Declined Organs for Liver Transplantation: A Right Decision or a Missed Opportunity for Patients with Hepatocellular Carcinoma?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Organ Allocation
2.1.1. Primary Allocation
2.1.2. Extended Allocation
2.1.3. Rescue Allocation
2.1.4. Assessing Transplant Candidate Condition
2.2. Reasons for Declining Organs for Transplantation
2.2.1. Major Extended Donor Criteria
2.2.2. Size Mismatch and Vascular Problems
2.2.3. Medical Reasons and Risk of Disease Transmission
2.2.4. Other Reasons
2.3. Fate of Declined Organs
2.4. Statistical Analysis
3. Results
3.1. Allocation Type, Donor, and Recipient Characteristics
3.2. Waiting List Dynamics
3.3. Reasons for Declining an Organ for Transplantation
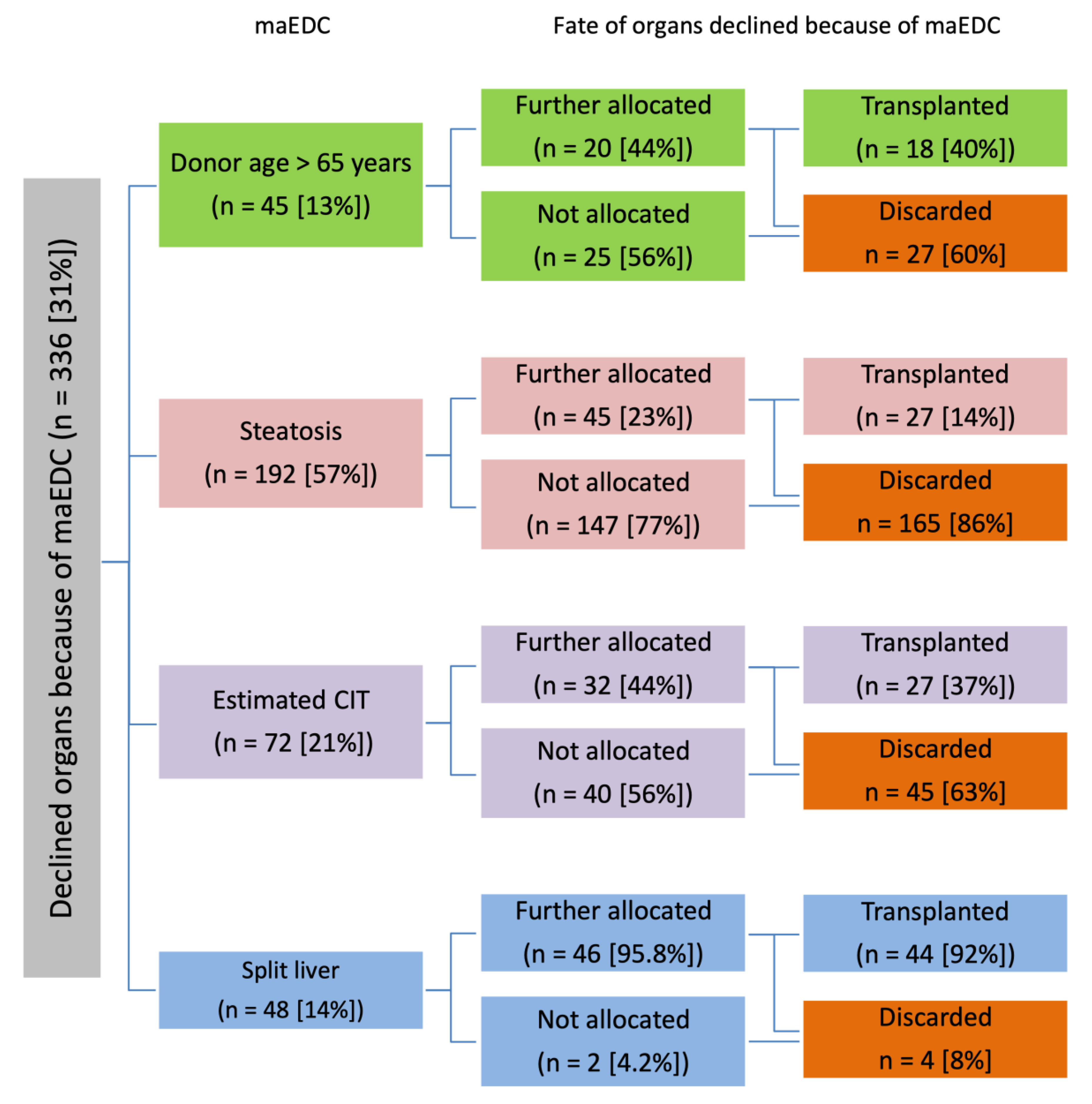
3.3.1. Major Extended Donor Criteria
3.3.2. Size Mismatch and Vascular Problems
3.3.3. Medical Reasons, Risk of Disease Transmission, and Other Reasons
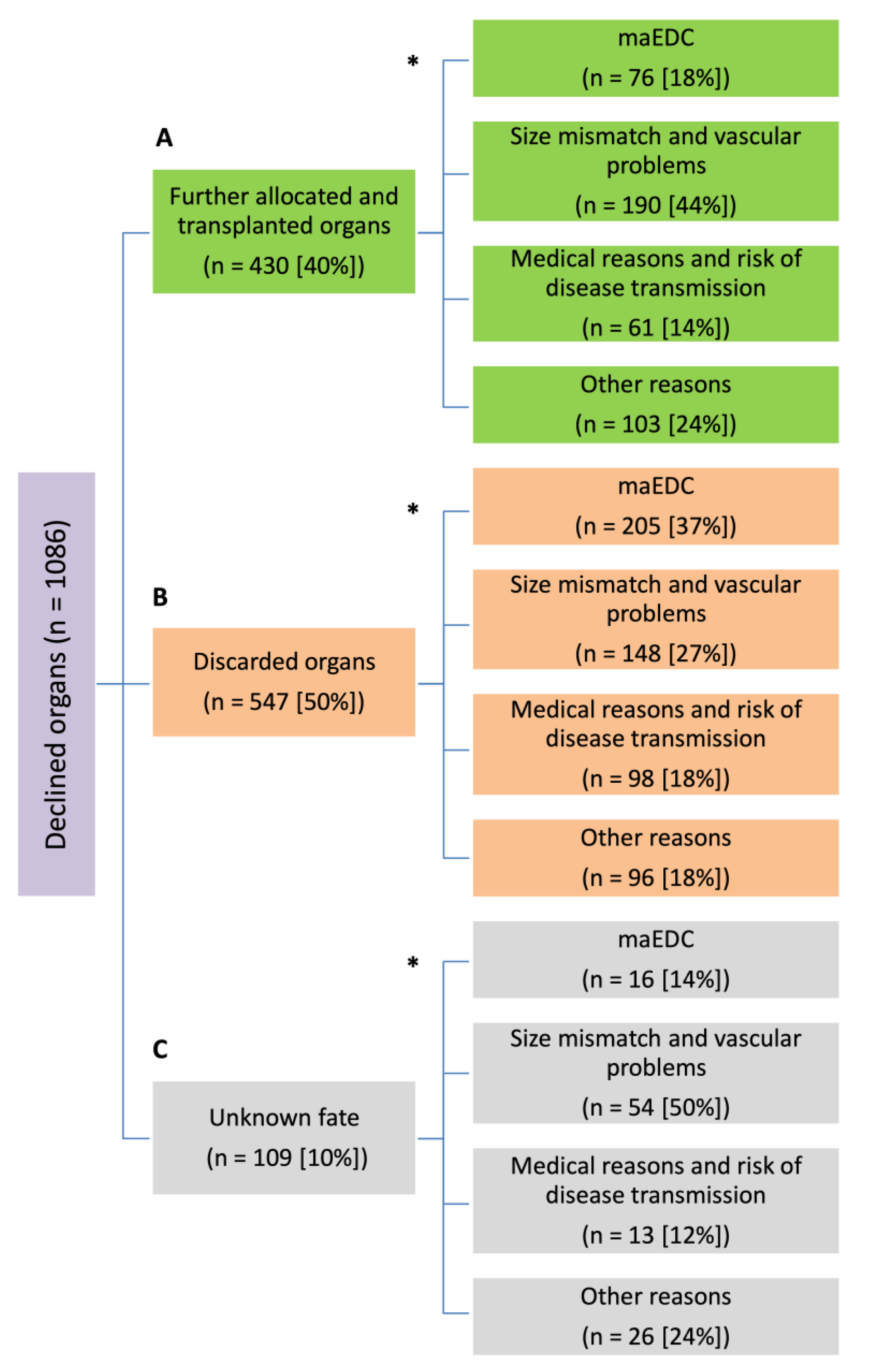
3.4. Fate of Declined Organs
3.4.1. Further Allocated and Transplanted Organs
3.4.2. Discarded Organs
3.4.3. Unknown Fate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Eurotransplant. Eurotransplant Annual Report; Eurotransplant: Leiden, The Netherlands, 2021. [Google Scholar]
- Umgelter, A.; Hapfelmeier, A.; Kopp, W.; van Rosmalen, M.; Rogiers, X.; Guba, M. For the Eurotransplant Liver Advisory Committee Disparities in Eurotransplant liver transplantation wait-list outcome between patients with and without model for end-stage liver disease exceptions. Liver Transplant. 2017, 23, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Organ Procurement and Transplantation Network (OPTN)—OPTN 2020 Annual Data Report. Available online: https://optn.transplant.hrsa.gov/data/view-data-reports/annual-report/ (accessed on 19 January 2021).
- Lai, J.C.; Feng, S.; Roberts, J.P. An Examination of Liver Offers to Candidates on the Liver Transplant Wait-List. Gastroenterology 2012, 143, 1261–1265. [Google Scholar] [CrossRef]
- Moosburner, S.; Raschzok, N.; Schleicher, C.; Boesebeck, D.; Gassner, J.M.; Ritschl, P.V.; Rahmel, A.; Sauer, I.M.; Pratschke, J. Declined Liver Grafts—Analysis of the German Donor Population from 2010 to 2018. Z. Gastroenterol. 2020, 58, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Emond, J.C. Measuring access to liver transplantation: An overdue metric for center quality and performance. J. Hepatol. 2016, 64, 766–767. [Google Scholar] [CrossRef] [PubMed]
- Garonzik-Wang, J.M.; James, N.T.; Arendonk, K.V.; Gupta, N.; Orandi, B.J.; Hall, E.C.; Massie, A.B.; Montgomery, R.A.; Dagher, N.N.; Singer, A.L.; et al. The aggressive phenotype revisited: Utilization of higher-risk liver allografts. Am. J. Transplant. 2013, 13, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Kahneman, D.; Tversky, A. Prospect Theory: An Analysis of Decision under Risk. Econometrica 1979, 47, 263. [Google Scholar] [CrossRef]
- White, S.L. Nudging the Organ Discard Problem. Transplantation 2017, 101, 1518–1519. [Google Scholar] [CrossRef]
- Maluf, D.G.; Edwards, E.B.; Kauffman, H.M. Utilization of extended donor criteria liver allograft: Is the elevated risk of failure independent of the model for end-stage liver disease score of the recipient? Transplantation 2006, 82, 1653–1657. [Google Scholar] [CrossRef]
- Volk, M.L.; Berg, C.L. Declined organ offers in liver transplantation: Careful timing or missed opportunity? Gastroenterology 2012, 143, 1141–1143. [Google Scholar] [CrossRef]
- Rahmel, A. Vermittlung postmortal gespendeter Lebern. Der Chir. 2013, 84, 372–379. [Google Scholar] [CrossRef]
- Bruns, H.; Lozanovski, V.J.; Schultze, D.; Hillebrand, N.; Hinz, U.; Büchler, M.W.; Schemmer, P. Prediction of Postoperative Mortality in Liver Transplantation in the Era of MELD-Based Liver Allocation: A Multivariate Analysis. PLoS ONE 2014, 9, e98782. [Google Scholar] [CrossRef] [PubMed]
- Jochmans, I.; Van Rosmalen, M.; Pirenne, J.; Samuel, U. Adult Liver Allocation in Eurotransplant. Transplantation 2017, 101, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Lozanovski, V.J.; Döhler, B.; Weiss, K.H.; Mehrabi, A.; Süsal, C. The Differential Influence of Cold Ischemia Time on Outcome After Liver Transplantation for Different Indications—Who Is at Risk? A Collaborative Transplant Study Report. Front. Immunol. 2020, 11, 892. [Google Scholar] [CrossRef]
- Lozanovski, V.J.; Kerr, L.T.; Khajeh, E.; Ghamarnejad, O.; Pfeiffenberger, J.; Hoffmann, K.; Chang, D.-H.; Mieth, M.; Longerich, T.; Strobel, O.; et al. Liver Grafts with Major Extended Donor Criteria May Expand the Organ Pool for Patients with Hepatocellular Carcinoma. J. Clin. Med. 2019, 8, 1692. [Google Scholar] [CrossRef] [PubMed]
- Lozanovski, V.J.; Khajeh, E.; Fonouni, H.; Pfeiffenberger, J.; von Haken, R.; Brenner, T.; Mieth, M.; Schirmacher, P.; Michalski, C.W.; Weiss, K.H.; et al. The impact of major extended donor criteria on graft failure and patient mortality after liver transplantation. Langenbeck’s Arch. Surg. 2018, 403, 719–731. [Google Scholar] [CrossRef]
- Lozanovski, V.J.; Probst, P.; Ramouz, A.; Arefidoust, A.; Ghamarnejad, O.; Aminizadeh, E.; Khajeh, E.; Mehrabi, A. Considering extended right lobe grafts as major extended donor criteria in liver transplantation is justified. Transpl. Int. 2021, 34, 622–639. [Google Scholar] [CrossRef]
- Lozanovski, V.J.; Unterrainer, C.; Döhler, B.; Süsal, C.; Mehrabi, A. Outcome of Extended Right Lobe Liver Transplantations. Liver Transplant. 2021, 28, 807–818. [Google Scholar] [CrossRef]
- Fukazawa, K.; Yamada, Y.; Nishida, S.; Hibi, T.; Arheart, K.L.; Pretto, E.A. Determination of the safe range of graft size mismatch using body surface area index in deceased liver transplantation. Transpl. Int. 2013, 26, 724–733. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & Healthcare. 7th Edition Guide to the Quality and Safety of Organs for Transplantation; European Directorate for the Quality of Medicines & Healthcare: Strasbourg, France, 2018. [Google Scholar]
- Volk, M.L.; Goodrich, N.; Lai, J.C.; Sonnenday, C.; Shedden, K. Decision support for organ offers in liver transplantation. Liver Transplant. 2015, 21, 784–791. [Google Scholar] [CrossRef]
- de Boer, J.D.; Blok, J.J.; Putter, H.; Koopman, J.J.; van Hoek, B.; Samuel, U.; van Rosmalen, M.; Metselaar, H.J.; Alwayn, I.P.; Guba, M.; et al. Optimizing the use of geriatric livers for transplantation in the Eurotransplant region. How to deal with an ageing donor population? Liver Transplant. 2018, 25, 260–274. [Google Scholar] [CrossRef]
- Houben, P.; Döhler, B.; Weiß, K.H.; Mieth, M.; Mehrabi, A.; Süsal, C. Differential Influence of Donor Age Depending on the Indication for Liver Transplantation—A Collaborative Transplant Study Report. Transplantation 2020, 104, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Haugen, C.E.; Bowring, M.G.; Holscher, C.M.; Jackson, K.R.; Garonzik-Wang, J.; Cameron, A.M.; Philosophe, B.; McAdams-DeMarco, M.; Segev, D.L. Survival benefit of accepting livers from deceased donors over 70 years old. Am. J. Transplant. 2019, 19, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Andrassy, J.; Wolf, S.; Lauseker, M.; Angele, M.; van Rosmalen, M.D.; Samuel, U.; Rogiers, X.; Werner, J.; Guba, M. Eurotransplant Liver Advisory Committee Higher retransplantation rate following extended right split-liver transplantation: An analysis from the Eurotransplant liver follow-up registry. Liver Transpl. 2018, 24, 26–34. [Google Scholar] [CrossRef]
- Herden, U.; Fischer, L.; Sterneck, M.; Grabhorn, E.; Nashan, B. Long-term follow-up after full-split liver transplantation and its applicability in the recent transplant era. Clin. Transplant. 2018, 32, e13205. [Google Scholar] [CrossRef]
- Sotiropoulos, G.C.; Paul, A.; Molmenti, E.; Lang, H.; Frilling, A.; Napieralski, B.P.; Nadalin, S.; Treckmann, J.; Brokalaki, E.I.; Gerling, T.; et al. Liver transplantation for hepatocellular carcinoma in cirrhosis within the Eurotransplant area: An additional option with “livers that nobody wants”. Transplantation 2005, 80, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Durand, F.; Renz, J.F.; Alkofer, B.; Burra, P.; Clavien, P.-A.; Porte, R.J.; Freeman, R.B.; Belghiti, J. Report of the Paris consensus meeting on expanded criteria donors in liver transplantation. Liver Transplant. 2008, 14, 1694–1707. [Google Scholar] [CrossRef]
- Flechtenmacher, C.; Schirmacher, P.; Schemmer, P. Donor liver histology—A valuable tool in graft selection. Langenbeck’s Arch. Surg. 2015, 400, 551–557. [Google Scholar] [CrossRef]
- Lozanovski, V.J.; Probst, P.; Arefidoust, A.; Ramouz, A.; Aminizadeh, E.; Nikdad, M.; Khajeh, E.; Ghamarnejad, O.; Shafiei, S.; Ali-Hasan-Al-Saegh, S.; et al. Prognostic role of the Donor Risk Index, the Eurotransplant Donor Risk Index and the Balance of Risk score on graft loss after liver transplantation. Transpl. Int. 2021, 34, 778–800. [Google Scholar] [CrossRef]
- Emond, J.C. Discarding Livers from Deceased Donors: Is It Ever OK? Liver Transpl. 2021, 27, 161–162. [Google Scholar] [CrossRef]
- Ceresa, C.D.L.; Nasralla, D.; Watson, C.J.E.; Butler, A.J.; Coussios, C.C.; Crick, K.; Hodson, L.; Imber, C.; Jassem, W.; Knight, S.R.; et al. Transient Cold Storage Prior to Normothermic Liver Perfusion May Facilitate Adoption of a Novel Technology. Liver Transplant. 2019, 25, 1503–1513. [Google Scholar] [CrossRef]
- Dutkowski, P.; Schlegel, A.; de Oliveira, M.; Müllhaupt, B.; Neff, F.; Clavien, P.-A. HOPE for human liver grafts obtained from donors after cardiac death. J. Hepatol. 2013, 60, 765–772. [Google Scholar] [CrossRef]
- Van Leeuwen, O.; De Vries, Y.; Matton, A.; Burlage, L.; Van Rijn, R.; Fujiyoshi, M.; Ubbink, R.; Pelgrim, G.; Werner, M.; De Boer, M.; et al. Biliary bicarbonate, pH and glucose are suitable biomarkers of biliary viability during Ex situ normothermic machine perfusion of human donor livers. HPB 2020, 22, S331–S332. [Google Scholar] [CrossRef]
- Mergental, H.; Laing, R.W.; Kirkham, A.J.; Perera, M.T.P.R.; Boteon, Y.L.; Attard, J.; Barton, D.; Curbishley, S.; Wilkhu, M.; Neil, D.A.H.; et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat. Commun. 2020, 11, 2939. [Google Scholar] [CrossRef]
- Patrono, D.; Surra, A.; Catalano, G.; Rizza, G.; Berchialla, P.; Martini, S.; Tandoi, F.; Lupo, F.; Mirabella, S.; Stratta, C.; et al. Hypothermic Oxygenated Machine Perfusion of Liver Grafts from Brain-Dead Donors. Sci. Rep. 2019, 9, 9337. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, O.B.; de Vries, Y.; Fujiyoshi, M.; Nijsten, M.W.; Ubbink, R.; Pelgrim, G.J.; Werner, M.J.; Reyntjens, K.M.; van den Berg, A.P.; de Boer, M.T.; et al. Transplantation of High-risk Donor Livers After Ex Situ Resuscitation and Assessment Using Combined Hypo- and Normothermic Machine Perfusion: A Prospective Clinical Trial. Ann. Surg. 2019, 270, 906–914. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, R.; Karimian, N.; Matton, A.P.; Burlage, L.C.; Westerkamp, A.C.; van den Berg, A.P.; de Kleine, R.H.; de Boer, M.T.; Lisman, T.; Porte, R.J. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br. J. Surg. 2017, 104, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Lozanovski, V.J.; Ramouz, A.; Aminizadeh, E.; Al-Saegh, S.A.; Khajeh, E.; Probst, H.; Picardi, S.; Rupp, C.; Chang, D.-H.; Probst, P.; et al. Prognostic role of selection criteria for liver transplantation in patients with hepatocellular carcinoma: A network meta-analysis. BJS Open 2022, 6, zrab130. [Google Scholar] [CrossRef] [PubMed]
- Briceño, J. Artificial intelligence and organ transplantation: Challenges and expectations. Curr. Opin. Organ Transplant. 2020, 25, 393–398. [Google Scholar] [CrossRef]
| Donor Characteristics | ||||
|---|---|---|---|---|
| Mean | Median | SD | Range (min–max) | |
| Age (years) | 57 | 59 | 20 | 0–96 |
| BMI (kg/m2) | 27.21 | 26.12 | 6.33 | 11–68 |
| Weight (kg) | 81 | 80 | 22.71 | 3–190 |
| Height (cm) | 171 | 174 | 17 | 52–200 |
| Gender Female (n, %) Male (n, %) | 475 (44) 611 (56) | |||
| Recipient characteristics | ||||
| Mean | Median | SD | Range (min–max) | |
| Age (years) | 49 | 52 | 12 | 15–74 |
| BMI (kg/m2) | 25.94 | 25.03 | 5.82 | 17–53 |
| Weight (kg) | 77.5 | 75 | 22.34 | 42–185 |
| Height (cm) | 172 | 170 | 10 | 147–205 |
| labMELD | 20.2 | 18 | 9.79 | 6–40 |
| eMELD | 27.41 | 27.53 | 6.15 | 2–40 |
| MatchMELD | 23.86 | 23 | 9.11 | 7–40 |
| Gender Female (n, %) Male (n, %) | 330 (49) 346 (51) | |||
| Donor Age > 65 Years | |||
|---|---|---|---|
| Yes (n = 45) | No (n = 1041) | p | |
| Female donor (n, %) | 31 (68.9) | 443 (42.6) | <0.001 |
| Donor age (mean ± SD) | 81 ± 7.2 | 56 ± 19.2 | <0.001 |
| Donor BMI (mean ± SD) | 26.2 ± 6 | 27.3 ± 6.3 | 0.25 |
| Liver steatosis (n, %) | 2 (4.4) | 190 (18.3) | 0.015 |
| Split organs (n, %) | 0 (0) | 48 (4.6) | 0.258 |
| Estimated CIT > 14 h (n, %) | 4 (8.9) | 68 (6.5) | 0.534 |
| Female recipient (n, %) | 21 (46.7) | 273 (26.2) | 0.005 |
| Recipient age (mean ± SD) | 46 ± 14 | 49 ± 12.5 | 0.174 |
| Recipient BMI (mean ± SD) | 26.4 ± 5.6 | 25.7 ± 5.6 | 0.53 |
| labMELD (mean ± SD) | 19.8 ± 8.7 | 20.8 ± 10 | 0.582 |
| eMELD (mean ± SD) | 28.2 ± 6 | 27.5 ± 5.9 | 0.774 |
| Liver steatosis | |||
| Yes (n = 192) | No (n = 894) | p | |
| Female donor (n, %) | 85 (44.3) | 389 (43.5) | 0.873 |
| Donor age (mean ± SD) | 62 ± 15.8 | 55 ± 20.1 | <0.001 |
| Donor age > 65 years (n, %) | 81 (42.2) | 294 (32.9) | 0.015 |
| Donor BMI (mean ± SD) | 29.5 ± 6.7 | 26.7 ± 6.1 | <0.001 |
| Split organs (n, %) | 0 (0) | 48 (5.4) | <0.001 |
| Estimated CIT > 14 h (n, %) | 6 (3.1) | 66 (7.4) | 0.036 |
| Female recipient (n, %) | 25 (29.1) | 269 (52.7) | <0.001 |
| Recipient age (mean ± SD) | 52 ± 9 | 49 ± 13 | 0.012 |
| Recipient BMI (mean ± SD) | 27.7 ± 5.1 | 25.4 ± 5.6 | <0.001 |
| labMELD (mean ± SD) | 18.3 ± 8.3 | 21.2 ± 10.1 | 0.007 |
| eMELD (mean ± SD) | 25.0 ± 3.2 | 27.8 ± 6.1 | 0.106 |
| Long estimated CIT | |||
| Yes (n = 72) | No (n = 1014) | p | |
| Female donor (n, %) | 27 (37.5) | 447 (44.1) | 0.325 |
| Donor age (mean ± SD) | 55 ± 17.3 | 57 ± 19.7 | 0.361 |
| Donor age > 65 years (n, %) | 22 (30.6) | 353 (34.8) | 0.522 |
| Donor BMI (mean ± SD) | 26.4 ± 4.4 | 27.3 ± 6.4 | 0.141 |
| Liver steatosis (n, %) | 6 (8.3) | 186 (18.3) | 0.036 |
| Split organs (n, %) | 8 (11.1) | 40 (3.9) | 0.011 |
| Female recipient (%) | 12 (40) | 282 (49.8) | 0.35 |
| Recipient age (mean ± SD) | 47 ± 13.7 | 49 ± 12.5 | 0.309 |
| Recipient BMI (mean ± SD) | 25.6 ± 4.4 | 25.8 ± 5.6 | 0.902 |
| labMELD (mean ± SD) | 21.7 ± 12 | 20.7 ± 9.8 | 0.676 |
| eMELD (mean ± SD) | 32.4 ± 7.3 | 27.3 ± 5.8 | 0.090 |
| Split livers | |||
| Female donor (n, %) | Yes (n = 48) | No (n = 1038) | p |
| Female donor (n, %) | 20 (41.7) | 454 (43.7) | 0.882 |
| Donor age (mean ± SD) | 36 ± 15.4 | 58 ± 19.2 | <0.001 |
| Donor age > 65 years (n, %) | 0 (0) | 375 (36.1) | <0.001 |
| Donor BMI (mean ± SD) | 23.1 ± 3.2 | 27.4 ± 6.4 | <0.001 |
| Liver steatosis (n, %) | 0 (0) | 192 (18.5) | <0.001 |
| Estimated CIT > 14 h (n, %) | 8 (16.7) | 64 (6.2) | 0.011 |
| Female recipient (n, %) | 25 (65.8) | 269 (48.2) | 0.044 |
| Recipient age (mean ± SD) | 44 ± 14 | 49 ± 12.4 | 0.010 |
| Recipient BMI (mean ± SD) | 24.8 ± 7.5 | 25.8 ± 5.4 | 0.289 |
| labMELD (mean ± SD) | 21 ± 11.7 | 20.7 ± 9.8 | 0.864 |
| eMELD (mean ± SD) | 31.3 ± 5.5 | 26.9 ± 5.8 | 0.006 |
| Size Mismatch n = 281 | Reasons to Decline Other than Size Mismatch n = 388 | p | |
|---|---|---|---|
| BSAi | |||
| BSAi < 0.78 (n, %) | 27 (9.6) | 19 (4.9) | <0.001 |
| BSAi = 0.78–1.24 (n, %) | 180 (64.1) | 359 (92.5) | |
| BSAi > 1.24 (n, %) | 74 (26.3) | 10 (2.6) | |
| BMI | |||
| BMI (mean ± SD) | 6.32 ± 5.96 | 4.1 ± 3.73 | <0.001 |
| BMI (median (range)) | 5.12 (0.11–35.88) | 3.27 (0.02–31.42) | |
| Reasons for Declining an Organ | Further Allocated and Transplanted Organs n = 430 | Discarded Organs n = 547 | p |
|---|---|---|---|
| maEDC (n, %) | 76 (17.7%) | 205 (37.5%) | <0.001 |
| Size mismatch and vascular problems (n, %) | 190 (44.2%) | 148 (27.1%) | <0.001 |
| Liver function and medical issues (n, %) | 61 (14.2%) | 98 (17.9%) | 0.138 |
| Other reasons (n, %) | 103 (24%) | 96 (17.6%) | 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozanovski, V.J.; Adigozalov, S.; Khajeh, E.; Ghamarnejad, O.; Aminizadeh, E.; Schleicher, C.; Hackert, T.; Müller-Stich, B.P.; Merle, U.; Picardi, S.; et al. Declined Organs for Liver Transplantation: A Right Decision or a Missed Opportunity for Patients with Hepatocellular Carcinoma? Cancers 2023, 15, 1365. https://doi.org/10.3390/cancers15051365
Lozanovski VJ, Adigozalov S, Khajeh E, Ghamarnejad O, Aminizadeh E, Schleicher C, Hackert T, Müller-Stich BP, Merle U, Picardi S, et al. Declined Organs for Liver Transplantation: A Right Decision or a Missed Opportunity for Patients with Hepatocellular Carcinoma? Cancers. 2023; 15(5):1365. https://doi.org/10.3390/cancers15051365
Chicago/Turabian StyleLozanovski, Vladimir J., Said Adigozalov, Elias Khajeh, Omid Ghamarnejad, Ehsan Aminizadeh, Christina Schleicher, Thilo Hackert, Beat Peter Müller-Stich, Uta Merle, Susanne Picardi, and et al. 2023. "Declined Organs for Liver Transplantation: A Right Decision or a Missed Opportunity for Patients with Hepatocellular Carcinoma?" Cancers 15, no. 5: 1365. https://doi.org/10.3390/cancers15051365
APA StyleLozanovski, V. J., Adigozalov, S., Khajeh, E., Ghamarnejad, O., Aminizadeh, E., Schleicher, C., Hackert, T., Müller-Stich, B. P., Merle, U., Picardi, S., Lund, F., Chang, D.-H., Mieth, M., Fonouni, H., Golriz, M., & Mehrabi, A. (2023). Declined Organs for Liver Transplantation: A Right Decision or a Missed Opportunity for Patients with Hepatocellular Carcinoma? Cancers, 15(5), 1365. https://doi.org/10.3390/cancers15051365






