Simple Summary
Whereas renal cell carcinoma (RCC) is the most common renal tumor in adults, pediatric RCC is a rare malignancy. The previous literature focusing on cross-sectional imaging of RCC concerns mainly computed tomography in adults, whereas in children, a different distribution of subtypes is seen, as well as a preference for magnetic resonance imaging (MRI). Therefore, the aim of this study was to identify MRI characteristics of pediatric and young-adult RCC through a case series and literature review focusing on translocation-type RCC (MiT-RCC) and the pediatric and young-adult population. In our review as well as in our case series T2-weighted hypo-intensity seems to be a potential discriminative characteristic. Moreover, an irregular growth pattern and limited diffusion restriction were often described. Nevertheless, we conclude the discrimination of RCC subtypes, and especially the differentiation of RCC from other pediatric renal tumors, remains difficult.
Abstract
Pediatric renal cell carcinoma (RCC) is a rare malignancy. Magnetic resonance imaging (MRI) is the preferred imaging modality for assessment of these tumors. The previous literature has suggested that cross-sectional-imaging findings differ between RCC and other pediatric renal tumors and between RCC subtypes. However, studies focusing on MRI characteristics are limited. Therefore, this study aims to identify MRI characteristics of pediatric and young-adult RCC, through a single-center case series and literature review. Six identified diagnostic MRI scans were retrospectively assessed, and an extensive literature review was conducted. The included patients had a median age of 12 years (63–193 months). Among other subtypes, 2/6 (33%) were translocation-type RCC (MiT-RCC) and 2/6 (33%) were clear-cell RCC. Median tumor volume was 393 cm3 (29–2191 cm3). Five tumors had a hypo-intense appearance on T2-weighted imaging, whereas 4/6 were iso-intense on T1-weighted imaging. Four/six tumors showed well-defined margins. The median apparent diffusion coefficient (ADC) values ranged from 0.70 to 1.20 × 10−3 mm2/s. In thirteen identified articles focusing on MRI characteristics of MiT-RCC, the majority of the patients also showed T2-weighted hypo-intensity. T1-weighted hyper-intensity, irregular growth pattern and limited diffusion–restriction were also often described. Discrimination of RCC subtypes and differentiation from other pediatric renal tumors based on MRI remains difficult. Nevertheless, T2-weighted hypo-intensity of the tumor seems a potential distinctive characteristic.
1. Introduction
Pediatric renal cell carcinoma (RCC) is a rare renal malignancy [1,2]. Although Wilms tumors (WTs) show the highest prevalence in young children, the incidence of RCC increases in the second decade of life [1,3,4]. Whereas in the Renal Tumor Study Group of the International Society of Pediatric Oncology (SIOP-RTSG), pre-operative chemotherapy is the standard of care for WTs, upfront surgery is recommended for localized RCC [5]. Invasive procedures to determine histology before the start of therapy in young children are discouraged [6,7]. Age and size of the tumor are important factors in the consideration of the diagnosis of pediatric renal tumors as well as in the consideration of performing a biopsy, indicating age >7 years as a criterion to consider tumor biopsy [6]. Thus far, no specific imaging characteristics discriminating RCC from WTs and other non-WTs have been identified [8,9,10].
Magnetic resonance imaging (MRI) is currently the preferred modality for the assessment of pediatric renal tumors within the SIOP-RTSG given its lack of ionizing radiation and excellent soft-tissue contrast. Furthermore, MRI is subject to continuous technical developments, such as the possibility of calculating the apparent diffusion coefficient (ADC) value using diffusion-weighted imaging (DWI) [6,11,12]. MRI could, therefore, play a potential role in the non-invasive discrimination of pediatric renal tumors [13,14,15,16,17].
Contrary to the rarity of RCC in children, this tumor type is the most common renal tumor in adolescents and adults [18,19,20,21]. Nevertheless, childhood RCC shows distinct histological characteristics, possibly related to the different distribution of RCC subtypes. Whereas translocation-type RCC (MiT-RCC), which has been officially recognized since 2004 by the World Health Organization, is the most frequent subtype in children, clear-cell RCC (ccRCC) is the predominant histological subtype in adults [2,5,22,23,24,25]. MiT-RCC is diagnosed based on translocations including transcription factor E3 (TFE3) and EB (TFEB), which are members of the family of microphthalmia transcription factors (MiT) [26,27]. Interestingly, the previous literature has suggested that cross-sectional imaging findings differ between RCC subtypes [13,28,29,30,31,32,33,34,35].
Until today, studies focusing on the MRI characteristics of pediatric RCC are limited in number, although identification of potential specific MRI characteristics of WTs and non-WTs is important for future validation studies [9,25,35]. Therefore, this study aims to retrospectively identify MRI characteristics of pediatric RCC patients at diagnosis through a case series in our center, including a literature review focusing on this topic.
2. Materials and Methods
2.1. Patients
Institutional Review Board approval was obtained. For this retrospective study, obtaining further formal consent was waived. All diagnostic MRI scans included were clinically indicated and were performed as the standard of care. Between 2014 and 2019, we identified 6 children with RCC that underwent an MRI scan at diagnosis.
The standard of care for localized pediatric RCC is upfront total nephrectomy [22,36]. Only in case of doubt of a WT diagnosis, based on predefined clinical and imaging characteristics, a core needle biopsy was performed. If there was no suspicion of a non-WT, the patients were pre-operatively treated with 4 weeks of vincristine/actinomycin-D (stage I-III) or 6 weeks of vincristine/actinomycin-D/doxorubicin (stage IV/V), according to the SIOP-RTSG protocol.
2.2. Magnetic Resonance Imaging Acquisition
Abdominal MRI for pediatric renal tumors in this study was performed using a 1.5T MRI system (Achieva, Philips Healthcare, Eindhoven, The Netherlands and Ingenia, Philips Healthcare, Eindhoven, The Netherlands). Two patients were scanned in external hospitals at diagnosis before referral to our center (Signa HDxt; GE Healthcare, Boston, USA and Magnetom Avanto; Siemens, Erlangen, Germany). Scan protocols slightly varied but at least consisted of coronal and axial T2-weighted imaging, axial T1-weighted turbo spin-echo and axial DWI with automatically generated ADC maps. Five patients underwent pre- and post-contrast T1-weighted imaging, whereas for one patient, contrast-enhanced MRI was not available (Table 1).

Table 1.
Scan parameters at 1.5-T MRI of the scanned sequences.
Children were awake, sedated or under general anesthesia depending on their ability to cooperate, according to the standard-of-care procedures. Gadobutrol (Gadovist; Bayer B.V., Leverkusen, Germany) was administered intravenously at a dose of 0.1 mL/kg body weight. Hyoscine butylbromide (Buscopan; Sanofi, Paris, France) was administered intravenously at a dose of 0.4 mg/kg body weight to reduce peristaltic artifacts, with a maximum of 10 mg in children ≥6 years and a maximum of 5 mg in children <6 years. All children were screened for contraindications for MRI and those concerning intravenous agents. For the two patients scanned at local hospitals, specifications of gadobutrol and hyoscine butylbromide were not available.
2.3. Image Analysis
The anonymized MRI datasets were transferred to DICOM software Osirix v. 5.5.2 (Pixmero, SARL, Bernex, Switzerland). Two pediatric radiologists (ASL with 13 years of experience and RAJN with 26 years of experience in body MRI, respectively), who were blinded to the histopathological subtype and clinical characteristics but were aware of the pediatric RCC diagnosis, reviewed the diagnostic MRI scans. All diagnostic scans were assessed using a case report form based on previous studies identifying potential specific imaging characteristics of different pediatric renal tumors [9]. The pediatric RCC cases were analyzed focusing on tumor presentation, growth pattern, characteristics of solid components and enhancement pattern, if available. Tumor volume was calculated based on the three dimensions of the tumor times 0.523. Moreover, up to four round-shaped ROIs containing solid areas of the tumor, mainly based on enhancement, were drawn in order to measure the ADC value of the most representative parts of the tumor. To limit inter-observer variability, an instruction form accompanying the case report form was provided.
2.4. Histopathological Review
Our national coordinating SIOP-RTSG histopathologist (RRK with 23 years of experience with pediatric renal tumor histopathology) reviewed the available macroscopy and microscopy from the surgically resected tumors and biopsies of all patients following the most recent WHO classification system [27,37].
Following protocol, the dorsal and ventral side and hilar region of the resected specimen were marked with varying color dyes following the instructions of the involved surgeons. The specimens were sliced free-handed in successive 10 to 20 mm transverse macroscopic slices in a cranial to caudal sequence or through longitudinal incision to bivalve the specimen.
2.5. Statistical Analyses
Due to the small number of patients, inter-observer agreement between the two pediatric radiologists was difficult to assess because Cohen’s kappa is affected by the prevalence of the finding under observation. Only six patients were included in this study, potentially resulting in low values or even an impossible calculation of kappa when focusing on separate characteristics [38,39]. Therefore, the inter-observer agreement was assessed using percentages of observed agreement, including the intra-class correlation coefficient (ICC) for the median ADC values including the regions of interest and for median tumor volumes. ICC values were interpreted as satisfactory >0.75 [40].
2.6. Literature Review
A literature review was performed following PRISMA guidelines to reflect on the case series and elaborate on the current knowledge about the MRI appearance of RCC by focusing on the predominant histological subtypes in the pediatric and young-adult population. For this purpose, PubMed, Embase/Medline and Cochrane libraries were searched in November 2021, using the main search terms ‘renal cell carcinoma’ and ‘magnetic resonance imaging’ (Table S1). The study has not been registered. Cross-referencing and a citation check of the included papers were executed using Scopus.
Articles were included when they (1) included MRI characteristics of patients with proven RCC; (2) were prospective or retrospective cohort studies, randomized controlled trials or case reports; (3) were written in the English language; and (4) were available in the full-text form. Subsequently, articles focusing on children (<19 years), potentially also including adolescents or young adults (≤35 years) and articles focusing on MiT-RCC were separated to serve as the focus of this literature review. Given the rarity of studies focusing on the MRI characteristics of MiT-RCC, articles focusing on adults were also included for this purpose. With this approach, we guaranteed identification of all relevant articles while subdividing their relevance for our study based on their full-text content. After removal of duplicates, 7012 articles were screened based on title and abstract, leaving 363 articles for full-text screening, resulting in the inclusion of 95 articles. Of these, 13 articles focused on pediatric, adolescent and young-adult RCC, and 13 articles focused on MiT-RCC, with an overlap of 6 articles (Figure 1). In November 2022, the search was updated, with no additional results for articles focusing on children and/or MiT-RCC.
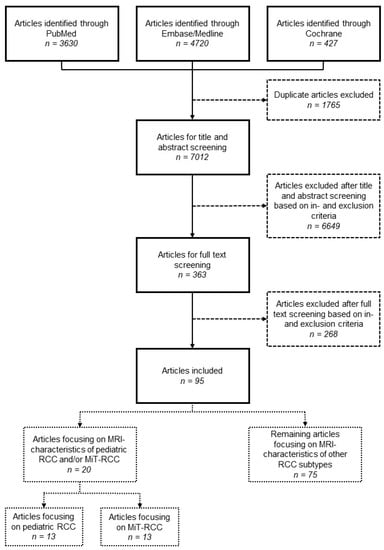
Figure 1.
Flow chart of the literature review.
3. Results
3.1. Case Presentation
3.1.1. Patient Characteristics
The six identified patients in our center had a median age of 12 years (range 63–193 months) (Table 2). Four patients were female, and half of the patients presented with a right-sided tumor. Two/six patients received pre-operative chemotherapy following suspicion of a WT, whereas 4/6 underwent upfront surgery. In one case, RCC was pre-operatively confirmed through tumor biopsy. Three patients had stage 1 disease, whereas the other patients had stage 2 (1/6) and stage 3 (2/6) disease (Table 2).

Table 2.
Characteristics of the included pediatric patients with RCC.
3.1.2. Histopathology
The average post-operative specimen weight was 898.6 g (range 210–2100 g), whereas the maximum post-operative tumor diameter ranged from 2.4 to 12.9 cm (median 6.8 cm). The post-operative weight of the specimen was missing for one patient, of which the largest tumor diameter was 9.5 cm (Table 2).
Five patients were tested for MiT-RCC, resulting in 2/5 MiT-RCC cases (Table 2, Figure 2 and Figure 3). In 4/5 cases, FISH was used, whereas in the two most recent cases, also RNA sequencing was performed, resulting in a rearrangement of TFE3 and SFPQ in the sixth patient. Two patients were diagnosed with ccRCC, and in one patient, the subtype could not be specified. The first patient, who was not tested for MiT-RCC, showed an FH mutation in the context of a hereditary leiomyomatosis and RCC cancer syndrome (Table 2) [41]. For the 5-year-old patient diagnosed with ccRCC, the FISH for MiT-RCC was not conclusive, and RNA sequencing for further analysis of TFEB was not available.
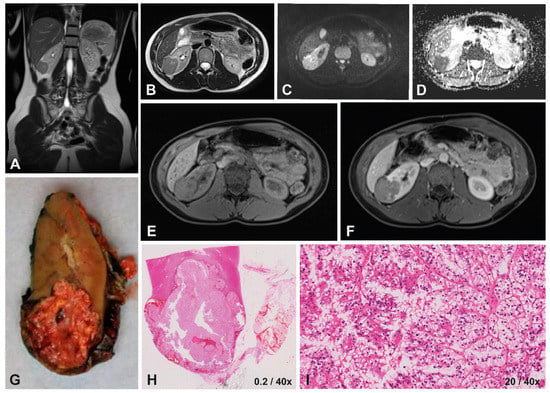
Figure 2.
Imaging and histopathology of a 14-year-old female patient with a right-sided translocation-type RCC (MiT-RCC). On T2-weighted imaging (A,B) the tumor appears hypo-intense with ill-defined margins, compared to the iso-intense homogeneous appearance on T1-weighted imaging (E) with relatively strong homogeneous enhancement on T1-weighted contrast-enhanced imaging (F). DWI showed restricted diffusion on the b500 scan (C), with a relatively high median ADC value of 0.98x10−3 mm2/s calculated based on the b0/b500s map (D). The macroscopic (G) and microscopic histopathology (H) showed an infiltrating tumor, detail showing tumor cells with hyperchromatic nuclei and papillary growth pattern (I).
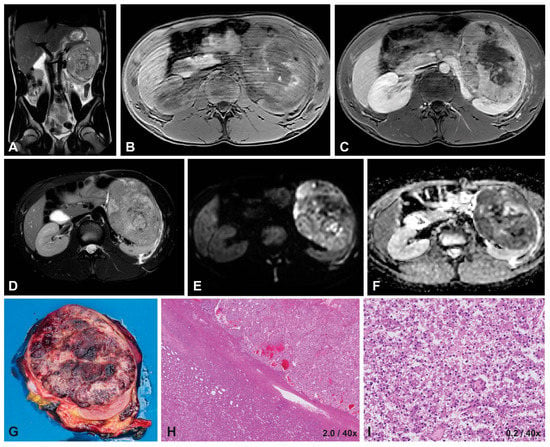
Figure 3.
Imaging and histopathology of a 16-year-old male patient with a left-sided translocation-type RCC (MiT-RCC). On T2-weighted imaging (A,D) the tumor appears hypo-intense and heterogeneous with well-defined margins, similar to a hypo-intense appearance on T1-weighted imaging (B) with mild, heterogeneous enhancement on T1-weighted contrast-enhanced imaging (C). DWI showed restricted diffusion on the b1000 scan (E), with a median ADC value of 0.80 ×10−3 mm2/s on the b0/b1000 map (F). The macroscopic histopathology (G) shows a large, round tumor, with little remaining normal renal tissue. The microscopic HE image (H) shows a capsule around the tumor, with a predominantly epithelial growth pattern in nests, often with cells with clear cytoplasm and mildly atypical nuclei (I).
3.1.3. Imaging Characteristics at Diagnosis
The median observed agreement between the two observers was 83% (range 33.3%–100%). The few imaging characteristics with low observed agreement were discussed between the two radiologists, and mismatching concepts were resolved (Table S2). Furthermore, the inter-reader agreement for median tumor volume was excellent, with an ICC of 0.991 (95% 0.941–0.999). Therefore, the imaging characteristics found by the first reader (ASL) were reported (Table 2).
Tumor volume ranged from 29 to 2191 cm3, with varying locations. The shape of the tumors was predominantly lobulated (4/6), and margins were well-defined in a majority of the patients (4/6). Capsule rupture was seen in only 2/6 cases, which was defined as an interruption of the hypo-intense capsule of the tumor. None of the cases presented with a tumor thrombus. Concerning hemorrhage and necrosis, these components were present in 3/6 and 1/6 cases, respectively. Cysts were present in 2/6 cases, whereas fatty tissue and subcapsular fluid were not observed (Table 2).
The tumors presented mainly homogeneously (4/6), with a predominant hypo-intense appearance on T2-weighted imaging and iso-intense appearance on T1-weighted imaging. Almost all cases showed a homogeneous enhancement pattern, varying from mild to strong enhancement (Table 2). There was no obvious consistency concerning MRI characteristics within patients based on histological subtype (Table 2, Figure 2, Figure 3).
3.1.4. Diffusion-Weighted Imaging
Inter-reader agreement was excellent for median ADC values with an ICC of 0.942 (95% CI 0.639–0.992) (Table S3). Therefore, only the median surfaces of ROIs and median ADC values measured by the first reader (ASL) were reported (Table 2). The median ADC values ranged from 0.70 to 1.20 × 10−3 mm2/s. The MiT-RCC cases and the case diagnosed as ccRCC but with inconclusive TFE results showed the lowest ADC values, ranging from 0.70 to 0.98 × 10−3 mm2/s (Table 2, Figure 2 and Figure 3).
3.2. Literature Review
3.2.1. Pediatric and Young-Adult RCC
We identified thirteen studies focusing on MRI findings of pediatric RCC, with a total of 25 patients (Figure 1, Table 3) [19,24,42,43,44,45,46,47,48,49,50,51,52]. Ages ranged from 4 to 33 years, with four studies also including young adults ≤35 years [19,24,48,51]. Six studies focused on MiT-RCC, whereas other histological subtypes represented ccRCC, papillary type RCC (pRCC), chromophobe RCC (chrRCC), renal medullary carcinoma (RMC) and other rare RCC types.

Table 3.
Review of the literature focusing on MRI characteristics of pediatric and young-adolescent renal cell carcinoma.
The location of all reported pediatric RCC tumors in the identified articles varied from central to peripheral (Table 3). On T1-weighted imaging and T2-weighted imaging, tumors appeared predominantly heterogeneously, whereas no clear predominant intensity was seen for one of these sequences. Accordingly, enhancement pattern on contrast-enhanced MRI was reported mostly as heterogeneous. Cysts, when specified, were found in only three cases, whereas the presence of necrosis and/or hemorrhage was often not specified [24,43,52].
Regional lymph node involvement and/or metastases to lymph nodes were reported in five studies (Table 3) [19,24,42,43,51]. In a study of seven patients, Wang et al. reported positive regional lymph node status in four patients and positive cervical lymph node status in one patient [19]. Blitman et al. reported two patients with vascular tumor involvement of the renal vein and three patients with encasement of the vascular pedicle out of a total of six patients, all with infiltrative tumors with ill-defined margins (Table 3) [51]. Only one study specified findings of DWI, reporting the iso-intense appearance of the tumor on the b500 DWI sequence compared to the renal parenchyma [50].
Concerning MRI characteristics of RCC subtypes other than MiT-RCC in children, Zou et al. reported a case of a 17-year-old male with von Hippel–Lindau disease with bilateral renal cysts and ccRCC (Table 3) [46]. This patient showed T2-weighted hyper-intensity and T1-weighted hypo-intensity, whereas enhancement was limited on contrast-enhanced imaging. Koetter et al. described a 16-year-old female at 31 weeks’ gestation presenting with a large, heterogeneous cystic–solid mass, which was histologically diagnosed as pRCC [43]. Another reported pRCC that presented as a complex cyst containing bloody elements, whereas a pediatric chrRCC showed a well-defined T1-weighted hypo-intense and T2-weighted hyper-intense tumor with necrosis (Table 3) [45,52].
Finally, RMC has been described as a very rare and malignant renal tumor, especially in children and young adults, and is often seen in RCC patients with sickle-cell traits [28,51,53]. Noreña-Rengifo et al. described a 12-year-old male with an intermediate enhancing mass on T1-weighted imaging with evident retroperitoneal lymphadenopathies, similar to the reported regional adenopathy identified on MRI in a retrospective study by Blitman et al. (Table 3) [42,51].
3.2.2. MiT-RCC
Thirteen studies focusing on MRI characteristics of MiT-RCC were identified, including the six identified studies focusing on pediatric patients with MiT-RCC (Figure 1, Table 3 and Table 4) [13,19,24,44,47,48,50,54,55,56,57,58,59]. There was a total of 46 patients, who were aged 4–76 years old, with MiT-RCC included in the identified articles. Whereas the tumor location was again highly variable among patients, overall, there was a majority showing hyper-intensity on T1-weighted imaging and hypo-intensity on T2-weighted imaging, with a heterogeneous enhancement pattern. Wang et al. reported 8/9 patients with necrosis, and 7/9 patients with hemorrhage, whereas in other studies, these characteristics were often not specified [19]. The tumor composition and growth pattern of MiT-RCC was very heterogeneous, although a substantial part of the cases seems to present with an infiltrative and/or irregular growth pattern. Fifteen patients presented with lymph node involvement; however, four studies lacked information concerning this characteristic. Reported metastatic sites were liver and/or lungs in a total of three patients [55,57].

Table 4.
Review of the literature focusing on MRI characteristics of translocation-type renal cell carcinoma (MiT-RCC).
DWI characteristics were reported in 5 studies for a total of 23 patients [13,50,54,55,57]. Overall, diffusion restriction seemed limited in these cases, with, for instance, Tohi et al. reporting no restriction and Chen et al. reporting a relatively high signal on the ADC map [54,57]. Razek et al. showed a mean ADC value of 1.50 ± 0.97 for four patients [13].
In our case series, the 14-year-old female patient in particular showed a typical presentation of MiT-RCC based on these findings in the previous literature. The tumor showed an ill-defined tumor with capsule invasion and an infiltrative growth pattern, appearing hypo-intense on T2-weighted imaging with a relatively high median ADC value (Table 2 and Table 4, Figure 2). The presentation of the 16-year-old male patient with MiT-RCC seemed less typical (Table 2 and Table 4, Figure 3).
3.2.3. Other Subtypes
The RCC subtypes most frequently occurring in children and adolescents besides MiT-RCC are ccRCC, pRCC and chrRCC (Table 3) [3,18]. Knowledge of MRI characteristics of these subtypes is based mainly on adult studies.
A retrospective study of Wang et al. focused on the MRI characteristics of 57 adult RCC patients, in which ccRCC and pRCC showed hemorrhage in 20–25% of the cases compared to no evidence of hemorrhage for chrRCC [60]. Moreover, a very high percentage of cystic necrosis was seen in ccRCC and pRCC, resulting in a significant difference of this characteristic compared to chrRCC, for which no cases were seen. Compared to ccRCC, other RCC subtypes often show a less aggressive growth pattern on MRI, which is illustrated by a higher numbers of cases with well-defined margins, less peripheral invasion and less extension of the tumor [60,61].
Oliva et al. described the MRI-features of 21 pRCCs and 28 ccRCCs, concluding that pRCC typically presents with T2 hypo-intensity, whereas ccRCC typically shows T2 hyper-intensity [62]. This finding, as well as the occurrence of increased enhancement in ccRCC compared to pRCC and chrRCC, has often been reported in the previous literature [35,63,64,65]. Furthermore, ccRCC seems to show significantly higher ADC values than pRCC and chrRCC [64,66,67].
4. Discussion
There seems to be a lack of specific imaging characteristics for discrimination of pediatric RCC and its subtypes based on MRI characteristics alone [6,9,10]. Nevertheless, imaging plays an increasingly important role in the diagnosis and follow-up of pediatric renal tumors and in the discrimination of different renal tumor types [28,68,69].
The heterogeneous diagnostic appearance of our patients was in line with findings in the identified literature and with previous studies stating that RCC is often indistinguishable from WTs based on MRI characteristics alone [70,71,72,73]. Part of the included patients showed cysts, necrosis and hemorrhage; however, none of these characteristics were explicitly found in all patients [74]. Calcifications have often been reported as common findings in pediatric RCC; however, MRI does not allow for a trustworthy assessment of calcifications and was, therefore, not included as an imaging characteristic in our case report form [28,69,75]. Despite the recommendation of the SIOP-RTSG to use MRI for cross-sectional imaging of renal tumors, various countries still perform abdominal CT scans in these patients. One of the largest studies focusing on CT characteristics of pediatric RCC to date also reported a widely variable radiological appearance, often with the presence of calcifications [49]. Nevertheless, calcifications can also be seen in WTs, making discrimination based on this imaging characteristic difficult given the rarity of pediatric RCC and other non-WTs [76,77]. Finally, the findings in our case series were in concordance with the frequently reported localized presentation and small size of pediatric RCC [6,75].
Whereas MiT-RCC is the most frequent histological subtype in children, we reported only two out of six patients with a proven TFE translocation. The MRI characteristics of these two patients were quite different from one another. MiT-RCC, similar to ccRCC, is often described as a relatively aggressive tumor in terms of growth pattern and tumor extension as well as prognosis [28,35,60,78,79,80,81]. Nevertheless, only one MiT-RCC case showed an infiltrative growth pattern with capsule rupture, whereas the second MiT-RCC case and both ccRCC cases had well-defined margins with the presence of a pseudocapsule, without any signs of aggressive growth. In general, capsule rupture remains difficult to assess. Concerning the discrimination between histological RCC subtypes, the predominantly reported T2-weighted hypo-intensity in MiT-RCC is also often described for pRCC and chrRCC, whereas ccRCC classically demonstrates high intrinsic T2-weighted signal intensity [31,33,35,82,83]. Nonetheless, knowledge of specific MRI characteristics of MiT-RCC remains limited, given the rarity of MiT-RCC in adult patients and its relatively recent recognition as an official subtype by the WHO [27].
Whereas in adult RCC, the main focus is often the discrimination of histological subtypes, in pediatric RCC, discrimination from the much more frequently occurring WTs in the early diagnostic stages is of great importance [6,7,9]. WTs have a very heterogeneous presentation at diagnosis and are, most often, large intra-renal tumors with a pseudocapsule [74,84,85]. Whereas an irregular growth pattern and absence of a capsule are often described as common for RCC in the previous literature, we observed a majority of well-defined margins and the presence of a pseudocapsule in our case series. Nonetheless, an enhancing capsule has also been reported as a characteristic of MiT-RCC [25,28,57]. MRI characteristics reported to be typical for RCC will still not be discriminative given the heterogeneous appearance of WTs. Nevertheless, WTs often appear hyper-intense on T2-weighted imaging, which is opposite to the T2-weighted hypo-intensity in a majority of our cases with RCC, as substantiated by the findings in the previous literature [28,69]. Finally, RCC is often reported to be smaller than WTs [7,10,57]. Following SIOP-RTSG protocols, based on the suspicion of a non-WT, a biopsy is recommended for children ≥10 years of age and for children between 7 and 10 years old with a tumor volume <200 mL [10]. In our case series, tumor volume was relatively low, except for the expected large FH-RCC case (case nr. 1). In the previous literature, tumor volume ranged widely; however, often only the largest diameter was reported [7,57,74,77,86].
Overall, there seems to remain a lack of pathognomonic MRI characteristics for the discrimination of pediatric RCC from other renal malignancies in children, as well as for the differentiation of histological subtypes [6,9,10]. Nevertheless, DWI has shown an increasing potential reliability for the non-invasive discrimination of renal lesions [15,16,87,88]. Whereas only one included pediatric study focused on the diffusion restriction of pediatric RCCs, our literature review confirmed results from previous overviews stating adult clear-cell RCC has shown significantly higher ADC values compared to non-clear-cell RCC [17,32,50,87,89]. In contrast, our case series showed the three lowest median ADC values in the ccRCC and MiT-RCC cases, whereas also relatively high ADC values were reported. In WTs, relatively low ADC values can be observed, varying among histological WT subtypes [12,16]. In children, discrimination of common histological RCC subtypes, as well as discrimination from WTs based on DWI, therefore, remains difficult. Nonetheless, the female patient with MiT-RCC in our case series appeared to have a typical presentation in the light of previous reports, showing potential discriminative MRI characteristics for TFE-positive tumors. Future studies may focus on validating adult findings in the pediatric population and explore the relationship between ADC values and common pediatric RCC subtypes combined with other typical MRI characteristics.
Over the past decades, differences between adult and pediatric RCC have increasingly been appreciated. Concerning imaging studies, the direct comparison of the pediatric and adult population has become even more complicated by the preference of CT in the adult population, whereas MRI has developed as the preferred imaging modality within the SIOP-RTSG [6,8]. Nevertheless, MRI also plays an increasingly important role in the adult population, mainly due to its ability to perform quantitative measurements [32,90]. Therefore, when searching the literature databases for MRI characteristics of pediatric RCC and MiT-RCC, the literature about the adolescent and adult population cannot be ignored. Not only because knowledge of MR imaging of these cases is scarce, but also because they are often embedded in studies focusing on adolescents and/or adults as well. Concerning cut-off values for age classification, we focused on the predefined range of 18–35 years for the ‘adolescents and young adults’ often used in Europe. However, this classification varies around the world [91,92].
Our study has a few limitations, mainly based on its retrospective nature and small study population. The limited number of patients did not allow any statistical analysis or strong conclusions. Furthermore, scan parameters were inconsistent due to not as yet centralized care. Nevertheless, these cases served mainly as an illustration accompanying the literature review in this developing field of research. In this way, this descriptive study contributes to the increasing knowledge of pediatric RCC and its diagnostic presentation on MRI. Concerning the reported imaging characteristics by two independent observers, there was excellent inter-observer agreement [39,40]. The small number of patients in this study does not allow for strong conclusions concerning validity of the use of the CRF in other populations.
5. Conclusions
For a few years, MRI has been the preferred imaging modality for imaging pediatric renal tumors within the SIOP-RTSG protocol. This case series represents one of the largest retrospective reports so far, including an extensive review focusing on MRI characteristics of RCC in the pediatric and young-adult population. The reported cases showed a varying presentation of different pediatric RCC subtypes on MRI, in line with the published literature. Nevertheless, based on this study, T2-weighted hypo-intensity of the tumor has been shown to be a potential distinctive characteristic for the discrimination of RCC from other renal tumors that are prevalent at this age, especially WTs. Future studies should focus on larger study populations through international collaboration, also exploring innovative techniques such as DWI as a non-invasive biomarker.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cancers15051401/s1, Table S1: Search strategy focusing on MRI characteristics of RCC; Table S2: Observed percentage agreement for dichotomous and categorical characteristics in the case report form for the two observers; Table S3: Median surface of ROI and median ADC values per patient for the two observers.
Author Contributions
Conceptualization, J.N.v.d.B., R.R.d.K., M.M.v.d.H.-E. and A.S.L.; methodology, J.N.v.d.B., R.R.d.K., R.A.J.N., A.B., A.J.K., M.M.v.d.H.-E. and A.S.L.; formal analysis, J.N.v.d.B., A.S.L.; investigation, J.N.v.d.B., R.R.d.K., R.A.J.N. and A.S.L.; resources, J.N.v.d.B., R.R.d.K., M.M.v.d.H.-E. and A.S.L.; writing—original draft preparation, J.N.v.d.B.; writing—review and editing, J.N.v.d.B., R.R.d.K., R.A.J.N., A.B., A.J.K., M.M.v.d.H.-E. and A.S.L.; visualization, J.N.v.d.B., R.R.d.K. and A.S.L.; supervision; R.R.d.K., M.M.v.d.H.-E. and A.S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant (grant number 341) from the Stichting Kinderen Kankervrij (KiKa).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approval from the Institutional Review Board of the University Medical Center Utrecht (WAG/mb/20/019804 20-332, 26-05-2020) was obtained. For this retrospective study, formal consent was waived.
Informed Consent Statement
Additional patient consent was waived due to the retrospective nature of this study. All diagnostic MRI scans included were clinically indicated and were performed as the standard of care.
Data Availability Statement
Restrictions apply to the availability of these data. The data that support the findings of this study are available in the Supplementary Materials and from the International Society of Pediatric Oncology–Renal Tumor Study Group office following standard access procedures upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nakata, K.; Colombet, M.; Stiller, C.A.; Pritchard-Jones, K.; Steliarova-Foucher, E. Incidence of childhood renal tumours: An international population-based study. Int. J. Cancer 2020, 147, 3313–3327. [Google Scholar] [CrossRef] [PubMed]
- van der Beek, J.N.; Geller, J.I.; de Krijger, R.R.; Graf, N.; Pritchard-Jones, K.; Drost, J.; Verschuur, A.C.; Murphy, D.; Ray, S.; Spreafico, F.; et al. Characteristics and Outcome of Children with Renal Cell Carcinoma: A Narrative Review. Cancers 2020, 12, 1776. [Google Scholar] [CrossRef]
- van der Beek, J.N.; Hol, J.A.; Coulomb-l’Hermine, A.; Graf, N.; van Tinteren, H.; Pritchard-Jones, K.; Houwing, M.E.; de Krijger, R.R.; Vujanic, G.M.; Dzhuma, K.; et al. Characteristics and outcome of pediatric renal cell carcinoma patients registered in the International Society of Pediatric Oncology (SIOP) 93-01, 2001 and UK-IMPORT database: A report of the SIOP-Renal Tumor Study Group. Int. J. Cancer 2021, 148, 2724–2735. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; van Peer, S.E.; de Witte, M.M.; Tytgat, G.A.M.; Karim-Kos, H.E.; van Grotel, M.; van de Ven, C.P.; Mavinkurve-Groothuis, A.M.C.; Merks, J.H.M.; Kuiper, R.P.; et al. Characteristics and outcome of children with renal tumors in the Netherlands: The first five-year’s experience of national centralization. PLoS ONE 2022, 17, e0261729. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Jones, R.; Pritchard-Jones, K.; Dzhuma, K.; van den Heuvel-Eibrink, M.; Tytgat, G.; van der Beek, J.; Oades, G.; Murphy, D. Pediatric and young adult renal cell carcinoma. Pediatr. Blood Cancer 2020, 67, e28675. [Google Scholar] [CrossRef]
- Watson, T.; Oostveen, M.; Rogers, H.; Pritchard-Jones, K.; Olsen, Ø. The role of imaging in the initial investigation of paediatric renal tumours. Lancet Child Adolesc. Health 2020, 4, 232–241. [Google Scholar] [CrossRef]
- de la Monneraye, Y.; Michon, J.; Pacquement, H.; Aerts, I.; Orbach, D.; Doz, F.; Bourdeaut, F.; Sarnacki, S.; Philippe-Chomette, P.; Audry, G.; et al. Indications and results of diagnostic biopsy in pediatric renal tumors: A retrospective analysis of 317 patients with critical review of SIOP guidelines. Pediatr. Blood Cancer 2019, 66, e27641. [Google Scholar] [CrossRef]
- Grover, S.B.; Antil, N.; Rajani, H.; Grover, H.; Kumar, R.; Mandal, A.K.; Bagga, D.; Katyan, A. Approach to pediatric renal tumors: An imaging review. Abdom. Radiol. 2019, 44, 619–641. [Google Scholar] [CrossRef]
- van der Beek, J.N.; Watson, T.A.; Nievelstein, R.A.J.; Brisse, H.J.; Morosi, C.; Lederman, H.M.; Coma, A.; Gavra, M.M.; Vult von Steyern, K.; Lakatos, K.; et al. MRI Characteristics of Pediatric Renal Tumors: A SIOP-RTSG Radiology Panel Delphi Study. J. Magn. Reson. Imaging 2021, 55, 543–552. [Google Scholar] [CrossRef]
- Jackson, T.J.; Brisse, H.J.; Pritchard-Jones, K.; Nakata, K.; Morosi, C.; Oue, T.; Irtan, S.; Vujanic, G.; van den Heuvel-Eibrink, M.M.; Graf, N.; et al. How we approach paediatric renal tumour core needle biopsy in the setting of preoperative chemotherapy: A Review from the SIOP Renal Tumour Study Group. Pediatr. Blood Cancer 2022, 69, e29702. [Google Scholar] [CrossRef]
- Littooij, A.S.; Nikkels, P.G.; Hulsbergen-van de Kaa, C.A.; van de Ven, C.P.; van den Heuvel-Eibrink, M.M.; Olsen, Ø.E. Apparent diffusion coefficient as it relates to histopathology findings in post-chemotherapy nephroblastoma: A feasibility study. Pediatr. Radiol. 2017, 47, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Hales, P.W.; Olsen, Ø.E.; Sebire, N.J.; Pritchard-Jones, K.; Clark, C.A. A multi-Gaussian model for apparent diffusion coefficient histogram analysis of Wilms’ tumour subtype and response to chemotherapy. NMR Biomed 2015, 28, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Razek, A.A.; Farouk, A.; Mousa, A.; Nabil, N. Role of diffusion-weighted magnetic resonance imaging in characterization of renal tumors. J. Comput. Assist. Tomogr. 2011, 35, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pan, J.; Shen, Y.; Bai, X.; Wang, Y.; Wang, H.; Ye, H. High signal renal tumors on DWI: The diagnostic value of morphological characteristics. Abdom. Radiol. 2019, 44, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Hötker, A.M.; Lollert, A.; Mazaheri, Y.; Müller, S.; Schenk, J.P.; Mildenberger, P.C.; Akin, O.; Graf, N.; Staatz, G. Diffusion-weighted MRI in the assessment of nephroblastoma: Results of a multi-center trial. Abdom. Radiol. 2020, 45, 3202–3212. [Google Scholar] [CrossRef]
- Littooij, A.S.; Sebire, N.J.; Olsen Ø, E. Whole-tumor apparent diffusion coefficient measurements in nephroblastoma: Can it identify blastemal predominance? J. Magn. Reson. Imaging 2017, 45, 1316–1324. [Google Scholar] [CrossRef]
- Wu, Y.; Kwon, Y.S.; Labib, M.; Foran, D.J.; Singer, E.A. Magnetic Resonance Imaging as a Biomarker for Renal Cell Carcinoma. Dis. Mrk. 2015, 2015, 648495. [Google Scholar] [CrossRef]
- Geller, J.I.; Cost, N.G.; Chi, Y.Y.; Perlman, E.J.; Kim, Y.; Cajaiba, M.; Mullen, E.A.; Glick, R.D.; Khanna, G.; Daw, N.C.; et al. A prospective study of pediatric renal cell carcinoma: A report from the Children’s Oncology Group study AREN0321. J. Clin. Oncol. 2018, 36, 10516. [Google Scholar] [CrossRef]
- Wang, W.; Ding, J.; Li, Y.; Wang, C.; Zhou, L.; Zhu, H.; Peng, W. Magnetic resonance imaging and computed tomography characteristics of renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion. PLoS ONE 2014, 9, e99990. [Google Scholar] [CrossRef]
- Murphy, G.; Jhaveri, K. The expanding role of imaging in the management of renal cell carcinoma. Expert. Rev. Anticancer. Ther. 2011, 11, 1871–1888. [Google Scholar] [CrossRef]
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Geller, J.I.; Ehrlich, P.F.; Cost, N.G.; Khanna, G.; Mullen, E.A.; Gratias, E.J.; Naranjo, A.; Dome, J.S.; Perlman, E.J. Characterization of adolescent and pediatric renal cell carcinoma: A report from the Children’s Oncology Group study AREN03B2. Cancer 2015, 121, 2457–2464. [Google Scholar] [CrossRef] [PubMed]
- Selle, B.; Furtwangler, R.; Graf, N.; Kaatsch, P.; Bruder, E.; Leuschner, I. Population-based study of renal cell carcinoma in children in Germany, 1980–2005: More frequently localized tumors and underlying disorders compared with adult counterparts. Cancer 2006, 107, 2906–2914. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xie, P.; Peng, W.; Zhou, Z. Renal carcinomas associated with Xp11.2 translocations/TFE3 gene fusions: Findings on MRI and computed tomography imaging. J. Magn. Reson. Imaging 2014, 40, 440–447. [Google Scholar] [CrossRef]
- He, M.; Cai, J.; Zhu, K.; Gu, W.; Li, M.; Xiong, J.; Guan, Z.; Wang, J.; Shu, Q. Renal cell carcinoma in children and adolescents: Single-center experience and literature review. Medicine 2021, 100, e23717. [Google Scholar] [CrossRef]
- Cajaiba, M.M.; Dyer, L.M.; Geller, J.I.; Jennings, L.J.; George, D.; Kirschmann, D.; Rohan, S.M.; Cost, N.G.; Khanna, G.; Mullen, E.A.; et al. The classification of pediatric and young adult renal cell carcinomas registered on the children’s oncology group (COG) protocol AREN03B2 after focused genetic testing. Cancer 2018, 124, 3381–3389. [Google Scholar] [CrossRef]
- Lopez-Beltran, A.; Scarpelli, M.; Montironi, R.; Kirkali, Z. 2004 WHO classification of the renal tumors of the adults. Eur. Urol. 2006, 49, 798–805. [Google Scholar] [CrossRef]
- Chung, E.M.; Lattin, G.E., Jr.; Fagen, K.E.; Kim, A.M.; Pavio, M.A.; Fehringer, A.J.; Conran, R.M. Renal Tumors of Childhood: Radiologic-Pathologic Correlation Part 2. The 2nd Decade: From the Radiologic Pathology Archives. Radiographics 2017, 37, 1538–1558. [Google Scholar] [CrossRef]
- Couvidat, C.; Eiss, D.; Verkarre, V.; Merran, S.; Correas, J.M.; Mejean, A.; Helenon, O. Renal papillary carcinoma: CT and MRI features. Diagn. Interv. Imaging 2014, 95, 1055–1063. [Google Scholar] [CrossRef]
- Schieda, N.; Lim, R.S.; McInnes, M.D.F.; Thomassin, I.; Renard-Penna, R.; Tavolaro, S.; Cornelis, F.H. Characterization of small (<4 cm) solid renal masses by computed tomography and magnetic resonance imaging: Current evidence and further development. Diagn. Interv. Imaging 2018, 99, 443–455. [Google Scholar] [CrossRef]
- Lopes Vendrami, C.; Parada Villavicencio, C.; DeJulio, T.J.; Chatterjee, A.; Casalino, D.D.; Horowitz, J.M.; Oberlin, D.T.; Yang, G.Y.; Nikolaidis, P.; Miller, F.H. Differentiation of Solid Renal Tumors with Multiparametric MR Imaging. Radiographics 2017, 37, 2026–2042. [Google Scholar] [CrossRef] [PubMed]
- Gurel, S.; Narra, V.; Elsayes, K.M.; Siegel, C.L.; Chen, Z.E.; Brown, J.J. Subtypes of renal cell carcinoma: MRI and pathological features. Diagn. Interv. Radiol. 2013, 19, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Muglia, V.F.; Prando, A. Renal cell carcinoma: Histological classification and correlation with imaging findings. Radiol. Bras. 2015, 48, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Sung, D.J.; Sim, K.C.; Han, N.Y.; Park, B.J.; Kim, M.J.; Cho, S.B. Renal tumors with low signal intensities on T2-weighted MR image: Radiologic-pathologic correlation. Abdom. Radiol. 2017, 42, 2108–2118. [Google Scholar] [CrossRef]
- Laguna, B.; Westphalen, A.C.; Guimarães, C.T.; Whang, Z.; Simko, J.; Zagoria, R. Uncommon malignant renal tumors and atypical presentation of common ones: A guide for radiologists. Abdom. Radiol. 2019, 44, 1430–1452. [Google Scholar] [CrossRef]
- Spreafico, F.; Collini, P.; Terenziani, M.; Marchiano, A.; Piva, L. Renal cell carcinoma in children and adolescents. Expert. Rev. Anticancer Ther. 2010, 10, 1967–1978. [Google Scholar] [CrossRef]
- Vujanic, G.M.; Gessler, M.; Ooms, A.; Collini, P.; Coulomb-l’Hermine, A.; D’Hooghe, E.; de Krijger, R.R.; Perotti, D.; Pritchard-Jones, K.; Vokuhl, C.; et al. The UMBRELLA SIOP-RTSG 2016 Wilms tumour pathology and molecular biology protocol. Nat. Rev. Urol. 2018, 15, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, A.R.; Cicchetti, D.V. High agreement but low kappa: I. The problems of two paradoxes. J. Clin. Epidemiol. 1990, 43, 543–549. [Google Scholar] [CrossRef]
- de Vet, H.C.; Mokkink, L.B.; Terwee, C.B.; Hoekstra, O.S.; Knol, D.L. Clinicians are right not to like Cohen’s κ. BMJ 2013, 346, f2125. [Google Scholar] [CrossRef]
- Hallgren, K.A. Computing Inter-Rater Reliability for Observational Data: An Overview and Tutorial. Tutor Quant. Methods Psychol. 2012, 8, 23–34. [Google Scholar] [CrossRef]
- Hol, J.A.; Jongmans, M.C.J.; Littooij, A.S.; de Krijger, R.R.; Kuiper, R.P.; van Harssel, J.J.T.; Mensenkamp, A.; Simons, M.; Tytgat, G.A.M.; van den Heuvel-Eibrink, M.M.; et al. Renal cell carcinoma in young FH mutation carriers: Case series and review of the literature. Fam. Cancer 2020, 19, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Noreña-Rengifo, B.D.; Ochoa-Gaviria, J.; Vélez-Escobar, A.; Muñoz, J.P.; Riveros-Ángel, M. Renal Medullary Carcinoma in an Adolescent With Unknown Sickle Cell Trait. Cureus 2021, 13, e14473. [Google Scholar] [CrossRef] [PubMed]
- Koetter, P.; Martin, K. Management of renal cell carcinoma presenting during teenage pregnancy. J. Pediatr. Surg. Case Rep. 2020, 63, 101664. [Google Scholar] [CrossRef]
- Schaefer, B.A.; Johnson, T.S.; Hooper, D.K.; Nathan, J.D.; Geller, J.I. TFE3-positive renal cell carcinoma occurring in three children with dysfunctional kidneys on immunosuppression. Pediatr. Transpl. 2017, 21, e12912. [Google Scholar] [CrossRef] [PubMed]
- Okabe, K.; Kitamura, H.; Nishiyama, N.; Masumori, N. A case of chromophobe renal cell carcinoma in a 12-year-old girl. Int. Cancer Conf. J. 2016, 5, 36–39. [Google Scholar] [CrossRef]
- Zou, Y.; Xu, J.; Zhang, M. Long-term follow-up and clinical course of a rare case of von Hippel-Lindau disease: A case report and review of the literature. Oncol. Lett. 2016, 11, 3273–3278. [Google Scholar] [CrossRef]
- Koo, H.J.; Choi, H.J.; Kim, M.H.; Cho, K.S. Radiologic-pathologic correlation of renal cell carcinoma associated with Xp11.2 translocation. Acta. Radiol. 2013, 54, 827–834. [Google Scholar] [CrossRef]
- Dang, T.T.; Ziv, E.; Weinstein, S.; Meng, M.V.; Wang, Z.; Coakley, F.V. Computed tomography and magnetic resonance imaging of adult renal cell carcinoma associated with Xp11.2 translocation. J. Comput. Assist. Tomogr. 2012, 36, 669–674. [Google Scholar] [CrossRef]
- Downey, R.T.; Dillman, J.R.; Ladino-Torres, M.F.; McHugh, J.B.; Ehrlich, P.F.; Strouse, P.J. CT and MRI appearances and radiologic staging of pediatric renal cell carcinoma. Pediatr. Radiol. 2012, 42, 410–417, quiz 513–414. [Google Scholar] [CrossRef]
- Kato, H.; Kanematsu, M.; Yokoi, S.; Miwa, K.; Horie, K.; Deguchi, T.; Hirose, Y. Renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion: Radiological findings mimicking papillary subtype. J. Magn. Reson. Imaging 2011, 33, 217–220. [Google Scholar] [CrossRef]
- Blitman, N.M.; Berkenblit, R.G.; Rozenblit, A.M.; Levin, T.L. Renal medullary carcinoma: CT and MRI features. AJR Am. J. Roentgenol. 2005, 185, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Nakatani, T.; Minami, H.; Ikemoto, S.; Esaki, K.; Morimoto, H.; Takase, T. Renal cell carcinoma with hemorrhagic cyst formation in a 4-year-old boy. Int. J. Urol. 2003, 10, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.J., Jr.; Mostofi, F.K.; Sesterhenn, I.A. Renal medullary carcinoma. The seventh sickle cell nephropathy. Am. J. Surg. Pathol. 1995, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tohi, Y.; Harada, S.; Kuroda, N.; Tanaka, K.; Inoue, K.; Kadota, K.; Haba, R.; Nishiyama, Y.; Ueda, N.; Sugimoto, M. 6p.21 translocation renal cell carcinoma in the elderly: Radiological findings mimicking fat poor angiomyolipoma or papillary renal cell carcinoma. Int. Cancer Conf. J. 2021, 10, 233–238. [Google Scholar] [CrossRef]
- Dai, C.; Sheng, R.; Ding, Y.; Yang, M.; Hou, J.; Zhou, J. Magnetic resonance imaging findings of renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion in adults: A pilot study. Abdom. Radiol. 2019, 44, 209–217. [Google Scholar] [CrossRef]
- Gong, P.; Zhuang, Q.; Wang, K.; Xu, R.; Chen, Y.; Wang, X.; Yin, S. Adult-onset renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion: 3 case reports and review of literature. Medicine 2018, 97, e11023. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Q.; Li, B.; Cui, W.; Zhou, H.; Duan, N.; Liu, Y.; Kundra, V.; Wang, Z. Renal cell carcinoma associated with Xp11.2 translocation/TFE gene fusion: Imaging findings in 21 patients. Eur. Radiol. 2017, 27, 543–552. [Google Scholar] [CrossRef]
- Yu, L.; Li, J.; Xu, S.; Navia Miranda, M.; Wang, G.; Duan, Y. An Xp11.2 translocation renal cell carcinoma with SMARCB1 (INI1) inactivation in adult end-stage renal disease: A case report. Diagn. Pathol. 2016, 11, 98. [Google Scholar] [CrossRef]
- D’Antonio, A.; Addesso, M.; Nappi, O.; Zeppa, P. Unsuspected Xp11 Translocation Renal Neoplasm Associated with Contralateral Clear Cell Carcinoma. Int. J. Surg. Pathol. 2016, 24, 248–252. [Google Scholar] [CrossRef]
- Wang, X.; Kong, W.; Wang, Y.; Wang, Y.; Chen, Y.; Shi, Z.; Liu, Y. Analysis of CT, MRI imaging features of renal cell carcinoma with different histopathological types. J. Buon 2021, 26, 2053–2058. [Google Scholar]
- Paschall, A.K.; Nikpanah, M.; Farhadi, F.; Jones, E.C.; Wakim, P.G.; Dwyer, A.J.; Gautam, R.; Merino, M.J.; Srinivasan, R.; Linehan, W.M.; et al. Hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome: Spectrum of imaging findings. Clin. Imaging 2020, 68, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.R.; Glickman, J.N.; Zou, K.H.; Teo, S.Y.; Mortelé, K.J.; Rocha, M.S.; Silverman, S.G. Renal cell carcinoma: t1 and t2 signal intensity characteristics of papillary and clear cell types correlated with pathology. AJR Am. J. Roentgenol. 2009, 192, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Hotker, A.M.; Mazaheri, Y.; Wibmer, A.; Karlo, C.A.; Zheng, J.; Moskowitz, C.S.; Tickoo, S.K.; Russo, P.; Hricak, H.; Akin, O. Differentiation of clear cell renal cell carcinoma from other renal cortical tumors by use of a quantitative multiparametric MRI approach. Am. J. Roentgenol. 2017, 208, W85–W91. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cheng, L.; Zhang, X.; Wang, D.; Guo, A.; Gao, Y.; Ye, H. Renal cell carcinoma: Diffusion-weighted MR imaging for subtype differentiation at 3.0 T. Radiology 2010, 257, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.; El Ghali, S.; Buy, X.; Lindner, V.; Gangi, A. Papillary renal cell carcinoma in allograft kidney. Eur. Radiol. 2005, 15, 661–665. [Google Scholar] [CrossRef]
- de Silva, S.; Lockhart, K.R.; Aslan, P.; Nash, P.; Hutton, A.; Malouf, D.; Lee, D.; Cozzi, P.; MacLean, F.; Thompson, J. The diagnostic utility of diffusion weighted MRI imaging and ADC ratio to distinguish benign from malignant renal masses: Sorting the kittens from the tigers. BMC Urol. 2021, 21, 67. [Google Scholar] [CrossRef]
- Paschall, A.K.; Mirmomen, S.M.; Symons, R.; Pourmorteza, A.; Gautam, R.; Sahai, A.; Dwyer, A.J.; Merino, M.J.; Metwalli, A.R.; Linehan, W.M.; et al. Differentiating papillary type I RCC from clear cell RCC and oncocytoma: Application of whole-lesion volumetric ADC measurement. Abdom. Radiol. 2018, 43, 2424–2430. [Google Scholar] [CrossRef]
- van der Beek, J.N.; Artunduaga, M.; Schenk, J.P.; Eklund, M.J.; Smith, E.A.; Lederman, H.M.; Warwick, A.B.; Littooij, A.S.; Khanna, G. Similarities and controversies in imaging of pediatric renal tumors: A SIOP-RTSG and COG collaboration. Pediatr. Blood Cancer 2022, e30080. [Google Scholar] [CrossRef]
- Chung, E.M.; Graeber, A.R.; Conran, R.M. Renal Tumors of Childhood: Radiologic-Pathologic Correlation Part 1. The 1st Decade: From the Radiologic Pathology Archives. Radiographics 2016, 36, 499–522. [Google Scholar] [CrossRef]
- Geller, E.; Kochan, P.S. Renal neoplasms of childhood. Radiol. Clin. N. Am. 2011, 49, 689–709. [Google Scholar] [CrossRef]
- Lonergan, G.J.; Martínez-León, M.I.; Agrons, G.A.; Montemarano, H.; Suarez, E.S. Nephrogenic rests, nephroblastomatosis, and associated lesions of the kidney. Radiographics 1998, 18, 947–968. [Google Scholar] [CrossRef]
- Stanescu, A.L.; Acharya, P.T.; Lee, E.Y.; Phillips, G.S. Pediatric Renal Neoplasms:: MR Imaging-Based Practical Diagnostic Approach. Magn. Reson. Imaging Clin. N. Am. 2019, 27, 279–290. [Google Scholar] [CrossRef]
- Hartman, D.S.; Davis, C.J., Jr.; Madewell, J.E.; Friedman, A.C. Primary malignant renal tumors in the second decade of life: Wilms tumor versus renal cell carcinoma. J. Urol. 1982, 127, 888–891. [Google Scholar] [CrossRef]
- Lowe, L.H.; Isuani, B.H.; Heller, R.M.; Stein, S.M.; Johnson, J.E.; Navarro, O.M.; Hernanz-Schulman, M. Pediatric renal masses: Wilms tumor and beyond. Radiographics 2000, 20, 1585–1603. [Google Scholar] [CrossRef] [PubMed]
- Swinson, S.; McHugh, K. Urogenital tumours in childhood. Cancer Imaging 2011, 11, S48–S64. [Google Scholar] [CrossRef] [PubMed]
- Riccabona, M. Imaging of renal tumours in infancy and childhood. Eur. Radiol. 2003, 13 (Suppl. 4), L116–L129. [Google Scholar] [CrossRef] [PubMed]
- Birkemeier, K.L. Imaging of solid congenital abdominal masses: A review of the literature and practical approach to image interpretation. Pediatr. Radiol. 2020, 50, 1907–1920. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Song, H. Nephron-sparing surgery in the treatment of pediatric renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusions. J. Pediatr. Surg. 2017, 52, 1492–1495. [Google Scholar] [CrossRef]
- Zhu, Q.Q.; Wang, Z.Q.; Zhu, W.R.; Chen, W.X.; Wu, J.T. The multislice CT findings of renal carcinoma associated with XP11.2 translocation/TFE gene fusion and collecting duct carcinoma. Acta. Radiol. 2013, 54, 355–362. [Google Scholar] [CrossRef]
- Ross, H.; Argani, P. Xp11 translocation renal cell carcinoma. Pathology 2010, 42, 369–373. [Google Scholar] [CrossRef]
- Camparo, P.; Vasiliu, V.; Molinie, V.; Couturier, J.; Dykema, K.J.; Petillo, D.; Furge, K.A.; Comperat, E.M.; Lae, M.; Bouvier, R.; et al. Renal translocation carcinomas: Clinicopathologic, immunohistochemical, and gene expression profiling analysis of 31 cases with a review of the literature. Am. J. Surg. Pathol. 2008, 32, 656–670. [Google Scholar] [CrossRef]
- Xu, H.S.; Balcacer, P.; Zhang, Z.; Zhang, L.; Yee, E.U.; Sun, M.R.; Tsai, L.L. Characterizing T2 iso- and hypo-intense renal masses on MRI: Can templated algorithms improve accuracy? Clin. Imaging 2021, 72, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Kay, F.U.; Canvasser, N.E.; Xi, Y.; Pinho, D.F.; Costa, D.N.; Diaz de Leon, A.; Khatri, G.; Leyendecker, J.R.; Yokoo, T.; Lay, A.H.; et al. Diagnostic Performance and Interreader Agreement of a Standardized MR Imaging Approach in the Prediction of Small Renal Mass Histology. Radiology 2018, 287, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Aslan, M.; Aslan, A.; Arıöz Habibi, H.; Kalyoncu Uçar, A.; Özmen, E.; Bakan, S.; Kuruğoğlu, S.; Adaletli, İ. Diffusion-weighted MRI for differentiating Wilms tumor from neuroblastoma. Diagn. Interv. Radiol. 2017, 23, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Schenk, J.P.; Graf, N.; Günther, P.; Ley, S.; Göppl, M.; Kulozik, A.; Rohrschneider, W.K.; Tröger, J. Role of MRI in the management of patients with nephroblastoma. Eur. Radiol. 2008, 18, 683–691. [Google Scholar] [CrossRef]
- Siegel, M.J.; Chung, E.M. Wilms’ tumor and other pediatric renal masses. Magn. Reson. Imaging Clin. N. Am. 2008, 16, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Hotker, A.M.; Mazaheri, Y.; Wibmer, A.; Zheng, J.; Moskowitz, C.S.; Tickoo, S.K.; Russo, P.; Hricak, H.; Akin, O. Use of DWI in the differentiation of renal cortical tumors. Am. J. Roentgenol. 2016, 206, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Meeus, E.M.; Zarinabad, N.; Manias, K.A.; Novak, J.; Rose, H.E.L.; Dehghani, H.; Foster, K.; Morland, B.; Peet, A.C. Diffusion-weighted MRI and intravoxel incoherent motion model for diagnosis of pediatric solid abdominal tumors. J. Magn. Reson. Imaging 2018, 47, 1475–1486. [Google Scholar] [CrossRef]
- Sobh, D.M.; El Hawary, G.E.S.M.; Abou El Ghar, M.; El-Diasty, T.A.E.M.; El-Sayed Settein, M.; ElShaer, S.; Tantawy, M.S.E. Role of diffusion weighted MR imaging in characterization of focal kidney and upper urinary tract lesions. Egypt. J. Radiol. Nucl. Med. 2016, 47, 1689–1700. [Google Scholar] [CrossRef]
- Zhu, J.; Luo, X.; Gao, J.; Li, S.; Li, C.; Chen, M. Application of diffusion kurtosis tensor MR imaging in characterization of renal cell carcinomas with different pathological types and grades. Cancer Imaging 2021, 21, 30. [Google Scholar] [CrossRef]
- Burkart, M.; Sanford, S.; Dinner, S.; Sharp, L.; Kinahan, K. Future health of AYA survivors. Pediatr. Blood Cancer 2019, 66, e27516. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults, 2020. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).