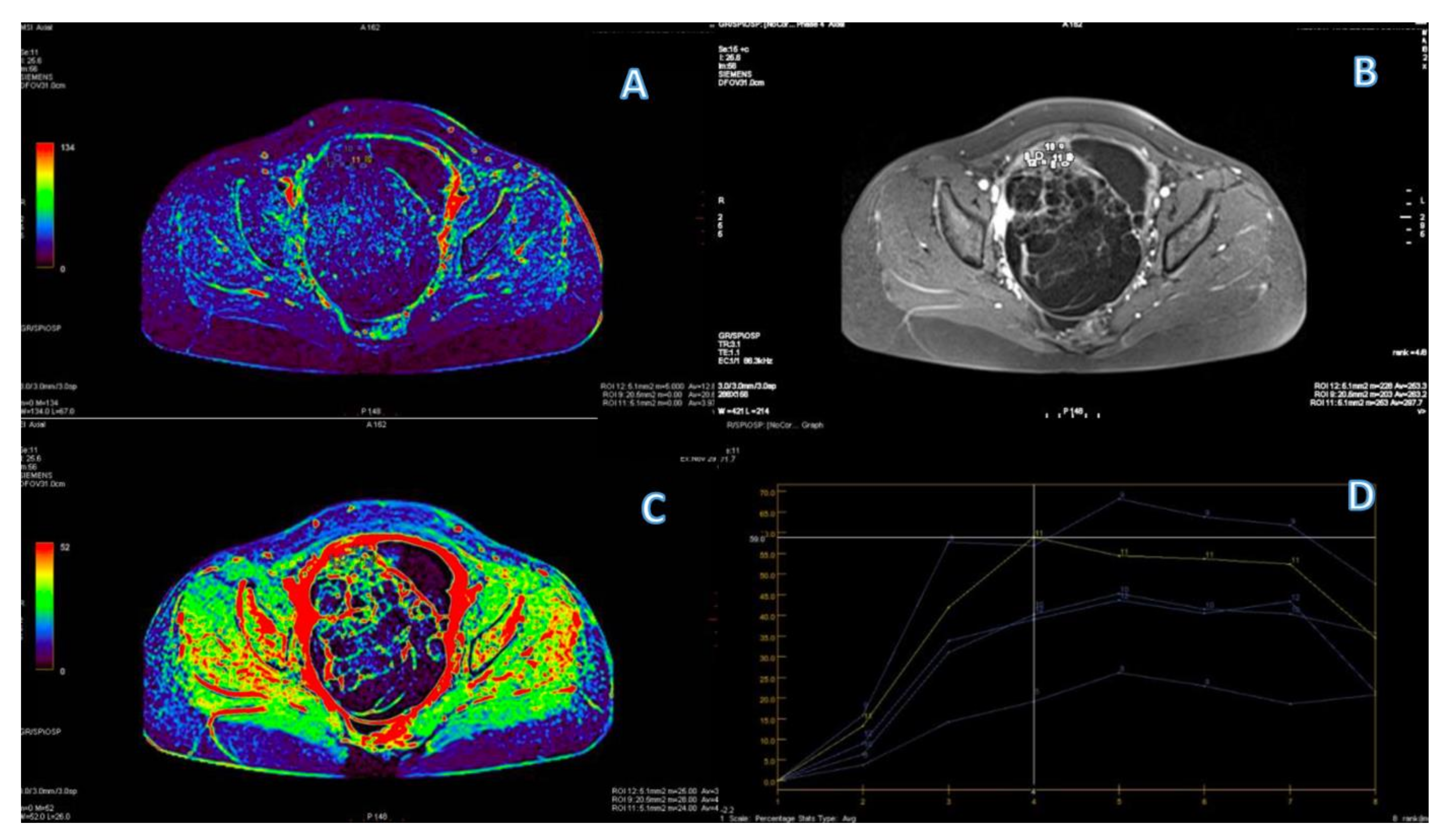

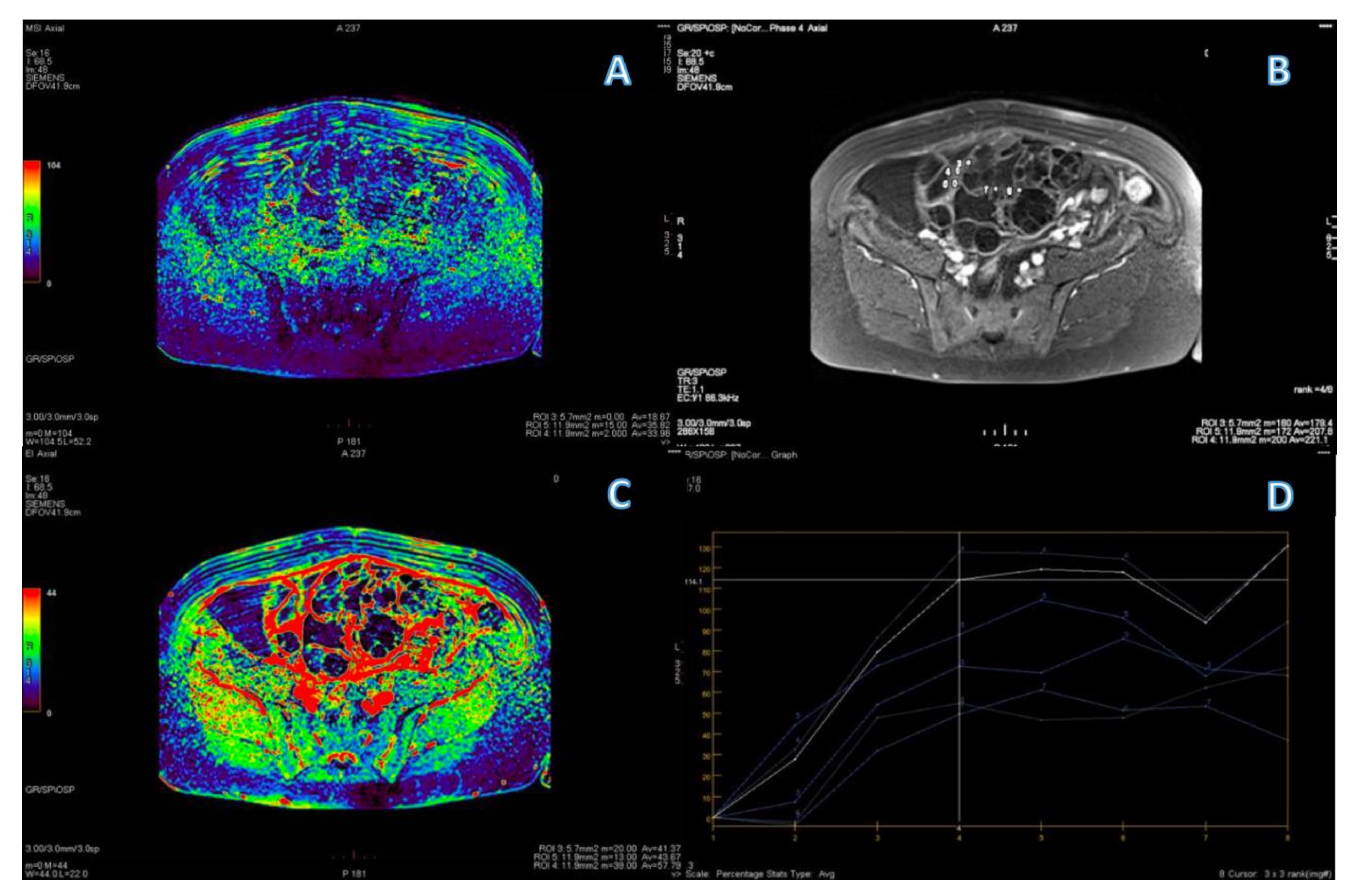
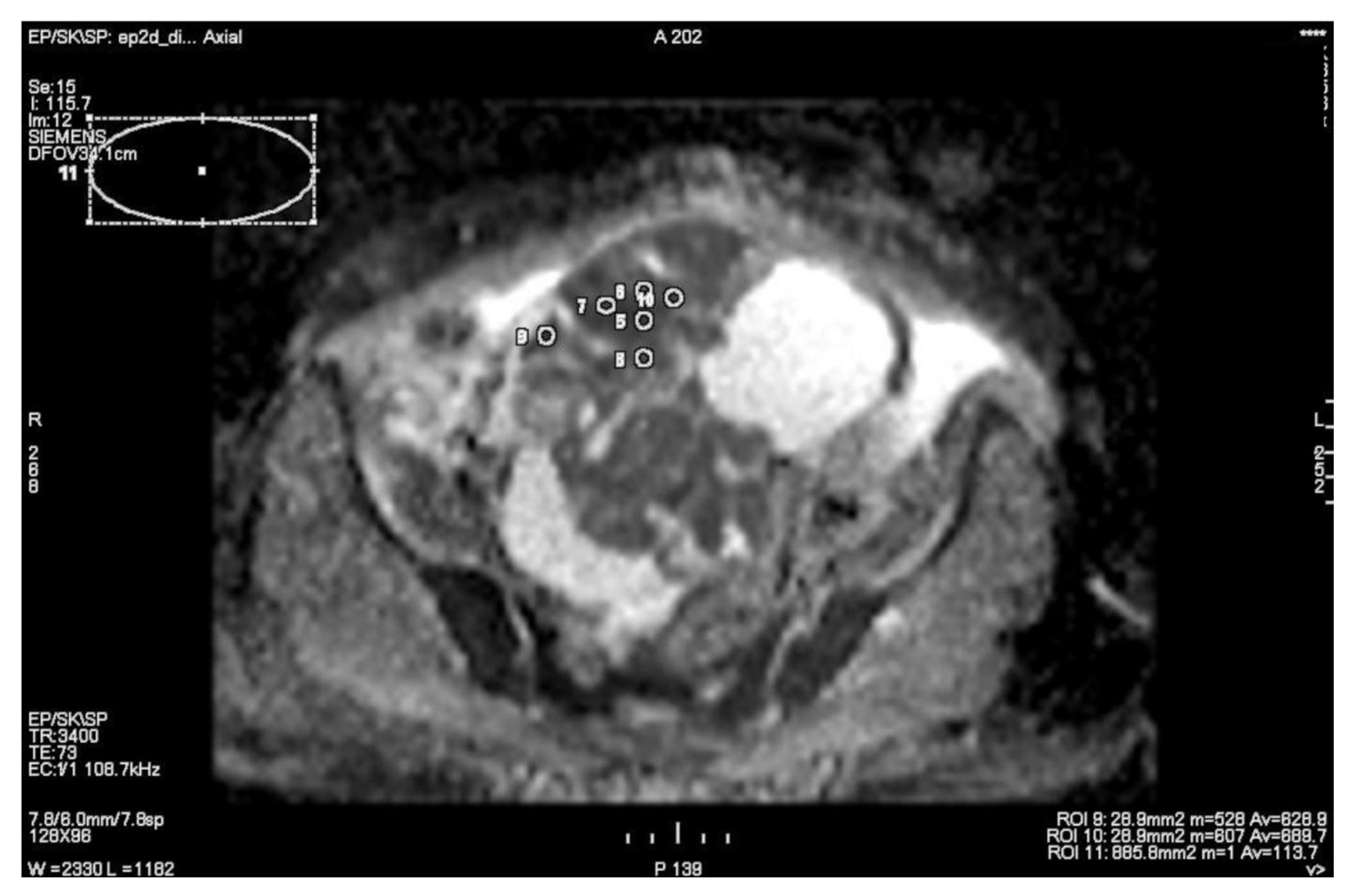
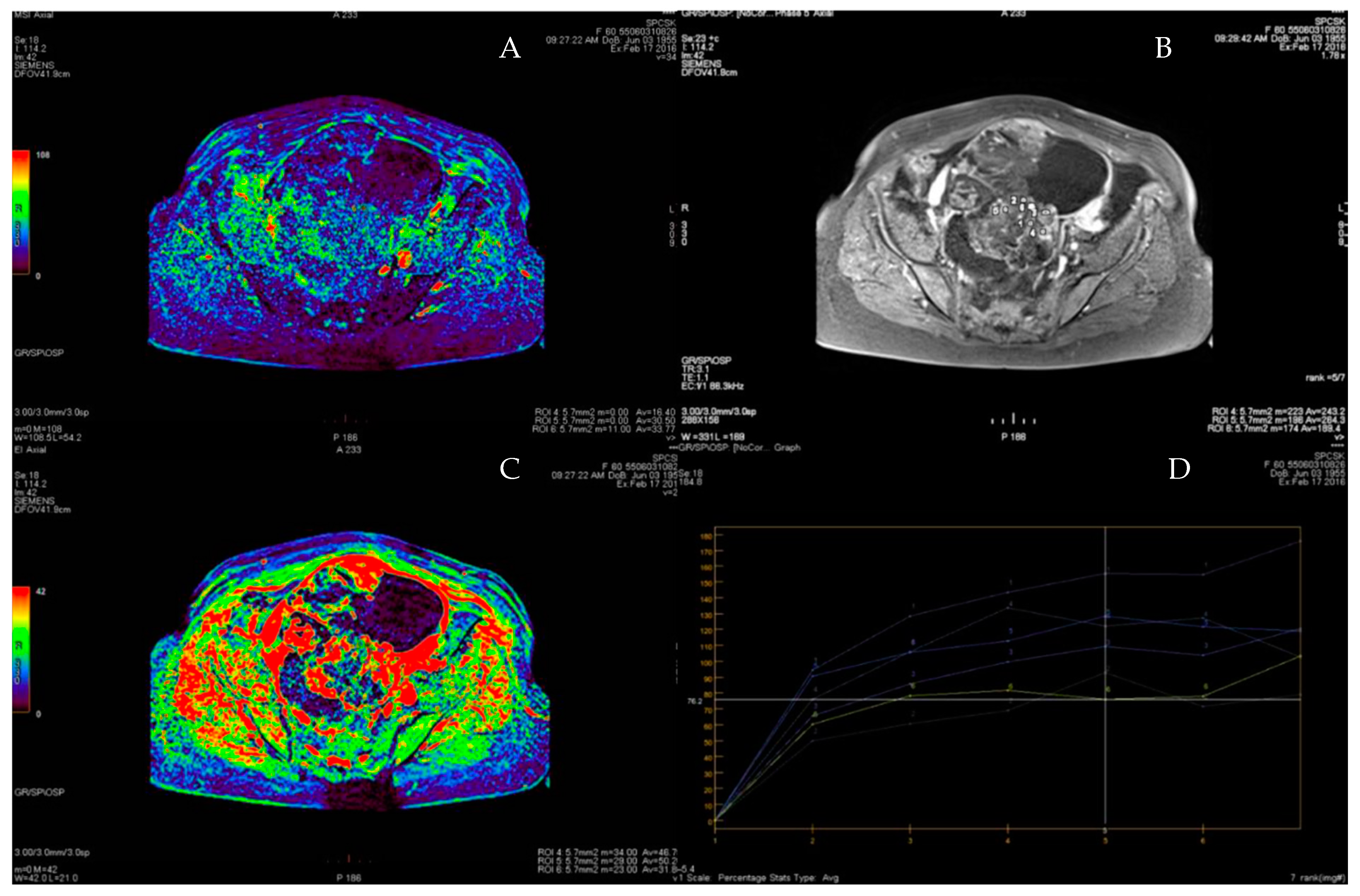

| Variable | n (%)/Mean (Range) |
|---|---|
| Age | 55.5 (31–78) |
| FIGO stage | |
| I | 14 |
| II | 3 |
| III | 44 |
| IV | 4 |
| Histological type | |
| Serous high-grade | 39 |
| Serous low-grade | 15 |
| Mucinous | 12 |
| Parameter | T2 TSE | T2 TSE Fat-Sat | DW EPI | T2 TIRM | Vibe 3D T1 GRE | T1 GRE (In- and Outphase) | T1 TSE Fat-Sat | T2 TSE (BLADE) Fat-Sat (SPAIR) |
|---|---|---|---|---|---|---|---|---|
| Repetition time (ms) | 4250 | 2110 | 3800 | 6100 | 3.05 | 125 | 510 | 2300 |
| Echo time (ms) | 117 | 123 | 73 | 39 | 1.13 | 1: 2.22 2: 4.92 | 9.6 | 116 |
| Flip angle (deg.) | 137 | 150 | 90 | 150 | 10 | 70 | 150 | 150 |
| iPAT factor | - | 2 | 2 | - | 2 | 2 | - | 2 |
| Plane | axial, sagital coronal | axial | axial | axial | axial | axial | axial, sagital coronal | axial, coronal |
| Number of signal averages | 1 | 1 | 4 | 1 | 1 | 1 | 1 | 1 |
| Field of view—FOV (mm) | 360 | 360 | 360 | 360 | 360 | 360 | 360 | 360 |
| Rectangular FOV (%) | 75, 100, 100 | 100 | 75 | 75 | 75 | 75 | 75 | 100 |
| Breath-hold | No | No | No | No | No | No | No | Yes |
| Resolution (mm) | 0.7 × 0.7 × 5 | 1.4 × 1.4 × 5 | B value: 0, 50, 500, 1000, 1500 | 0.9 × 0.9 × 5 | 1.7 × 1.3 × 3 | 1.3 × 1.3 × 5 | 0.9 × 0.9 × 5 | 1.4 × 1 × 4 × 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabowska-Derlatka, L.; Derlatka, P.; Hałaburda-Rola, M. Characterization of Primary Mucinous Ovarian Cancer by Diffusion-Weighted and Dynamic Contrast Enhancement MRI in Comparison with Serous Ovarian Cancer. Cancers 2023, 15, 1453. https://doi.org/10.3390/cancers15051453
Grabowska-Derlatka L, Derlatka P, Hałaburda-Rola M. Characterization of Primary Mucinous Ovarian Cancer by Diffusion-Weighted and Dynamic Contrast Enhancement MRI in Comparison with Serous Ovarian Cancer. Cancers. 2023; 15(5):1453. https://doi.org/10.3390/cancers15051453
Chicago/Turabian StyleGrabowska-Derlatka, Laretta, Pawel Derlatka, and Marta Hałaburda-Rola. 2023. "Characterization of Primary Mucinous Ovarian Cancer by Diffusion-Weighted and Dynamic Contrast Enhancement MRI in Comparison with Serous Ovarian Cancer" Cancers 15, no. 5: 1453. https://doi.org/10.3390/cancers15051453
APA StyleGrabowska-Derlatka, L., Derlatka, P., & Hałaburda-Rola, M. (2023). Characterization of Primary Mucinous Ovarian Cancer by Diffusion-Weighted and Dynamic Contrast Enhancement MRI in Comparison with Serous Ovarian Cancer. Cancers, 15(5), 1453. https://doi.org/10.3390/cancers15051453






