Simple Summary
Heat-shock proteins (HSPs) are molecular chaperones overexpressed in tumor cells and are necessary for their survival. In leukemia and lymphoma, HSPs have been reported to have unique cytoprotective effects on different cell death and growth pathways. In this review, we describe the implication of HSPs in those pathways in hematological malignancies and discuss the pertinence of detecting and targeting them for future innovative treatment strategies.
Abstract
Heat-shock proteins (HSPs) are powerful chaperones that provide support for cellular functions under stress conditions but also for the homeostasis of basic cellular machinery. All cancer cells strongly rely on HSPs, as they must continuously adapt to internal but also microenvironmental stresses to survive. In solid tumors, HSPs have been described as helping to correct the folding of misfolded proteins, sustain oncogenic pathways, and prevent apoptosis. Leukemias and lymphomas also overexpress HSPs, which are frequently associated with resistance to therapy. HSPs have therefore been proposed as new therapeutic targets. Given the specific biology of hematological malignancies, it is essential to revise their role in this field, providing a more adaptable and comprehensive picture that would help design future clinical trials. To that end, this review will describe the different pathways and functions regulated by HSP27, HSP70, HSP90, and, not least, HSP110 in leukemias and lymphomas.
1. Introduction
Leukemias and lymphomas represent 6.5% of cancers worldwide [1]. They emerge from a wide array of cells at different stages of hematopoietic maturation, from hematopoietic stem cells and myeloid and lymphoid progenitors to mature B and T cells. Treatment breakthroughs have been made in recent years thanks to the comprehensive identification of survival pathways and oncogenes, leading to targeted therapies such as kinase inhibitors or anti-apoptotic protein inhibitors [2,3]. At the same time, recent progress in high-throughput molecular analysis combined with bioinformatic power has revealed a more diverse and complex picture of cancer sub-populations than expected, challenging the too simplistic “one treatment fits all” [4]. Furthermore, natural mechanisms of cell death resistance and escape from therapy occur in many patients either from the start of the treatment or after several cycles of chemotherapy, pushing for more specific and innovative drug use in the clinic. Therefore, cancer initiation, development, and cell death resistance are still intense fields of research to identify new therapeutic targets that could foster the development of new treatments.
HSPs have been involved in many cellular processes as they are important housekeeping proteins. Their chaperone function is observed in every cell compartment and is intrinsically associated with protein translation, migration, localization, stability, and degradation. Traditionally, they have been classified by their molecular weight, with the most studied members in cancer being HSP90, HSP70, HSP27, and, more recently, HSP110. Cell stress is a powerful initiator and booster of HSP expression as it leads to multiple protein structural alterations, such as aggregation, unfitted conformations, and accelerated protein synthesis. Because tumor cells must rewire their metabolism in permanence, they are like highly stressed cells and therefore rely on the strong expression of HSPs for their survival. Consequently, they become more resistant to cell death mechanisms, either of natural origin or therapeutically induced. HSP expression profiles in newly diagnosed patients compared to healthy controls in various hematological malignancies reveal a frequent but heterogeneous expression. In acute myeloid leukemia (AML), HSP70 was strongly expressed in 58% of patients, compared to 26% for HSP60 [5]. Despite this variability of expression, the global HSP family proteome provides widespread protection to tumor cells, as the overall survival of AML patients was inversely correlated to HSP expression. Moreover, when other prognosis factors were considered, HSP expression always an additive pejorative value for the patients’ outcomes [5].
Similarly, in chronic lymphocytic leukemia (CLL) and chronic myeloid leukemia (CML), HSP70 is significantly more expressed in patients than in healthy controls. High levels of HSP70 correlate with resistance to tyrosine kinase inhibitors [6,7]. In lymphoma, a high expression of the mitochondrial HSP70 (mortalin) correlates with treatment resistance and poor survival of patients [8]. As HSP expression is highly modulated by environmental aggressions, HSPs further accumulate in cancer cells after anti-cancer treatments through the induction/activation of the HSPs’ transcription factor HSF1. In CLL, increased HSP70 and HSF1 were observed in response to Ibrutinib treatment and signaled a failure of clinical improvement [9]. Conversely, only responders show a decrease in HSP70 expression [7]. HSP27 has been associated with clinical responses as it is highly expressed in pediatric acute leukemia and provides resistance to chemotherapy [10]. HSPs are also secreted, free or with nanovesicules. Some of them, such as HSP70, have a membrane location and can be found both in the cancer microenvironment and in the blood [11]. High HSP70 levels have been detected at the cell surface and in the serum in AML and correlate with limited survival [12,13]. Extracellular HSPs, also described in many solid tumors, can have an immunosuppressive function mostly through macrophage polarization and myeloid suppressive cell activation [14]. The importance of the immune microenvironment in leukemia and lymphoma [15] and the capacity of HSPs to diffuse largely through the body as free proteins or embedded in extracellular vesicles provide solid grounds for studies of their extracellular functions. The most abundant HSP, HSP90, also shows elevated levels in various types of leukemias and lymphomas and could serve as a prognostic marker. In addition, its elevated expression is necessary for the survival and propagation of those cancer cells [16,17,18,19,20,21]. Finally, the expression of the high-molecular-weight HSP110, a long-forgotten chaperone, has recently been revealed to correlate with the aggressiveness of non-Hodgkin lymphoma (NHL) [22,23,24].
HSPs are therefore strongly associated with hematological malignancy aggressiveness and responses to therapies. In this review, we will present the most recent advances in understanding the different HSP functions in this specific family of tumors, ranging from their BCR signaling pathway to their metabolic regulation.
2. HSP Families’ Overview
2.1. Heat-Shock Factor
In humans, there are six members forming the HSF family: HSF1, HSF2, HSF4, HSF5, HSFX, and HSFY. HSF proteins have specific and overlapping roles. HSF1 is the most studied member of the family and is known to be involved in the expression of HSPs during a response to different types of stresses. However, it is now well established that HSFs are involved in the regulation of a broader array of genes that are not related to HSPs in normal and pathological hematopoiesis [25]. HSF1 is also involved in immune responses and aging. Together with HSF2, both transcription factors are involved in the development of gametes (sperm and oocytes) and the nervous system. HSF4 has been described to cooperate with HSF1 and is involved in the development and homeostasis of the lens, the survival of the lens cells, and the genesis of neurons [26]. It is also expressed in the brain, muscles, heart, and pancreas. HSF5 is one of the newest members of the family, and although it is expressed in humans, its roles are better understood in animals: in the zebrafish, HSF5 loss of function leads to male infertility, and in mice, HSF5 is known to be involved in spermatogenesis. HSFX is localized on the X chromosome, but its roles are unknown. Finally, HSFY, located on the Y chromosome, has been described as being involved in spermatogenesis, with its deletion causing infertility [27].
HSPs are transcriptionally regulated by two members of the heat shock factor family, HSF1 and HSF2. Various stresses can activate HSF1 and thereby induce HSP expression: an increase in the cytosolic levels of misfolded proteins, a rise in temperature, or an alteration in the cellular pH or redox state. Under normal conditions, HSF1 is restrained in the cytosol by HSP90 or HSP70, and with one of the cell stresses quoted above, HSF1 will dissociate from those HSPs [28]. This will allow HSF1 to phosphorylate and trimerize, and so it will enter the nucleus, binding the HSE sequences and permitting the expression of HSP genes [29]. In general, HSF1 recognizes and binds the heat-shock element (HSE) sequence (nTTCnnGAAnnTTCn) in various regions of HSP genes upon activating signals and promotes their transcription. HSF1 is involved in the proteostasis of the earliest stage of blood cell development. Notably, HSF1 ensures protein synthesis and proteostasis in hematopoietic stem cells [30]. Since HSF1 is a central regulator of stress responses, various studies have demonstrated its implication in cancer development, showing it to be an important target in cancer therapy. In AML, the inhibition of HSF1 with the chemical compound DTHIB decreases the growth and engraftment of AML cells [31]. It has also been shown that the inhibition of the translational factor eIF4a inactivates HSF1 and thereby has anti-leukemic effects both in vitro and in vivo [32]. In diffuse large B-cell lymphomas (DLBCL), HSF1 mRNA is upregulated compared with regular B-cells, which can be one of the reasons for the strong expression of a wide variety of HSPs in this type of cancer [33]. Cooperating with STAT3, HSF1 is able to maintain the cancerous phenotype of liver cancer cells [34], and the inhibition of HSF1 in pancreatic cancer decreases their stemness and sensitizes them to gemcitabine [35]. HSF1 knock-down in prostate cancer cell lines sensitizes them to chemotherapy [36]. Recently, it has been shown that HSF2, in an interplay role with HSF1, can also induce the expression of HSPs [37]. HSF2 does not have a significant role in the heat-shock response, but it is able to regulate a common set of genes with HSF1, and among them are the HSP genes. Using HSF1 and/or HSF2 knockout cell lines, dysregulated responses to nutrient stress could be observed, as well as a reduction in tumor progression, making HSF2 a critical co-factor of HSF1 [38]. HSF2 is involved in various types of carcinomas. Notably, HSF2 is known to be constitutively active in embryonic carcinoma cells, leading to the overexpression of HSPs. It is also implicated in the development of breast, lung, thyroid, oesophageal, and prostate cancers [39].
2.2. HSP110 Family
The HSP family with the highest molecular weight is composed of four members: HSP110 (HSP105, NY-CO-25, or HSPH1), APG2 (HSPA4 or HSPH2), APG1 (HSPA4L, Osp94, or HSPH3), and GRP170 (ORP150, HYOU1, or HSPH4). The roles of APG1 and APG2 are not well known. APG1 is involved in testis homeostasis, and APG2 has roles in spermatogenesis and is also expressed in the brain. GRP170 is an endoplasmic reticulum (ER) chaperon induced by glucose deprivation [40]. HSP110 is coded by the gene HSPH1 and was first described as a cochaperone of HSP70 and HSC70. More specifically, it was characterized as a nucleotide exchange factor (NEF) for those HSPs, carrying ATP and so enhancing their chaperoning function [41,42]. HSP110 is known to recognize and bind denatured or misfolded proteins for other HSPs, such as HSP70, to convert them into fully functional proteins [42]. Other HSPs, such as HSP70 or HSC70, are able to perform this function, but HSP110 is four times more efficient [40]. Therefore, it was thought that HSP110 was dependent on HSP70/HSC70 for its refolding role, but it has been discovered that HSP110, with the help of HSP40, can unfold and refold misfolded proteins on its own [42]. Together with HSP70 and HSP40, the HSP110-HSP70-HSP40 complex is an important chaperone network that disaggregates and refolds aggregates of denatured proteins [40].
2.3. HSP90 Family
HSP90 is probably the best-known family of HSPs involved in hematopoietic malignancies. Its members are well represented in cells, since they constitute between 1% and 2% of whole cellular proteins in mammals [43]. HSP90 family members are spread into different cell compartments: HSP90α A1 (also called HSPC1, HSP90A, HSP90AA1, or HSP86), HSP90α A2 (HSPC2 or HSP90AA2), and HSP90β (HSPC3, HSP90B, HSP90AB1, or HSP84) are cytosolic, while Grp94 (called HSPC4, GRP94, gp96, or HSP90B1) is restrained to the endoplasmic reticulum but can also be in the cell membrane. Finally, the last member, TRAP1 (or HSPC5, HSP90L, or HSP75), is mainly located in the mitochondria. More than 200 cellular proteins have been described as HSP90 client proteins. From proximal molecules of the BCR to transcription factors within the nucleus, HSP90 is a systematic organizer of protein transcription, translation, and activation. Abundant literature illustrates this “hub” function of HSP90 within several signaling pathways. From the cell surface, where tonic or chronic BCR stimulation occurs, HSP90 is present and interacts with BCR components to sustain signals.
2.4. HSP70 Family
The HSP70 family is, along with the HSP90 family, the most studied group of chaperones. There are thirteen members of the HSP70 family, all coded by the HSPA genes. Five of them are particularly studied: HSP70 (also called HSPA1 or HSP72) and HSPA6 (also called HSPB’), which are stress-induced, but also three other constitutively expressed HSP70 members, which are HSC70 (also called HSPA8 or HSP73), mortalin (also called HSPA9), and GRP78 (also called HSPA5 or BiP) [44]. HSP70s are involved in multiple cellular functions, such as the folding of unfolded or misfolded proteins, the maintenance of protein homeostasis, the transport of proteins to different subcellular fractions, and cell survival after stress [44,45]. With the help of its cochaperones, HSP40 and HSP110, HSP70 is able to desegregate and fold denatured proteins and aggregates [40]. The HSP70 family of chaperones is known for its diverse roles in cancer [44]. When it comes to lymphoid malignancies, four of these HSPs are described in the literature: HSP70 (or HSP72), HSC70, Grp78, and mortalin.
2.5. Small HSPs Family
The small HSP family has ten members. The best-known is HSP27 (HSPB1, HSP25, or HSP28), which is cytoplasmic but can translocate to the nucleus under stress, and it has been reported to be involved in cancer development. MKBP (HSPB2) is mainly expressed in the heart and muscles, helping with their structure and functions. HSPL27 (HSPB3, DHMN2C, or HMN2C) is also an HSP involved in muscle functions and is specific to this type of tissue. αA-crystallin (HSPB4, CRYAA, CRYA1, or CTRCT9) is principally expressed in the eye and most specifically in the lens. Unlike most chaperones, it does not refold proteins but holds them, maintaining the solubility of aggregates to maintain cell survival. The αB-crystallin (HSPB5, CRYAB, CRYA2, or MFM2) is a ubiquitous chaperone involved in the development of neurological diseases, certain myopathies, and cancers. HSP20 (HSPB6 or HEL55), like HSPB2 and HSPB3, is involved in muscle and heart functions. HSPB7 (or cvHSP) is known to be involved in heart failures and is suspected of acting as a tumor suppressor, associated with renal carcinomas. HSP22 (HSPB8, H11, HMN2, or E2IG1) has an estrogen-dependent expression in estrogen receptor-positive breast cancer cells and in some neuromuscular diseases. HSPB9 (or CT51) is the least well-known member of the family, with a cytoplasmic and nuclear location in cells. Finally, ODF1 (or HSPB10) is a chaperone involved in the cytoskeleton structures of sperm tails. In general, small HSPs have been shown to have roles in cell proliferation, survival, and the progression of cancer cells. Notably, HSPB1 is involved in breast cancer cell lines proliferation [46], and HSPB5 is associated with breast carcinoma progression [47], making HSPB5 a biomarker for the diagnosis of breast cancers [48]. High levels of HSPB5 are associated with low survival rates in hepatocellular, lung, and prostatic carcinomas [49,50,51].
3. HSPs as Chaperons of Cell Signaling Proteins
3.1. Chaperoning the B-Cell Receptor and the T-Cell-Receptor
The B-cell receptor (BCR) and T-cell receptor (TCR) are essential detectors of antigens on B and T cells, whose binding initiates multiple downstream signaling pathways leading to activation, survival, and differentiation. Their activations are also crucial in the development of leukemias and lymphomas, whose aberrant proliferation is often due to BCR pathway mutations [52]. Several HSPs have been involved in the proximal signaling of the BCR and TCR (Figure 1).
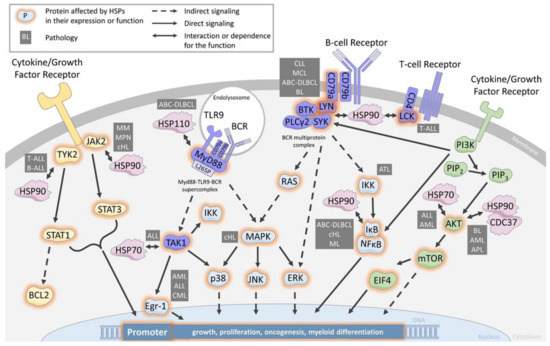
Figure 1.
HSP are chaperones of cell signaling components in the BCR/TCR, JAK/STAT and PI3K/AKT pathways. Schematic of a leukemia/lymphoma cell showing the main actors involved in the major signaling pathways BCR/TCR, JAK/STAT and PI3K/AKT, as well as the involvement of the major heat shock protein families. CLL: chronic lymphocytic leukemia, MCL: mantle cell lymphoma, ABC-DLBCL: active B-cell diffuse large lymphoma, ALL: acute lymphoblastic leukemia, AML: acute myeloid leukemia, BL: Burkitt lymphoma, APL: acute promyelocytic leukemia, cHL: classical Hodgkin lymphoma, CML: chronic myeloid leukemia, MM: multiple myeloma MPN: myeloproliferative neoplasms.
SRC refers to a family of proto-oncogenes encoding the lymphocyte-specific SRC family kinases (SFK). In this family, LCK (for lymphocyte-specific protein tyrosine kinase) is highly expressed by T-cell lymphoblastic leukemia (T-ALL) and is essential for TCR signaling [53,54]. Glucocorticoid resistance is reversed by LCK inhibition in pediatric T-ALL [55]. The inhibition of LCK by preventing its phosphorylation is an important strategy for the treatment of malignant hematopoiesis such as T-ALL, particularly with the use of bosutinib, dasatinib, or daracatinib, which affect the proliferation of leukemia cells [55,56,57]. Although it was shown several years ago that LCK interacts with HSP90 [58], only recently has the use of HSP90-specific inhibitors demonstrated the dependence of LCK expression on HSP90 in T-ALL [59]. The use of patient-derived models (PDX) has further shown the negative impact of HSP90 neutralization on primary leukemic cell survival [59].
LCK homologue protein LYN (Lck/Yes-related novel protein tyrosine kinase) is more specifically expressed by B-cell lymphoblastic leukemia (B-ALL) and B-cell lymphoma and is important for BCR signaling. The inhibition of LYN, notably with Dasatinib, is an important strategy for the treatment of B-ALL and B-cell lymphoma. LCK protects cells from glucocorticoid-induced apoptosis, and its inhibition enhances sensitivity to dexamethasone in lymphoma cells [60]. Lymphomas resistant to proteasome inhibitors show increased expression in BCR and activation of the BCR signaling pathway enhances the activity of SFK, especially LYN, and downstream kinases PI3K/AKT/mTOR in proteasome inhibitor-resistant lymphoma cells. Therefore, targeting BCR signaling with dasatinib could be a novel therapeutic approach for patients with mantle cell lymphoma that are refractory to proteasome inhibition with bortezomib. LYN is predominantly expressed in B-lymphocytes and plays a central role in initiating B-cell signaling. Evidence is mounting that strongly implicates an important role for LYN in several types of leukemia and lymphoma, particularly in B-ALL, where studies have confirmed the overexpression of LYN and its critical role in maintaining proliferation and antiapoptotic pathways in leukemic cells.
In CLL and MCL, high HSP90 expression correlates with overexpression of BCR signalosome proteins, including CD79a, PLCg2, LYN, BTK, and SYK. These proteins are associated with HSP90 in a multiprotein complex [21,61,62]. Pharmacological inhibition of HSP90 dislocates the complex and induces cell death, suggesting a critical role for maintenance of the tonic BCR signaling. Similarly, in ABC-DLBCL, HSP90 is a member of the BCR signalosome, and the HSP90 inhibitor PUH71 decreases SYK and BTK phosphorylation [63].
Considering ibrutinib resistance and given this BCR-complex, HSP90 targeting could be envisioned. Indeed, the HSP90 inhibitors SNX-5422 and AUY922 were recently shown to induce cell death in B cell lines expressing BTK C815S [62]. Combining drugs with ibrutinib to increase survival rates is being explored [64], and its association with PUH71 induced the synergistic killing of lymphoma cells [63]. In ABC-DLBCL, only 37% of patients respond to ibrutinib [65]. Phelan et al. have identified that most of these patients harbor dual CD79a and MyD88L265P mutations leading to the formation of a cytoplasmic MyD88-TLR9-BCR supercomplex driving IκB phosphorylation and thereby to cell survival and proliferation [66]. We have shown that HSP110 expression correlates with MyD88 in patients’ lymph node biopsies and that HSP110, by chaperoning MyD88 and MyD88L265P, enhances ABC-DLBCL cell lines survival [24]. Therefore, specific inhibitors of HSP110, like the small chemical compound recently reported by us [67], would destabilize MyD88L265P and subsequently the MyD88-TLR9-BCR super complex. This strategy might be a therapeutic alternative for ibrutinib-resistant patients.
In addition to chronic BCR-activated leukemia and lymphoma, HSP90 also cooperates with BCR signaling in Burkitt lymphoma (BL) as it interacts with SYK in a BCR Y197-dependent manner [68]. SYK/HSP90 interaction seems to play a particularly important role in BL cell survival, as LYN and BTK knock-down by shRNA do not alter the cells’ survival, unlike SYK knock-down.
BCR downstream pathways are subsequently impacted by HSP90 inhibition, as demonstrated in MCL, classical Hodgkin lymphoma (cHL), and ABC-DLBCL, where IkB degradation and reduction of NFkB signaling are observed [62,63,69]. In addition to indirect BCR effects, other client proteins of multiple signaling pathways are also impacted. In cHL, ERK/MAPK and RAS were reported to be down-regulated, and in ATL, AKT and the NfkB kinase complex IKKa, b, and d were degraded [70,71].
The involvement of HSP70 is less well documented than that of HSP90. However, in ALL it seems to play an important role. In this disease, the transforming growth factor-β-Activated Kinase 1 (TAK1), a member of the mitogen-activated protein kinase (MAP3K) family that has serine/threonine protein kinase activity, activates several pathways, including MAPK p38, JNK, and IKK [72]. HSP70 silencing leads to a decrease in the quantity of TAK1, which consequently derepresses Egr-1 expression, a tumor suppressor gene that leads to cell death [73]. Egr-1 low expression plays a major role in normal HSC long-term maintenance and localization; in contrast, its dysregulation is linked to hematopoietic aging [74]. This suggests that HSP70 would favor normal hematopoiesis through HSC regulation and the TAK1/Egr-1 pathway. Conversely, the Egr-1 down-expression observed in AML, ALL, and CML could be reversed by targeting HSP70 [75].
3.2. Chaperoning the JAK/STAT Pathway
Aberrant activation of the Janus-activated kinase (JAK) tyrosine kinase family plays an oncogenic role in leukemias, lymphomas, and other hematopoietic disorders such as myeloproliferative neoplasms (MPN). TYK2 was the first identified JAK family member aberrantly activated in T-ALL and B-ALL. It results in STAT1 activation and up-regulation of the anti-apoptotic protein BCL2 [76,77]. TYK2 has been identified as a HSP90 client protein [58], and in T-ALL, pharmacological inhibition of HSP90 led to TYK2 degradation, reduced STAT1 phosphorylation, and subsequent BCL2 down-expression [78].
In MPN, the JAK2 mutation has been observed in most patients, ranging from 50% with essential thrombocythemia and myelofibrosis to 95% in polycythemia vera [79]. Therefore, JAK inhibitors like ruxolitinib are being used in the clinic. In patients, JAK2 inhibition has been shown to decrease splenomegaly but cannot eradicate the MPN clone [80], leading to the search for alternative targeted therapies. Of note, JAK2 is a client protein of HSP90, and its inhibition leads to JAK2 degradation, followed by abrogation of the JAK/STAT signaling in vitro and in vivo [81,82]. Murine models of MPN treated with a HSP90 inhibitor led to global improvements in the mice with significant survival benefits. This prompted a clinical pilot study with HSP90 inhibitors [83], which showed an in vivo reduction in the spleen’s size in all patients. Although these results need to be confirmed in a more ambitious clinical trial, they suggest that HSP90 targeting would be a valuable therapy in MPN. An additional interest in HSP90 inhibitors would be in patients resistant to JAK inhibitors due to JAK2 mutations (G935R, Y931C, and E864K), because those mutant JAK2 are still client proteins of HSP90 [84].
Concerning small HSPs, HSP27 also plays a role in the myelofibrosis form of MPN as it is strongly expressed in bone marrow biopsies of patients in contrast to HSP70 and HSP90 [85]. HSP27, indeed, binds directly to JAK2 and STAT5 to protect STAT5 from its dephosphorylation. It might also favor the molecular assembly JAK2/STAT5. Furthermore, treatment of murine models of myelofibrosis with OGX-427, an antisense oligonucleotide against HSP27, limited the progression of bone marrow fibrosis, normalized the platelet and white blood cell counts.
In cHL, JAK2 and TYK2 activation are also observed and correlate with STAT signaling and cell survival [86]. As in MPN, HSP90 inhibition in cHL and multiple myeloma (MM) leads to loss of STAT3 and 5 tyrosine phosphorylation due to JAK1,3 and TYK2 down-regulation [86,87]. Receptor expression involved in the JAK/STAT3 pathway activation, such as IL6-R and IGRI-R, are also decreased upon HSP90 inhibition in MM [88]. In conclusion, several cancers with JAK2/STAT overactivation, either induced by growth factor stimulation or by receptor mutations, could benefit from HSP90 or HSP27 targeting alone or in combination with JAK2 targeting therapies.
3.3. Chaperoning the PI3K/AKT Pathway
The phosphatidylinositol 3-kinase (PI3K)/AKT regulates many cellular processes that favor cell survival and proliferation in normal and pathological hematopoietic cells. The hyperactivation of this pathway is frequently observed in leukemia and lymphomas due to either mutations or gene amplifications of PI3K or AKT, but also due to the loss of regulatory proteins such as PTEN [89]. Given the importance of this pathway, many inhibitory compounds have been developed for clinical use and are now being tested [90]. HSP90 and HSP70 have been shown to regulate this pathway.
HSP90 not only binds to AKT with CDC37 in an active complex to promote AKT activity and PI3K signaling [91,92], but also binds to multiple components of the PI3K/AKT/mTOR pathway (the PI3K catalytic and regulatory subunits, as well as downstream factors mTOR and EIF4E), as shown in BL [93]. As mentioned previously for the association of other signaling pathway inhibitors with HSP90 inhibitors, a synergistic anti-tumor effect was observed with PU-H71 and PI3K/mTOR inhibitors in BL [93]. In AML, the combination of ganetespib with cytarabine also provides synergistic cytotoxicity and is characterized by AKT disappearance [94]. For GC-DLBCL that shows a great dependency on AKT mediated by SYK activation [95,96], HSP90 targeting seems a good option to neutralize two client proteins essential for lymphoma survival. This goal is, however, reversed in acute promyelocytic leukemia (APL), which lacks AKT activation and shows a reduction in HSP90 expression. In this situation, ATRA treatment reverses HSP90 expression and AKT phosphorylation, suggesting a role for HSP90 in restarting myeloid differentiation. Regarding HSP70, the HSP70 inhibitor PFT-μ has been shown to reduce AKT expression in ALL and AML, also suggesting a chaperoning role in this pathway [97]. However, solid demonstrations of direct interaction and regulation are still lacking in those pathologies.
4. HSPs Are Chaperones of Oncogenes
Fusion proteins play a critical role in many leukemia initiations, such as the well-known BCR-ABL fusion in CML. As they are not naturally synthesized and folded proteins, HSPs are mandatory to maintain their correct conformation (Figure 2). BCR-ABL is a client protein for HSP90. The chaperone interacts with its coiled-coil domain in the N-terminal part, thereby preventing the transport of BCR-ABL to the nucleus and permitting the cytosolic promotion of signaling [98]. Conversely, HSP90 inhibition leads to the nuclear transport of BCR-ABL, its degradation, and a subsequent decrease of the downstream signaling molecules P-AKT and P-STAT5 [98,99]. Recently, a newly identified HSP90-specific inhibitor that targets HSP90 dimerization sites, aminoxyrone, has shown a similar effect on BCR-ABL down-expression in both leukemic stem cells and bulk fraction [100]. Other fusion proteins like FOP2-FGFR1, FLT3-ITD, and BCR-FGFR1 in AML are also client proteins of HSP90/CDC37, which holds them in a permanently active conformation [101,102,103]. HSP90 inhibitors alone or in combination with cytarabine show FP2-FGFR1 downregulation and anti-leukemic activity [104]. Similarly, the association of HSP90 inhibitors with a protein translation inhibitor (homoharringtonine) shows synergistic apoptosis and cell cycle arrest effects in FLT3-ITD AML [105]. Furthermore, this strategy is still valid in patients previously treated in the long term with TK inhibitors for FLT3-ITD and developing resistance through acquired mutations in FLT3-ITD, as HSP90 inhibitors are still very effective in those mutated cells [106]. Cooperation between different HSP family members is sometimes necessary to achieve the correct folding of aberrant proteins. This is the case for the AML1-ETO fusion proteins, the most important oncogenes in AML, whose correct folding is enabled by the associated work of both HSP70 and the chaperonin TRiC [107].
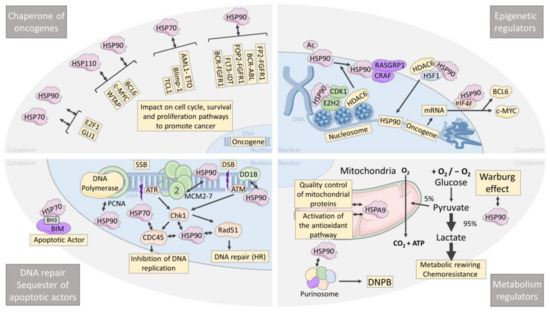
Figure 2.
HSP are chaperones of oncogenes, epigenetic regulators, metabolism regulators, chaperones of DNA repair, and sequesters of apoptotic actors in leukemia and lymphoma. Schematic of a leukemia/lymphoma cell showing the four major roles of HSPs in the global cellular homeostasis, which are: chaperones of oncogenes, chaperones of epigenetic regulators, chaperones of DNA repair actors, and their involvement in metabolism. SSB: single-strand break, DSB: double-strand break, DNPB: de novo purine biosynthetic pathway, HR: homologous recombination.
Aberrant transcriptional regulation by either hyperactive transcription factors or transcriptional repressors is also a hallmark of leukemia/lymphoma cells in which HSPs are involved. Bcl6, the transcriptional suppressor highly expressed in GC-DLBCL, and c-MYC, the master cell cycle regulator that promotes BL development, are both sustained by HSP90 and HSP110 (Figure 2). Indeed, HSP90 was shown to bind to BCL6 at the promotor of the targeted genes, stabilizing BCL6 and facilitating BCL6 transcriptional activity [108]. HSP110 expression correlates with c-MYC and Bcl6 protein expression in tumor biopsies of BL, DLBCL, FL, and MCL, and HSP110 knockdown leads to decreases in both oncogenes [23]. As described for the contribution of HSP90 to various signaling pathways, HSP90 is also embedded in multiprotein complexes in the nucleus as it is associated with BCL6 and WTAP (Wil’s tumor-1-associated protein) [109]. C-MYC is also a HSP90 client protein in BL and MCL [110,111], and therefore its degradation by the proteasome is protected by the chaperone.
Although BCL6 and c-MYC are promising therapeutic targets, effective inhibitors have not yet reached clinical efficacy, suggesting that indirect inhibition through the destabilization of their protein complexes with HSPs could be an alternative strategy. This is supported by reports of the degradation of BCL6 and c-MYC upon HSP90 or HSP110 inhibition, which is followed by increased cell death. Of note, in MCL, the MYC transcriptional program was particularly and specifically suppressed upon HSP90 inhibition compared to other pathways, confirming the importance of the peculiar HSP90/c-MYC relationship [110]. Many other transcription factors might be regulated by HSP70, HSP90, and HSP110, and future research will probably confirm this. For instance, E2F1, a central factor involved in cell cycle in MCL, and GLI1, the main effector of the hedgehog pathway in AML, have been identified as new HSP90 client proteins that are down-expressed upon HSP90 and HSP70 inhibition [112,113]. Conversely, the accelerated cytosolic degradation of the mutated transcription factors that would, in their wild-type form, normally alleviate the aborted differentiation is a not-so-well-studied mechanism of aberrant HSP chaperoning. This has been demonstrated in ABC-DLBCL, where unstable N-terminally misfolded Blimp-1 mutants are recognized by HSP70 and brought to Hrd1-mediated cytosolic sequestration, ubiquitination, and proteasomal degradation. In this context, HSP70 inhibition restored Blimp-1 mutants’ nuclear localization and function [114]. This mechanism of HSP70-mediated aberrant sequestration of a transcription factor is reminiscent of what we observed for GATA-1 in myelodysplastic syndromes [115].
T-cell leukemia/lymphoma 1 (Tcl1) is overexpressed in aggressive CLL and other human B-cell lymphomas. It is known to be a co-activator of AKT and a transactivator of NFkB [116,117]. HSP70 inhibition induces the proteasomal degradation of TCL1, prevents NFkB activation, and decreases tumor growth in mice xenografted with Daudi and Raji BL cell lines [118]. Therefore, not only transcription factors but also oncogenes that serve as signaling pathway transactivators, such as Tcl1, are stabilized by HSPs, expanding the number of putative therapeutic targets (Figure 2).
5. HSPs Are Epigenetic Regulators
Epigenetic regulators are also masters of gene expression, and their down- or over-expression is frequently associated with chemotherapy resistance [119]. In particular, the histone methyltransferase EZH2 is lost in 50% of AML patients and induces cytarabine resistance [120]. This reduction in EZH2 protein is due to CDK1-dependent phosphorylation within a complex involving HSP90. Accordingly, HSP90 inhibition restores EZH2 and drug sensitivity [121]. The histone deacetylase (HDAC) proteins are powerful epigenetic translational regulators. They mediate chromatin compacting and gene expression by the deacetylation of histones and regulate both the protein stability and activity of non-histone proteins. HDAC6 is a client protein of HSP90 and mediates its deacetylation [122,123]. Therefore, HDAC inhibition leads to HSP90 hyperacetylation and degradation, with all the cascade of client protein disappearance that follows (Figure 2). Particularly, in B-cell lymphomas and T-cell leukemias, RASGRP1 and CRAF, two HSP90 client proteins, are degraded upon HDAC6 inhibition, leading to RASGRP1/CRAF-dependent apoptosis [124]. Being an HSP90 client protein, however, HDAC6 is also degraded upon HSP90 inhibition; therefore, mutual regulation does exist and is a tempting target for combinational therapies. Furthermore, in AML and CML, HDAC inhibition leads to stronger binding of HSP90 to its inhibitor, 17-allylamino-17-demethoxygeldanamycin (17-AAG), fostering HSP90 degradation. Accordingly, combinations of HDAC inhibitors and 17-AAG logically result in increased cell death [125]. Finally, the transcriptional regulation of HSP90 is also modulated by HDAC6, first by facilitating HSF1 nuclear translocation, and second by deacetylating histone at the HSP90 gene locus. In conclusion, the search for dual HDAC and HSP90 inhibitors is a promising field to synergistically improve therapeutic strategies [126]. Transcriptional regulation of many oncogenic mRNAs is also controlled by the eukaryotic initiation complex eIF4F, which binds to HSP90 [127]. As in DLBCL, BCL6 and cMYC mRNAs are exported and translated under the control of eIF4F; targeting HSP90 would also make it possible to blunt the production of these oncogenes. In conclusion, HSP90 is known to be a direct regulator of gene expression as a chromatin-bound protein [128].
6. HSPs Are Leukemia and Lymphoma Metabolism Regulators
Metabolism rewiring allows tumor cells to adapt their biosynthesis to the nutrient availability in their microenvironment. Therefore, aggressive lymphomas and leukemias both use oxidative phosphorylation and glycolysis to fulfill their need in ATP. The latter process is amplified and known as the Warburg effect, which contributes to chemoresistance [129]. Oncogenes and aberrant cell signaling such as c-MYC, HIF1a, and the PI3K/AKT/mTOR pathway contribute to the promotion of the Warburg effect, the conversion of pyruvate to lactate, and fatty acid synthesis. By sustaining these pathways and oncogenes directly or indirectly, HSPs can modulate such metabolic rewiring (Figure 2). Recently, it has been reported that HSP90 is involved in this process as a stabilizer of protein complexes rather than as signaling pathway promoters [130]. Indeed, HSP90 coordinates and sustains several metabolic pathways, such as enzymes involved in the metabolism of nucleotides, carbohydrates, and amino acids, by promoting the formation of multienzymatic complexes. More recently, this metabolic function of HSP90 has also been shown in the process of de novo purine biosynthesis (DNPB) under high purine demands during metabolic stress [131]. Purinosome, a multienzymatic complex that drives DNPB, requires HSP90 to maintain its physical properties and thereby promote purine production. Although demonstrated in HeLa cells and not yet in hematological malignancies, this finding supports the role of HSP90 as a metabolic regulator and encourages future research in this field.
Mitochondria are multicompetent actors in cellular metabolism, and mitochondrial chaperones are important factors. HSPA9 (Mortalin), a mitochondrial HSP70 family member, is involved in the quality control of proteins imported into the mitochondrial matrix. HSPA9 also senses oxidative stress and controls the activation of the antioxidant pathway [132], therefore playing a central role in the mitochondrial stress response. The targeting of mitochondrial chaperones could be envisioned as sensitivity to proteostasis inhibition strategies, commonly used in relapsed MM or MCL patients through bortezomib treatment, which relies on mitochondrial metabolism [133]. This hypothesis is sustained by the fact that inhibition of HSPA9 by HSP70 allosteric inhibitors alleviates proteasome inhibitor resistance in MM cells [134,135]. Globally, HSP70 family members are also good target candidates, as resistance to Bortezomib therapy is associated with their up-regulation [135,136].
7. HSP Are Chaperones of DNA Repair and Sequesters of Apoptotic Actors
DNA damage can result from environmental or endogenous stress, consequently initiating several mechanisms of DNA repair to maintain cells’ integrity and survival. The ability of HSPs to promote DNA repair in several cancers has been recently reviewed [137,138]. Here, we will focus on their role in leukemia and lymphomas (Figure 2). Several mechanisms are involved in DNA repair, i.e., DNA damage detection, cell cycle arrest, and DNA synthesis [139]. PCNA (proliferating cell nuclear antigen), which favors the recruitment of DNA polymerase to DNA strand breaks, DDB1 (damage-specific DNA binding protein), which recognizes UV-induced DNA lesions, and MC2, a substrate of ATM and ATR upon DNA damage, are reduced upon HSP90 inhibition by SNX-7081 in CLL [140]. This promotion of DNA repair by HSP90 favors resistance to DNA-damaging drugs like fludarabine. Therefore, treatment with an HSP90 inhibitor restores the cells’ sensitivity [141,142]. Similarly, in AML, whose treatment relies mostly on replicative stress induced by nucleoside analogues, HSP90 inhibition reduces Chk1 and Rad51, two HSP90 client proteins that induce cell cycle arrest and promote homologous reparation repair (HR), respectively. Accordingly, treatment of HSP90 inhibitors with cytarabine leads to a stronger cell cycle arrest [143]. Therefore, associating an HSP90 inhibitor with nucleoside analogues for the treatment of AML would permit increasing leukemic cells’ responses and limiting the progression of the disease. Regulation of DNA replication and repair could also be modulated at the transcriptional level by E2F1, a client protein of HSP90 and HSP70 that assures the transcription of CDC6, CDC45, MCM4, MCM7, RIM1, and RIM2 in CLL [112,113]. The subtle equilibrium between pro- and anti-apoptotic proteins must be finely tuned. HSP are involved in this adjustment and could shift the balance toward cell survival in cancer cells through direct interaction with key apoptotic proteins. In hematopoietic cells, interaction of HSP70 with the BH3-only pro-apoptotic protein BIM via a BH3 domain has been shown [144]. A dual mechanism simultaneously appears; first, sequestration of BIM from Bcl2, which contributes to cell survival, and second, promotion of the chaperone activity of HSP70 with the aid of BIM, which serves as a co-chaperone. Recently, a specific inhibitor that targets the BH3 domain involved in HSP70-BIM interaction has been shown to disrupt this association and thereby overcome the BCR-ABL independent TKI resistance in CML [145]. Venetoclax is a Bcl2-selective inhibitor approved in 2016 as a treatment of CLL, AML, and shows promising results in MM. Recent studies have shown the efficacy of this inhibitor as a treatment for MCL patients refractory to ibrutinib [146]. However, the development of acquired resistance in some patients remains inevitable. It has been shown that HSP27 may be the cause of this relapse in patients treated with venetoclax for MCL, suggesting that targeting HSP27 could overcome this resistance [147]. Furthermore, in MCL, a robust synergy was observed between the HSP90 inhibitor, LAM-003 and venetoclax in FLT3-ITD AML cells, a particularly aggressive and resistant cell type [148].
8. Recent Progress in Clinical Targeting of HSP90
As said previously, HSP90 is known to facilitate the maturation, stabilization, and activation of over 200 client proteins, covering all cellular processes in cancers. Therefore, huge efforts have been made over the past decades to develop selective inhibitors that directly target HSP90. Most of the HSP90 inhibitors that are currently available and all that have been clinically assessed both in solid and hematological tumors, bind to the nucleotide binding pocket of the N-terminal domain and block the processing of client proteins by preventing ATP binding and hydrolysis.
HSP90 inhibition has shown, for example, some efficacy for the treatment of lymphomas. HSP90 inhibitor ganetespib enhances the sensitivity of MCL to Bruton’s Tyrosine Kinase inhibitor ibrutinib [149]. Aberrant HSP90 expression in lymphocytes and HSP90 response to anti-PD-1 therapy in lymphoma patients have also been shown [150]. HSP90 inhibition sensitizes diffuse large B-cell lymphoma cells to Cisplatin [151]. Regarding AML, several HSP90 inhibitors affect cancer cells’ growth. For instance, HSP90 inhibitors overcome the resistance to Fms-like tyrosine kinase 3 (FLT3) inhibitors in AML [106]. HSP90 inhibitors NVP-AUY922 and ganetespib (STA-9090) have shown synergistic anti-leukemic activity with cytarabine in AML [94,104]. Alvespimycin (17-DMAG), administered intravenously twice weekly to AML patients, was also found to be effective [152]. Co-treatments with 17-AAG and a FLT3 kinase inhibitor or a histone deacetylase inhibitor are highly effective against human AML cells with mutant FLT3 [153,154]. Finally, HSP90 inhibition depletes DNA repair proteins to sensitize AML to nucleoside analog chemotherapeutics [143].
Other studies have also confirmed that there is an elevated expression of HSP90 in CML, suggesting that HSP90 could serve as a prognostic marker [20]. This also explains why several chemical inhibitors of HSP90 have been tested to treat CML [155]. In addition, targeting HSP90 dimerization is effective in imatinib-resistant CML [100]. ACY-1215 suppresses the proliferation and induces the apoptosis of CML cells via the ROS/PTEN/Akt pathway [156]. Preclinical evaluation of the HSP90 inhibitor SNX-5422 in Ibrutinib-resistant CLL has furthermore been described, and destabilization of ROR1 enhances the activity of Ibrutinib against CLL in vivo [64,157].
Regarding ALL, the HSP90 inhibitor PU-H71 has also been shown to be effective in treating T-ALL patient samples that express a high level of NOTCH1 (notch receptor 1) [158]. TAS-116 (pimitespib), a HSP90 inhibitor, shows efficacy in preclinical models of adult T-ALL [159].
It is well known that HSP90 is involved in many essential cellular processes in non-malignant cells, such as protein maturation and stabilization, chaperoning of kinases, transcription factors, and other major signaling proteins [160,161]. Fortunately, tumor cells rely more on HSP for survival and proliferation than normal cells, limiting the inhibitors’ side effects [161]. However, to limit these adverse events, one must pay attention to each HSP90 inhibitor’s selectivity towards normal cells, as AUY922 does not preferentially impair the proliferation of cancer cells over normal cells, whereas the C-terminal Hsp90 modulator SM258 only impacts colon cancer cell lines [162]. In monotherapies, HSP90 inhibitors have shown some toxicity, limiting their efficacy, and tumor cells manage to develop resistance [161]. Therefore, combining low doses of HSP90 inhibitors with other anti-cancer drugs would be a solution in the future. Development of inhibitors that would target the tumor-specific conformation of HSP90 would also be a goal [162,163]. Current HSP inhibitors and their stage of clinical development are shown in Table 1.

Table 1.
List of HSP inhibitors presented in this review, showing their targets, mechanisms of action, and clinical phases of development.
9. Discussion
Leukemia and lymphoma represent a diverse, although particular, group of cancers that poses specific challenges to researchers. We have presented here the diverse and major roles of HSPs in these diseases, which could help to better target oncogenic processes and contribute to improving therapies. Typical of B- and T-cell populations, BCR and TCR signaling networks are frequently and heavily mutated in lymphoid leukemias and lymphomas and strongly rely on HSPs to transmit sustained signaling. We have also described the stabilization of many oncogenes by HSPs that would otherwise endanger the viability of tumor cells.
We must not forget that the roles of HSP and HSF are not limited to cell signaling but provide critical support to gene expression and epigenetic regulation, particularly through HDAC6. In solid tumors, HSF1, the major HSP transcription factor, also controls various transcriptional programs such as those for cell-cycle regulation, RNA splicing, and resistance to apoptosis [164]. Very few studies have explored these non-classical functions of HSF1 in hematological malignancies. We have shown that HSF1 is highly expressed during myeloid differentiation, modulating PU.1 expression [165]. Of note, chronic myelomonocytic leukemia cells that are frequently associated with defective monocyte differentiation were lacking both HSF1 and PU1 expression [165]. HSF1 is also known to be involved in metabolic regulation in hepatocellular carcinoma [166]. This could be explored in the context of leukemia and lymphomas as metabolic rewiring is modulated by HSP90 and HSPA9, promoting the production of large amounts of needed ATP [130]. Targeting HSF1 has been difficult to date due to the lack of specific inhibitors. However, recently, Dong et al. [167] identified a small molecule that directly binds to HSF1 and promotes the degradation of its nuclear and active forms. This promising new tool should soon be tested in leukemias and in lymphomas murine models to validate its clinical perspectives.
So far, most studies on the role of HSPs have focused on HSP90 and HSP70 for obvious reasons of cellular abundance and inhibitor availability. This must not overshadow the importance of other HSPs such as HSP27, mitochondrial HSPA9, or HSP110. The latter starts to reveal its critical involvement in the oncogenesis of lymphoma, such as the stabilization of several oncogenes like BCL6, C-MYC, and MyD88. The recent discovery of the first HSP110 inhibitors [67] would probably foster research in other types of hematological malignancies and the identification of more functions.
In this review, we focused on the intracellular functions of HSPs, but one must not forget the extracellular HSPs, either membrane-bound or secreted in the extracellular milieu. HSP70 and HSP110 are expressed on the surface of NHL or released within extracellular vesicles such as exosomes and have the ability to interact with various ligands expressed on immune cells like TLR2 or TLR4 [22,168,169]. These extracellular HSPs are mainly observed in tumors, both in cells and their microenvironment, and have the capacity to activate immune cells associated with immunosuppression [170,171,172]. Therefore, their targeting or neutralization should be envisioned. This could be achieved by CAR-T cell technology as proposed by Smith J. et al. for HSP70 (patent: EP3250605).
In B cell lymphomas, exosomes from plasma samples can be easily isolated and characterized, and their content might be used as a predictor of response to therapy [173]. Moreover, HSP27, HSP70 and HSP110 exosomes can be easily detected in body fluids, and their content is associated with a bad prognosis in several solid tumors [85,174,175,176]. Therefore, their detection in plasma samples from patients with leukemia and lymphoma may also be used as biomarkers to predict tumoral HSP expression and to monitor responses to therapies.
10. Conclusions
In conclusion, HSPs appears to play multiple and crucial roles in every aspect of leukemia and lymphoma biology, from specific cell signalling to epigenetic regulations. Identification of specific functions related to the oncogenesis of these cells must be pursued to provide better targeted and personalized therapies. Identification of new and more specific inhibitors will also be beneficial for the entire anti-cancer field of research as HSPs are hallmarks of cancer resistance to intra- and extracellular stresses.
Author Contributions
Writing, Review and Editing, V.C.-G., M.D., R.Q., F.G., C.G. and G.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by La Ligue Nationale contre le Cancer EL2020.LNCC/CaG (VCG, CG and GJ), La fondation ARC pour la recherche sur la cancer (RQ, GJ), Ligue Nationale contre le Cancer comité régional (R.Q., G.J.), the French National Research Agency (C.G.), Institut National du Cancer PLBIO22-093 (C.G.), Fondation Ruban Rose (C.G.), Regional Council Burgundy-Franche-Comté FEDER BG24709 (HoST-110) (C.G.), Des tulipes contre le cancer, Châlon sur Saône (F.G.).
Acknowledgments
C.G. team is labeled by La Ligue Nationale contre le Cancer. We thank all volunteers and patients from “La ligue Nationale contre le Cancer”, “la fondation ARC”, and “Des tulipes contre le cancer” for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Moraes Hungria, V.T.; Chiattone, C.; Pavlovsky, M.; Abenoza, L.M.; Agreda, G.P.; Armenta, J.; Arrais, C.; Flores, O.A.; Barroso, F.; Basquiera, A.L.; et al. Epidemiology of hematologic malignancies in real-world settings: Findings from the hemato-oncology Latin america observational registry study. J. Glob. Oncol. 2019, 2019, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Lagarón, N.O.; McGowan, E.M.; Parmar, I.; Jha, A.; Hubbard, B.P.; Rupasinghe, H.P.V. Kinase-targeted cancer therapies: Progress, challenges and future directions. Mol. Cancer 2018, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Sinha, S.; Aldape, K.; Hannenhalli, S.; Sahinalp, C.; Ruppin, E. Big data in basic and translational cancer research. Nat. Rev. Cancer 2022, 22, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Thomas, X.; Campos, L.; Mounier, C.; Cornillon, J.; Flandrin, P.; Le, Q.H.; Piselli, S.; Guyotat, D. Expression of heat-shock proteins is associated with major adverse prognostic factors in acute myeloid leukemia. Leuk. Res. 2005, 29, 1049–1058. [Google Scholar] [CrossRef]
- Pocaly, M.; Lagarde, V.; Etienne, G.; Ribeil, J.A.; Claverol, S.; Bonneu, M.; Moreau-Gaudry, F.; Guyonnet-Duperat, V.; Hermine, O.; Melo, J.V.; et al. Overexpression of the heat-shock protein 70 is associated to imatinib resistance in chronic myeloid leukemia. Leukemia 2007, 21, 93–101. [Google Scholar] [CrossRef]
- Frezzato, F.; Raggi, F.; Martini, V.; Severin, F.; Trimarco, V.; Visentin, A.; Scomazzon, E.; Accordi, B.; Bresolin, S.; Piazza, F.; et al. HSP70/HSF1 axis, regulated via a PI3K/AKT pathway, is a druggable target in chronic lymphocytic leukemia. Int. J. Cancer 2019, 145, 3089–3100. [Google Scholar] [CrossRef]
- Sun, Q.; Ye, Y.; Gui, A.; Sun, X.; Xie, S.; Zhan, Y.; Chen, R.; Yan, Y.; Gu, J.; Qiu, S.; et al. MORTALIN-Ca2+ axis drives innate rituximab resistance in diffuse large B-cell lymphoma. Cancer Lett. 2022, 537, 215678. [Google Scholar] [CrossRef]
- Frezzato, F.; Visentin, A.; Severin, F.; Pizzo, S.; Ruggeri, E.; Mouawad, N.; Martinello, L.; Pagnin, E.; Trimarco, V.; Tonini, A.; et al. Targeting of HSP70/HSF1 axis abrogates in vitro ibrutinib-resistance in chronic lymphocytic leukemia. Cancers 2021, 13, 5453. [Google Scholar] [CrossRef]
- Yang, L.; Cao, L.; Yang, M.; Tang, D.; Kang, R.; Min, X.; Zhu, S.; Yu, Y. Hsp27: A novel therapeutic target for pediatric M4/M5 acute myeloid leukemia. Oncol. Rep. 2013, 29, 1459–1466. [Google Scholar] [CrossRef]
- Shevtsov, M.; Huile, G.; Multhoff, G. Membrane heat shock protein 70: A theranostic target for cancer therapy. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160526. [Google Scholar] [CrossRef]
- Steiner, K.; Graf, M.; Hecht, K.; Reif, S.; Rossbacher, L.; Pfister, K.; Kolb, H.J.; Schmetzer, H.M.; Multhoff, G. High HSP70-membrane expression on leukemic cells from patients with acute myeloid leukemia is associated with a worse prognosis. Leukemia 2006, 20, 2076–2079. [Google Scholar] [CrossRef]
- Yeh, C.H.; Tseng, R.; Hannah, A.; Estrov, Z.; Estey, E.; Kantarjian, H.; Albitar, M. Clinical correlation of circulating heat shock protein 70 in acute leukemia. Leuk. Res. 2010, 34, 605–609. [Google Scholar] [CrossRef]
- Berthenet, K.; Boudesco, C.; Collura, A.; Svrcek, M.; Richaud, S.; Hammann, A.; Causse, S.; Yousfi, N.; Wanherdrick, K.; Duplomb, L.; et al. Extracellular HSP110 skews macrophage polarization in colorectal cancer. Oncoimmunology 2016, 5, e1170264. [Google Scholar] [CrossRef]
- Höpken, U.E.; Rehm, A. Targeting the Tumor Microenvironment of Leukemia and Lymphoma. Trends Cancer 2019, 5, 351–364. [Google Scholar] [CrossRef]
- Yufu, Y.; Nishimura, J.; Nawata, H. High constitutive expression of heat shock protein 90α in human acute leukemia cells. Leuk. Res. 1992, 16, 597–605. [Google Scholar] [CrossRef]
- Trentin, L.; Frasson, M.; Donella-Deana, A.; Frezzato, F.; Pagano, M.A.; Tibaldi, E.; Gattazzo, C.; Zambello, R.; Semenzato, G.; Brunati, A.M. Geldanamycin-induced Lyn dissociation from aberrant Hsp90-stabilized cytosolic complex is an early event in apoptotic mechanisms in B-chronic lymphocytic leukemia. Blood 2008, 112, 4665–4674. [Google Scholar] [CrossRef]
- Hacıhanefioglu, A.; Gonullu, E.; Mehtap, O.; Keski, H.; Yavuz, M.; Ercin, C. Effect of heat shock protein-90 (HSP90) and vascular endothelial growth factor (VEGF) on survival in acute lymphoblastic leukemia: An immunohistochemical study. Med. Oncol. 2011, 28, 846–851. [Google Scholar] [CrossRef]
- Milani, M.; Laranjeira, A.B.A.; De Vasconcellos, J.F.; Brandalise, S.R.; Nowill, A.E.; Yunes, J.A. Plasma Hsp90 Level as a Marker of Early Acute Lymphoblastic Leukemia Engraftment and Progression in Mice. PLoS ONE 2015, 10, e0129298. [Google Scholar]
- Žáčková, M.; Moučková, D.; Lopotová, T.; Ondráčková, Z.; Klamová, H.; Moravcová, J. Hsp90—A potential prognostic marker in CML. Blood Cells, Mol. Dis. 2013, 50, 184–189. [Google Scholar] [CrossRef]
- Guo, A.; Lu, P.; Lee, J.; Zhen, C.; Chiosis, G.; Wang, Y.L. HSP90 stabilizes B-cell receptor kinases in a multi-client interactome: PU-H71 induces CLL apoptosis in a cytoprotective microenvironment. Oncogene 2017, 36, 3441–3449. [Google Scholar] [CrossRef] [PubMed]
- Zappasodi, R.; Bongarzone, I.; Ghedini, G.C.; Castagnoli, L.; Cabras, A.D.; Messina, A.; Tortoreto, M.; Tripodo, C.; Magni, M.; Carlo-Stella, C.; et al. Serological identification of HSP105 as a novel non-Hodgkin lymphoma therapeutic target. Blood 2011, 118, 4421–4430. [Google Scholar] [CrossRef] [PubMed]
- Zappasodi, R.; Ruggiero, G.; Guarnotta, C.; Tortoreto, M.; Tringali, C.; Cavanè, A.; Cabras, A.D.; Castagnoli, L.; Venerando, B.; Zaffaroni, N.; et al. HSPH1 inhibition downregulates Bcl-6 and c-Myc and hampers the growth of human aggressive B-cell non-Hodgkin lymphoma. Blood 2015, 125, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Boudesco, C.; Verhoeyen, E.; Martin, L.; Chassagne-Clement, C.; Salmi, L.; Mhaidly, R.; Pangault, C.; Fest, T.; Ramla, S.; Jardin, F.; et al. HSP110 sustains chronic NF-kB signaling in activated B-cell diffuse large B-cell lymphoma through MyD88 stabilization. Blood 2018, 132, 510–520. [Google Scholar] [CrossRef]
- Boudesco, C.; Rattier, T.; Garrido, C.; Jego, G. Do not stress, just differentiate: Role of stress proteins in hematopoiesis. Cell Death Dis. 2015, 6. [Google Scholar] [CrossRef]
- Syafruddin, S.E.; Ling, S.; Low, T.Y.; Aiman Mohtar, M. More Than Meets the Eye: Revisiting the Roles of Heat Shock Factor 4 in Health and Diseases. Biomolecules 2021, 11, 523. [Google Scholar] [CrossRef]
- Gomez-Pastor, R.; Burchfiel, E.T.; Thiele, D.J. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 4. [Google Scholar] [CrossRef]
- Morán Luengo, T.; Mayer, M.P.; Rüdiger, S.G.D. The Hsp70–Hsp90 Chaperone Cascade in Protein Folding. Trends Cell Biol. 2019, 29, 164–177. [Google Scholar] [CrossRef]
- Lang, B.J.; Guerrero, M.E.; Prince, T.L.; Okusha, Y.; Bonorino, C.; Calderwood, S.K. The functions and regulation of heat shock proteins; key orchestrators of proteostasis and the heat shock response. Arch. Toxicol. 2021, 95, 1943–1970. [Google Scholar] [CrossRef]
- Kruta, M.; Sunshine, M.J.; Chua, B.A.; Fu, Y.; Chawla, A.; Dillingham, C.H.; Hidalgo San Jose, L.; De Jong, B.; Zhou, F.J.; Signer, R.A.J. Hsf1 promotes hematopoietic stem cell fitness and proteostasis in response to ex vivo culture stress and aging. Cell Stem Cell 2021, 28, 1950–1965.e6. [Google Scholar] [CrossRef]
- Dong, Q.; Xiu, Y.; Wang, Y.; Hodgson, C.; Borcherding, N.; Jordan, C.; Buchanan, J.; Taylor, E.; Wagner, B.; Leidinger, M.; et al. HSF1 is a driver of leukemia stem cell self-renewal in acute myeloid leukemia. Nat. Commun. 2022, 13, 6107. [Google Scholar] [CrossRef]
- Nishida, Y.; Zhao, R.; Heese, L.E.; Akiyama, H.; Patel, S.; Jaeger, A.M.; Jacamo, R.O.; Kojima, K.; Ma, M.C.J.; Ruvolo, V.R.; et al. Inhibition of translation initiation factor eIF4a inactivates heat shock factor 1 (HSF1) and exerts anti-leukemia activity in AML. Leukemia 2021, 35, 2469–2481. [Google Scholar] [CrossRef]
- Cyran, A.M.; Zhitkovich, A. Heat Shock Proteins and HSF1 in Cancer. Front. Oncol. 2022, 12, 860320. [Google Scholar] [CrossRef]
- Yang, Z.; Wan, W.; Zhang, P.; Wang, S.; Zhao, Z.; Xue, J.; Yao, M.; Zhao, Y.; Zheng, W.; Niu, B.; et al. Crosstalk between HSF1 and STAT3 mediated by IL-8 autocrine signaling maintains the cancer stem cell phenotype in liver cancer. J. Gastroenterol. Hepatol. 2022, 38, 138–152. [Google Scholar] [CrossRef]
- Qin, T.; Chen, K.; Li, J.; Qian, W.; Xiao, Y.; Wu, E.; Ma, J.; Chen, Z.; Wang, Z.; Ma, Q.; et al. Heat shock factor 1 inhibition sensitizes pancreatic cancer to gemcitabine via the suppression of cancer stem cell-like properties. Biomed. Pharmacother. 2022, 148, 112713. [Google Scholar] [CrossRef]
- Kabiri Renani, M.; Yousefi, R.; Koohkan, F.; Heidari, M.; Asad, S.; Hosseinzadeh, S.; Nazaran, M.H. Knockdown of HSF1 sensitizes resistant prostate cancer cell line to chemotherapy. Mod. Med. Lab. J. 2022, 5, 47–55. [Google Scholar] [CrossRef]
- Östling, P.; Björk, J.K.; Roos-Mattjus, P.; Mezger, V.; Sistonen, L. Heat Shock Factor 2 (HSF2) contributes to inducible expression of hsp genes through interplay with HSF1. J. Biol. Chem. 2007, 282, 7077–7086. [Google Scholar] [CrossRef]
- Smith, R.S.; Takagishi, S.R.; Amici, D.R.; Metz, K.; Gayatri, S.; Alasady, M.J.; Wu, Y.; Brockway, S.; Taiberg, S.L.; Khalatyan, N.; et al. HSF2 cooperates with HSF1 to drive a transcriptional program critical for the malignant state. Sci. Adv. 2022, 8, 6526. [Google Scholar] [CrossRef]
- Tokunaga, Y.; Otsuyama, K.-I.; Kakuta, S.; Hayashida, N. Heat Shock Transcription Factor 2 Is Significantly Involved in Neurodegenerative Diseases, Inflammatory Bowel Disease, Cancer, Male Infertility, and Fetal Alcohol Spectrum Disorder: The Novel Mechanisms of Several Severe Diseases. Int. J. Mol. Sci. 2022, 23, 13763. [Google Scholar] [CrossRef]
- Wang, X.Y.; Subjeck, J.R. High molecular weight stress proteins: Identification, cloning and utilisation in cancer immunotherapy. Int. J. Hyperth. 2013, 29, 364–375. [Google Scholar] [CrossRef]
- Liu, T.; Daniels, C.K.; Cao, S. Comprehensive review on the HSC70 functions, interactions with related molecules and involvement in clinical diseases and therapeutic potential. Pharmacol. Ther. 2012, 136, 354–374. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, R.U.H.; Sharma, S.K.; Priya, S.; Finka, A.; Goloubinoff, P. Hsp110 is a bona fide chaperone using ATP to unfold stable misfolded polypeptides and reciprocally collaborate with Hsp70 to solubilize protein aggregates. J. Biol. Chem. 2013, 288, 21399–21411. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, L.; Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 2005, 5, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, T.; Rios, Z.; Mei, Q.; Lin, X.; Cao, S. Heat Shock Proteins and Cancer. Trends Pharmacol. Sci. 2017, 38, 226–256. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.; Jiang, Z.; You, Q. Modulation of protein fate decision by small molecules: Targeting molecular chaperone machinery. Acta Pharm. Sin. B 2020, 10, 1904–1925. [Google Scholar] [CrossRef]
- Treweek, T.M.; Meehan, S.; Ecroyd, H.; Carver, J.A. Small heat-shock proteins: Important players in regulating cellular proteostasis. Cell. Mol. Life Sci. 2015, 72, 429–451. [Google Scholar] [CrossRef]
- Chelouche-Lev, D.; Kluger, H.M.; Berger, A.J.; Rimm, D.L.; Price, J.E. αB-crystallin as a marker of lymph node involvement in breast carcinoma. Cancer 2004, 100, 2543–2548. [Google Scholar] [CrossRef]
- Ivanov, O.; Chen, F.; Wiley, E.L.; Keswani, A.; Diaz, L.K.; Memmel, H.C.; Rademaker, A.; Gradishar, W.J.; Morrow, M.; Khan, S.A.; et al. αB-crystallin is a novel predictor of resistance to neoadjuvant chemotherapy in breast cancer. Breast Cancer Res. Treat. 2008, 111, 411–417. [Google Scholar] [CrossRef]
- Takashi, M.; Katsuno, S.; Sakata, T.; Ohshima, S.; Kato, K. Different concentrations of two small stress proteins, alphaB crystallin and HSP27 in human urological tumor tissues. Urol. Res. 1998, 26, 395–399. [Google Scholar] [CrossRef]
- Liu, X.L.; Guo, K.P.; Ma, F.; Xie, G.Y.; He, Y.; Hu, C.H. Expression profile of heat shock proteins in tissues and cells of lung adenocarcinoma. J. Cent. South Univ. Medical Sci. 2007, 32, 660–664. [Google Scholar]
- Tang, Q.; Liu, Y.F.; Zhu, X.J.; Li, Y.H.; Zhu, J.; Zhang, J.P.; Feng, Z.Q.; Guan, X.H. Expression and prognostic significance of the alpha B-crystallin gene in human hepatocellular carcinoma. Hum. Pathol. 2009, 40, 300–305. [Google Scholar] [CrossRef]
- Profitós-Pelejà, N.; Santos, J.C.; Marín-Niebla, A.; Roué, G.; Ribeiro, M.L. Regulation of B-Cell Receptor Signaling and Its Therapeutic Relevance in Aggressive B-Cell Lymphomas. Cancers 2022, 14, 860. [Google Scholar] [CrossRef]
- Löwenberg, M.; Verhaar, A.P.; Bilderbeek, J.; van Marle, J.; Buttgereit, F.; Peppelenbosch, M.P.; van Deventer, S.J.; Hommes, D.W. Glucocorticoids cause rapid dissociation of a T-cell-receptor-associated protein complex containing LCK and FYN. EMBO Rep. 2006, 7, 1023–1029. [Google Scholar] [CrossRef]
- Nika, K.; Soldani, C.; Salek, M.; Paster, W.; Gray, A.; Etzensperger, R.; Fugger, L.; Polzella, P.; Cerundolo, V.; Dushek, O.; et al. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity 2010, 32, 766–777. [Google Scholar] [CrossRef]
- Serafin, V.; Lissandron, V.; Buldini, B.; Bresolin, S.; Paganin, M.; Grillo, F.; Andriano, N.; Palmi, C.; Cazzaniga, G.; Marmiroli, S.; et al. Phosphoproteomic analysis reveals hyperactivation of mTOR/STAT3 and LCK/Calcineurin axes in pediatric early T-cell precursor ALL. Leukemia 2017, 31, 1007–1011. [Google Scholar] [CrossRef]
- Buffière, A.; Accogli, T.; Saint-Paul, L.; Lucchi, G.; Uzan, B.; Ballerini, P.; Bastie, J.N.; Delva, L.; Pflumio, F.; Quéré, R. Saracatinib impairs maintenance of human T-ALL by targeting the LCK tyrosine kinase in cells displaying high level of lipid rafts. Leukemia 2018, 32, 2062–2065. [Google Scholar] [CrossRef]
- Serafin, V.; Capuzzo, G.; Milani, G.; Minuzzo, S.A.; Pinazza, M.; Bortolozzi, R.; Bresolin, S.; Porcù, E.; Frasson, C.; Indraccolo, S.; et al. Glucocorticoid resistance is reverted by LCK inhibition in pediatric T-cell acute lymphoblastic leukemia. Blood 2017, 130, 2750–2761. [Google Scholar] [CrossRef]
- Taipale, M.; Krykbaeva, I.; Koeva, M.; Kayatekin, C.; Westover, K.D.; Karras, G.I.; Lindquist, S. Quantitative Analysis of Hsp90-Client Interactions Reveals Principles of Substrate Recognition. Cell 2012, 150, 987–1001. [Google Scholar] [CrossRef]
- Mshaik, R.; Simonet, J.; Georgievski, A.; Jamal, L.; Bechoua, S.; Ballerini, P.; Bellaye, P.S.; Mlamla, Z.; Pais de Barros, J.P.; Geissler, A.; et al. HSP90 inhibitor NVP-BEP800 affects stability of SRC kinases and growth of T-cell and B-cell acute lymphoblastic leukemias. Blood Cancer J. 2021, 11, 61. [Google Scholar] [CrossRef]
- Harr, M.W.; Caimi, P.F.; McColl, K.S.; Zhong, F.; Patel, S.N.; Barr, P.M.; Distelhorst, C.W. Inhibition of Lck enhances glucocorticoid sensitivity and apoptosis in lymphoid cell lines and in chronic lymphocytic leukemia. Cell Death Differ. 2010, 17, 1381–1391. [Google Scholar] [CrossRef]
- Mahmud, H.; Mendez, M.; Mukhopadhyay, B.; Holter-Chakrabarty, J.; Ghosh, A.K. HSP90 overexpression potentiates the B-cell receptor and fibroblast growth factor receptor survival signals in chronic lymphocytic leukemia cells. Oncotarget 2020, 11, 2037. [Google Scholar] [CrossRef]
- Jacobson, C.; Kopp, N.; Layer, J.V.; Redd, R.A.; Tschuri, S.; Haebe, S.; Van Bodegom, D.; Bird, L.; Christie, A.L.; Christodoulou, A.; et al. HSP90 inhibition overcomes ibrutinib resistance in mantle cell lymphoma. Blood 2016, 128, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.L.; Yang, S.N.; Taldone, T.; Chang, B.; Gerecitano, J.; Elenitoba-Johnson, K.; Shaknovich, R.; Tam, W.; Leonard, J.P.; Chiosis, G.; et al. Pharmacoproteomics identifies combinatorial therapy targets for diffuse large B cell lymphoma. J. Clin. Invest. 2015, 125, 4559–4571. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.L.; Harrington, B.; Truxall, J.; Wasmuth, R.; Prouty, A.; Sloan, S.; Lehman, A.M.; Sampath, D.; Orlemans, E.; Baiocchi, R.A.; et al. Preclinical evaluation of the Hsp90 inhibitor SNX-5422 in ibrutinib resistant CLL. J. Hematol. Oncol. 2021, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.H.; Young, R.M.; Schmitz, R.; Yang, Y.; Pittaluga, S.; Wright, G.; Lih, C.J.; Williams, P.M.; Shaffer, A.L.; Gerecitano, J.; et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat. Med. 2015, 21, 922–926. [Google Scholar] [CrossRef]
- Phelan, J.D.; Young, R.M.; Webster, D.E.; Roulland, S.; Wright, G.W.; Kasbekar, M.; Shaffer, A.L.; Ceribelli, M.; Wang, J.Q.; Schmitz, R.; et al. A multiprotein supercomplex controlling oncogenic signalling in lymphoma. Nature 2018, 560, 387–391. [Google Scholar] [CrossRef]
- Gozzi, G.J.; Gonzalez, D.; Boudesco, C.; Dias, A.M.M.; Gotthard, G.; Uyanik, B.; Dondaine, L.; Marcion, G.; Hermetet, F.; Denis, C.; et al. Selecting the first chemical molecule inhibitor of HSP110 for colorectal cancer therapy. Cell Death Differ. 2019, 27, 117–129. [Google Scholar] [CrossRef]
- Walter, R.; Pan, K.T.; Doebele, C.; Comoglio, F.; Tomska, K.; Bohnenberger, H.; Young, R.M.; Jacobs, L.; Keller, U.; Bönig, H.; et al. HSP90 promotes Burkitt lymphoma cell survival by maintaining tonic B-cell receptor signaling. Blood 2017, 129, 598–608. [Google Scholar] [CrossRef]
- Segges, P.; Corrêa, S.; Du Rocher, B.; Vera-Lozada, G.; Krsticevic, F.; Arce, D.; Sternberg, C.; Abdelhay, E.; Hassan, R. Targeting Hodgkin and Reed–Sternberg Cells with an Inhibitor of Heat-Shock Protein 90: Molecular Pathways of Response and Potential Mechanisms of Resistance. Int. J. Mol. Sci. 2018, 19, 836. [Google Scholar] [CrossRef]
- Taniguchi, H.; Hasegawa, H.; Sasaki, D.; Ando, K.; Sawayama, Y.; Imanishi, D.; Taguchi, J.; Imaizumi, Y.; Hata, T.; Tsukasaki, K.; et al. Heat shock protein 90 inhibitor NVP-AUY922 exerts potent activity against adult T-cell leukemia-lymphoma cells. Cancer Sci. 2014, 105, 1601–1608. [Google Scholar] [CrossRef]
- Hertlein, E.; Wagner, A.J.; Jones, J.; Lin, T.S.; Maddocks, K.J.; Towns, W.H.; Goettl, V.M.; Zhang, X.; Jarjoura, D.; Raymond, C.A.; et al. 17-DMAG targets the nuclear factor-κB family of proteins to induce apoptosis in chronic lymphocytic leukemia: Clinical implications of HSP90 inhibition. Blood 2010, 116, 45–53. [Google Scholar] [CrossRef]
- Chen, I.T.; Hsu, P.H.; Hsu, W.C.; Chen, N.J.; Tseng, P.H. Polyubiquitination of Transforming Growth Factor β-activated Kinase 1 (TAK1) at Lysine 562 Residue Regulates TLR4-mediated JNK and p38 MAPK Activation. Sci. Rep. 2015, 5, 12300. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, A.; Huang, J.; Suo, M.; Zhong, Y.; Liang, Y. Suppression of HSP70 inhibits the development of acute lymphoblastic leukemia via TAK1/Egr-1. Biomed. Pharmacother. 2019, 119, 109399. [Google Scholar] [CrossRef]
- Desterke, C.; Bennaceur-Griscelli, A.; Turhan, A.G. EGR1 dysregulation defines an inflammatory and leukemic program in cell trajectory of human-aged hematopoietic stem cells (HSC). Stem Cell Res. Ther. 2021, 12, 419. [Google Scholar] [CrossRef]
- Kulkarni, R. Early Growth Response Factor 1 in Aging Hematopoietic Stem Cells and Leukemia. Front. Cell Dev. Biol. 2022, 10, 925761. [Google Scholar] [CrossRef]
- Sanda, T.; Tyner, J.W.; Gutierrez, A.; Ngo, V.N.; Glover, J.; Chang, B.H.; Yost, A.; Ma, W.; Fleischman, A.G.; Zhou, W.; et al. TYK2-STAT1-BCL2 pathway dependence in T-cell acute lymphoblastic leukemia. Cancer Discov. 2013, 3, 564–577. [Google Scholar] [CrossRef]
- Wöss, K.; Simonović, N.; Strobl, B.; Macho-Maschler, S.; Müller, M. Tyk2: An upstream kinase of stats in cancer. Cancers 2019, 11, 1728. [Google Scholar] [CrossRef]
- Akahane, K.; Sanda, T.; Mansour, M.R.; Radimerski, T.; Deangelo, D.J.; Weinstock, D.M.; Look, A.T. HSP90 inhibition leads to degradation of the TYK2 kinase and apoptotic cell death in T-cell acute lymphoblastic leukemia. Leukemia 2016, 30, 219–228. [Google Scholar] [CrossRef]
- Baxter, E.J.; Scott, L.M.; Campbell, P.J.; East, C.; Fourouclas, N.; Swanton, S.; Vassiliou, G.S.; Bench, A.J.; Boyd, E.M.; Curtin, N.; et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005, 365, 1054–1061. [Google Scholar] [CrossRef]
- Brkic, S.; Stivala, S.; Santopolo, A.; Szybinski, J.; Jungius, S.; Passweg, J.R.; Tsakiris, D.; Dirnhofer, S.; Hutter, G.; Leonards, K.; et al. Dual targeting of JAK2 and ERK interferes with the myeloproliferative neoplasm clone and enhances therapeutic efficacy. Leukemia 2021, 35, 2875–2884. [Google Scholar] [CrossRef] [PubMed]
- Fiskus, W.; Verstovsek, S.; Manshouri, T.; Rao, R.; Balusu, R.; Venkannagari, S.; Nalabothula, N.R.; Ha, K.; Smith, J.E.; Hembruff, S.L.; et al. Heat shock protein 90 inhibitor is synergistic with JAK2 inhibitor and overcomes resistance to JAK2-TKI in human myeloproliferative neoplasm cells. Clin. Cancer Res. 2011, 17, 7347–7358. [Google Scholar] [CrossRef] [PubMed]
- Marubayashi, S.; Koppikar, P.; Taldone, T.; Abdel-Wahab, O.; West, N.; Bhagwat, N.; Caldas-Lopes, E.; Ross, K.N.; Gönen, M.; Gozman, A.; et al. HSP90 is a therapeutic target in JAK2-dependent myeloproliferative neoplasms in mice and humans. J. Clin. Invest. 2010, 120, 3578–3593. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, G.; Litvin, R.; Ahn, J.; Mckenney, A.S.; Mauro, M.J.; Tallman, M.S.; Berman, E.; Heaney, M.L.; Levine, R.L.; Rampal, R.K. AUY922, a Heat Shock Protein 90 (Hsp90) Inhibitor, Demonstrates Activity in Patients with Myeloproliferative Neoplasms (MPNs). Blood 2015, 126, 4075. [Google Scholar] [CrossRef]
- Weigert, O.; Lane, A.A.; Bird, L.; Kopp, N.; Chapuy, B.; van Bodegom, D.; Toms, A.V.; Marubayashi, S.; Christie, A.L.; McKeown, M.; et al. Genetic resistance to JAK2 enzymatic inhibitors is overcome by HSP90 inhibition. J. Exp. Med. 2012, 209, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Sevin, M.; Kubovcakova, L.; Pernet, N.; Causse, S.; Vitte, F.; Villeval, J.L.; Lacout, C.; Cordonnier, M.; Rodrigues-Lima, F.; Chanteloup, G.; et al. HSP27 is a partner of JAK2-STAT5 and a potential therapeutic target in myelofibrosis. Nat. Commun. 2018, 9, 1431. [Google Scholar] [CrossRef]
- Schoof, N.; Von Bonin, F.; Trümper, L.; Kube, D. HSP90 is essential for Jak-STAT signaling in classical Hodgkin lymphoma cells. Cell Commun. Signal. 2009, 7, 17. [Google Scholar] [CrossRef]
- Khong, T.; Spencer, A. Targeting HSP 90 induces apoptosis and inhibits critical survival and proliferation pathways in multiple myeloma. Mol. Cancer Ther. 2011, 10, 1909–1917. [Google Scholar] [CrossRef]
- Richardson, P.G.; Mitsiades, C.S.; Laubach, J.P.; Lonial, S.; Chanan-Khan, A.A.; Anderson, K.C. Inhibition of heat shock protein 90 (HSP90) as a therapeutic strategy for the treatment of myeloma and other cancers. Br. J. Haematol. 2011, 152, 367–379. [Google Scholar] [CrossRef]
- Xu, W.; Berning, P.; Lenz, G. Targeting B-cell receptor and PI3K signaling in diffuse large B-cell lymphoma. Blood 2021, 138, 1110–1119. [Google Scholar] [CrossRef]
- Shouse, G.; Danilova, O.V.; Danilov, A.V. Current status of phosphoinotiside-3 kinase inhibitors in blood cancers. Curr. Opin. Oncol. 2022, 34, 540–545. [Google Scholar] [CrossRef]
- Sato, S.; Fujita, N.; Tsuruo, T. Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl. Acad. Sci. USA 2000, 97, 10832–10837. [Google Scholar] [CrossRef]
- Basso, A.D.; Solit, D.B.; Chiosis, G.; Giri, B.; Tsichlis, P.; Rosen, N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J. Biol. Chem. 2002, 277, 39858–39866. [Google Scholar] [CrossRef]
- Giulino-Roth, L.; Van Besien, H.J.; Dalton, T.; Totonchy, J.E.; Rodina, A.; Taldone, T.; Bolaender, A.; Erdjument-Bromage, H.; Sadek, J.; Chadburn, A.; et al. Inhibition of Hsp90 suppresses PI3K/AKT/mTOR signaling and has antitumor activity in Burkitt lymphoma. Mol. Cancer Ther. 2017, 16, 1779–1790. [Google Scholar] [CrossRef]
- Lazenby, M.; Hills, R.; Burnett, A.K.; Zabkiewicz, J. The HSP90 inhibitor ganetespib: A potential effective agent for Acute Myeloid Leukemia in combination with cytarabine. Leuk. Res. 2015, 39, 617–624. [Google Scholar] [CrossRef]
- Chen, L.; Monti, S.; Juszczynski, P.; Daley, J.; Chen, W.; Witzig, T.E.; Habermann, T.M.; Kutok, J.L.; Shipp, M.A. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood 2008, 111, 2230–2237. [Google Scholar] [CrossRef]
- Havranek, O.; Xu, J.; Köhrer, S.; Wang, Z.; Becker, L.; Comer, J.M.; Henderson, J.; Ma, W.; Ma, J.M.C.; Westin, J.R.; et al. Tonic B-cell receptor signaling in diffuse large B-cell lymphoma. Blood 2017, 130, 995–1006. [Google Scholar] [CrossRef]
- Kaiser, M.; Kühnl, A.; Reins, J.; Fischer, S.; Ortiz-Tanchez, J.; Schlee, C.; Mochmann, L.H.; Heesch, S.; Benlasfer, O.; Hofmann, W.K.; et al. Antileukemic activity of the HSP70 inhibitor pifithrin-l in acute leukemia. Blood Cancer J. 2011, 1, e28. [Google Scholar] [CrossRef]
- Peng, Y.; Huang, Z.; Zhou, F.; Wang, T.; Mou, K.; Feng, W. Effect of HSP90AB1 and CC domain interaction on Bcr-Abl protein cytoplasm localization and function in chronic myeloid leukemia cells. Cell Commun. Signal. 2021, 19, 71. [Google Scholar] [CrossRef]
- Ju, H.Q.; Wang, S.X.; Xiang, Y.F.; Liu, Z.; Liu, J.Y.; Chen, Z.P.; Zeng, F.L.; Xia, M.; Liu, Z.H.; Xing, G.W.; et al. BJ-B11, a novel Hsp90 inhibitor, induces apoptosis in human chronic myeloid leukemia K562 cells through the mitochondria-dependent pathway. Eur. J. Pharmacol. 2011, 666, 26–34. [Google Scholar] [CrossRef]
- Bhatia, S.; Diedrich, D.; Frieg, B.; Ahlert, H.; Stein, S.; Bopp, B.; Lang, F.; Zang, T.; Kröger, T.; Ernst, T.; et al. Targeting HSP90 dimerization via the C terminus is effective in imatinib-resistant CML and lacks the heat shock response. Blood 2018, 132, 307–320. [Google Scholar] [CrossRef]
- Jinwal, U.K.; Trotter, J.H.; Abisambra, J.F.; Koren, J.; Lawson, L.Y.; Vestal, G.D.; O’Leary, J.C.; Johnson, A.G.; Jin, Y.; Jones, J.R.; et al. The Hsp90 kinase co-chaperone Cdc37 regulates tau stability and phosphorylation dynamics. J. Biol. Chem. 2011, 286, 16976–16983. [Google Scholar] [CrossRef] [PubMed]
- Reikvam, H.; Hatfield, K.J.; Ersvær, E.; Hovland, R.; Skavland, J.; Gjertsen, B.T.; Petersen, K.; Bruserud, Ø. Expression profile of heat shock proteins in acute myeloid leukaemia patients reveals a distinct signature strongly associated with FLT3 mutation status—Consequences and potentials for pharmacological intervention. Br. J. Haematol. 2012, 156, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Peiris, M.N.; Meyer, A.N.; Nelson, K.N.; Bisom-Rapp, E.W.; Donoghue, D.J. Oncogenic fusion protein BCR-FGFR1 requires the breakpoint cluster region-mediated oligomerization and chaperonin Hsp90 for activation. Haematologica 2020, 105, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Wendel, T.; Zhen, Y.; Suo, Z.; Bruheim, S.; Wiedlocha, A. The novel HSP90 inhibitor NVP-AUY922 shows synergistic anti-leukemic activity with cytarabine in vivo. Exp. Cell Res. 2016, 340, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhuang, H.; Yu, Q.; Zhang, X.; Jiang, X.; Lu, X.; Xu, Y.; Yang, L.; Wu, B.; Ma, A.; et al. Homoharringtonine Combined with the Heat Shock Protein 90 Inhibitor IPI504 in the Treatment of FLT3-ITD Acute Myeloid Leukemia. Transl. Oncol. 2019, 12, 801–809. [Google Scholar] [CrossRef]
- Katayama, K.; Noguchi, K.; Sugimoto, Y. Heat shock protein 90 inhibitors overcome the resistance to Fms-like tyrosine kinase 3 inhibitors in acute myeloid leukemia. Oncotarget 2018, 9, 34240–34258. [Google Scholar] [CrossRef]
- Roh, S.H.; Kasembeli, M.; Galaz-Montoya, J.G.; Trnka, M.; Lau, W.C.Y.; Burlingame, A.; Chiu, W.; Tweardy, D.J. Chaperonin TRiC/CCT modulates the folding and activity of leukemogenic fusion oncoprotein AML1-ETO. J. Biol. Chem. 2016, 291, 4732–4741. [Google Scholar] [CrossRef]
- Cerchietti, L.C.; Lopes, E.C.; Yang, S.N.; Hatzi, K.; Bunting, K.L.; Tsikitas, L.A.; Mallik, A.; Robles, A.I.; Walling, J.; Varticovski, L.; et al. A purine scaffold Hsp90 inhibitor destabilizes BCL-6 and has specific antitumor activity in BCL-6-dependent B cell lymphomas. Nat. Med. 2009, 15, 1369–1376. [Google Scholar] [CrossRef]
- Kuai, Y.; Gong, X.; Ding, L.; Li, F.; Lei, L.; Gong, Y.; Liu, Q.; Tan, H.; Zhang, X.; Liu, D.; et al. Wilms’ tumor 1-associating protein plays an aggressive role in diffuse large B-cell lymphoma and forms a complex with BCL6 via Hsp90. Cell Commun. Signal. 2018, 16, 50. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, L.L.; Wu, W.; Guo, H.; Li, Y.; Sukhanova, M.; Venkataraman, G.; Zhang, H.; Alikhan, M.; Lu, P.; et al. Activation of MYC, a bona fide client of HSP90, contributes to intrinsic ibrutinib resistance in mantle cell lymphoma. Blood Adv. 2018, 2, 2039–2051. [Google Scholar] [CrossRef]
- Poole, C.J.; Zheng, W.; Lee, H.; Young, D.; Lodh, A.; Chadli, A.; van Riggelen, J. Targeting the MYC oncogene in burkitt lymphoma through HSP90 inhibition. Cancers 2018, 10, 448. [Google Scholar] [CrossRef]
- Liu, H.; Lu, Z.; Shi, X.; Liu, L.; Zhang, P.; Golemis, E.A.; Tu, Z. HSP90 inhibition downregulates DNA replication and repair genes via E2F1 repression. J. Biol. Chem. 2021, 297, 100996. [Google Scholar] [CrossRef]
- Freisleben, F.; Modemann, F.; Muschhammer, J.; Stamm, H.; Brauneck, F.; Krispien, A.; Bokemeyer, C.; Kirschner, K.N.; Wellbrock, J.; Fiedler, W. Mebendazole mediates proteasomal degradation of GLI transcription factors in acute myeloid leukemia. Int. J. Mol. Sci. 2021, 22, 10670. [Google Scholar] [CrossRef]
- Wang, W.F.; Yan, L.; Liu, Z.; Liu, L.X.; Lin, J.; Liu, Z.Y.; Chen, X.P.; Zhang, W.; Xu, Z.Z.; Shi, T.; et al. HSP70-Hrd1 axis precludes the oncorepressor potential of N-terminal misfolded Blimp-1s in lymphoma cells. Nat. Commun. 2017, 8, 363. [Google Scholar] [CrossRef]
- Frisan, E.; Vandekerckhove, J.; De Thonel, A.; Pierre-Eugène, C.; Sternberg, A.; Arlet, J.B.; Floquet, C.; Gyan, E.; Kosmider, O.; Dreyfus, F.; et al. Defective nuclear localization of Hsp70 is associated with dyserythropoiesis and GATA-1 cleavage in myelodysplastic syndromes. Blood 2012, 119, 1532–1542. [Google Scholar] [CrossRef]
- Pekarsky, Y.; Palamarchuk, A.; Maximov, V.; Efanov, A.; Nazaryan, N.; Santanam, U.; Rassenti, L.; Kipps, T.; Croce, C.M. Tcl1 functions as a transcriptional regulator and is directly involved in the pathogenesis of CLL. Proc. Natl. Acad. Sci. USA 2008, 105, 19643–19648. [Google Scholar] [CrossRef]
- Paduano, F.; Gaudio, E.; Mensah, A.A.; Pinton, S.; Bertoni, F.; Trapasso, F. T-Cell Leukemia/Lymphoma 1 (TCL1): An oncogene regulating multiple signaling pathways. Front. Oncol. 2018, 8, 317. [Google Scholar] [CrossRef]
- Gaudio, E.; Paduano, F.; Ngankeu, A.; Lovat, F.; Fabbri, M.; Sun, H.L.; Gasparini, P.; Efanov, A.; Peng, Y.; Zanesi, N.; et al. Heat shock protein 70 regulates Tcl1 expression in leukemia and lymphomas. Blood 2013, 121, 351–359. [Google Scholar] [CrossRef]
- Cheng, Y.; He, C.; Wang, M.; Ma, X.; Mo, F.; Yang, S.; Han, J.; Wei, X. Targeting epigenetic regulators for cancer therapy: Mechanisms and advances in clinical trials. Signal Transduct. Target. Ther. 2019, 4, 62. [Google Scholar] [CrossRef]
- Kempf, J.M.; Weser, S.; Bartoschek, M.D.; Metzeler, K.H.; Vick, B.; Herold, T.; Völse, K.; Mattes, R.; Scholz, M.; Wange, L.E.; et al. Loss-of-function mutations in the histone methyltransferase EZH2 promote chemotherapy resistance in AML. Sci. Rep. 2021, 11, 5838. [Google Scholar] [CrossRef]
- Göllner, S.; Oellerich, T.; Agrawal-Singh, S.; Schenk, T.; Klein, H.U.; Rohde, C.; Pabst, C.; Sauer, T.; Lerdrup, M.; Tavor, S.; et al. Loss of the histone methyltransferase EZH2 induces resistance to multiple drugs in acute myeloid leukemia. Nat. Med. 2017, 23, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Bali, P.; Pranpat, M.; Bradner, J.; Balasis, M.; Fiskus, W.; Guo, F.; Rocha, K.; Kumaraswamy, S.; Boyapalle, S.; Atadja, P.; et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: A novel basis for antileukemia activity of histone deacetylase inhibitors. J. Biol. Chem. 2005, 280, 26729–26734. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xiao, J.; Wang, Y.; Song, X.; Huang, L.; Ren, Z.; Kitazato, K.; Wang, Y. Posttranslational modification and beyond: Interplay between histone deacetylase 6 and heat-shock protein 90. Mol. Med. 2021, 27, 110. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Peterson, K.L.; Correia, C.; Koh, B.; Schneider, P.A.; Nowakowski, G.S.; Kaufmann, S.H. Histone deacetylase inhibitors interrupt HSP90•RASGRP1 and HSP90•CRAF interactions to upregulate BIM and circumvent drug resistance in lymphoma cells. Leukemia 2017, 31, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Fiskus, W.; Yang, Y.; Lee, P.; Joshi, R.; Fernandez, P.; Mandawat, A.; Atadja, P.; Bradner, J.E.; Bhalla, K. HDAC6 inhibition enhances 17-AAG mediated abrogation of hsp90 chaperone function in human leukemia cells. Blood 2008, 112, 1886–1893. [Google Scholar] [CrossRef]
- Ojha, R.; Huang, H.L.; HuangFu, W.C.; Wu, Y.W.; Nepali, K.; Lai, M.J.; Su, C.J.; Sung, T.Y.; Chen, Y.L.; Pan, S.L.; et al. 1-Aroylindoline-hydroxamic acids as anticancer agents, inhibitors of HSP90 and HDAC. Eur. J. Med. Chem. 2018, 150, 667–677. [Google Scholar] [CrossRef]
- Culjkovic-Kraljacic, B.; Fernando, T.M.; Marullo, R.; Calvo-Vidal, N.; Verma, A.; Yang, S.N.; Tabbò, F.; Gaudiano, M.; Zahreddine, H.; Goldstein, R.L.; et al. Combinatorial targeting of nuclear export and translation of RNA inhibits aggressive B-cell lymphomas. Blood 2016, 127, 858–868. [Google Scholar] [CrossRef]
- Isaacs, J.S. Hsp90 as a “Chaperone” of the Epigenome: Insights and Opportunities for Cancer Therapy. In Advances in Cancer Research; Academic Press: Cambridge, MA, USA, 2016; Volume 129, pp. 107–140. ISBN 9780128022900. [Google Scholar]
- Kirsch, B.J.; Chang, S.J.; Betenbaugh, M.J.; Le, A. Non-Hodgkin Lymphoma Metabolism. Adv. Exp. Med. Biol. 2021, 1311, 103–116. [Google Scholar]
- Calvo-Vidal, M.N.; Zamponi, N.; Krumsiek, J.; Stockslager, M.A.; Revuelta, M.V.; Phillip, J.M.; Marullo, R.; Tikhonova, E.; Kotlov, N.; Patel, J.; et al. Oncogenic HSP90 facilitates metabolic alterations in aggressive B-cell lymphomas. Cancer Res. 2021, 81, 5202. [Google Scholar] [CrossRef]
- Pedley, A.M.; Boylan, J.P.; Chan, C.Y.; Kennedy, E.L.; Kyoung, M.; Benkovic, S.J. Purine biosynthetic enzymes assemble into liquid-like condensates dependent on the activity of chaperone protein HSP90. J. Biol. Chem. 2022, 298, 101845. [Google Scholar] [CrossRef]
- Tai-Nagara, I.; Matsuoka, S.; Ariga, H.; Suda, T. Mortalin and DJ-1 coordinately regulate hematopoietic stem cell function through the control of oxidative stress. Blood 2014, 123, 41–50. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Detappe, A.; Cai, K.; Keys, H.R.; Brune, Z.; Ying, W.; Thiru, P.; Reidy, M.; Kugener, G.; Rossen, J.; et al. Mitochondrial metabolism promotes adaptation to proteotoxic stress. Nat. Chem. Biol. 2019, 15, 681–689. [Google Scholar] [CrossRef]
- Ferguson, I.D.; Lin, Y.H.T.; Lam, C.; Shao, H.; Tharp, K.M.; Hale, M.; Kasap, C.; Mariano, M.C.; Kishishita, A.; Patiño Escobar, B.; et al. Allosteric HSP70 inhibitors perturb mitochondrial proteostasis and overcome proteasome inhibitor resistance in multiple myeloma. Cell Chem. Biol. 2022, 29, 1288–1302.e7. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Y.; Bai, J.; Yang, Y.; Wang, F.; Feng, Y.; Zhang, R.; Li, F.; Zhang, P.; Lv, N.; et al. Blockade of HSP70 by VER-155008 synergistically enhances bortezomib-induced cytotoxicity in multiple myeloma. Cell Stress Chaperones 2020, 25, 357–367. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Shen, H.; Guo, J.; Wang, X.; Liu, J. Bortezomib promotes apoptosis of multiple myeloma cells by regulating HSP27. Mol. Med. Rep. 2019, 20, 2410–2418. [Google Scholar] [CrossRef]
- Dubrez, L.; Causse, S.; Borges Bonan, N.; Dumétier, B.; Garrido, C. Heat-shock proteins: Chaperoning DNA repair. Oncogene 2020, 39, 516–529. [Google Scholar] [CrossRef]
- Sottile, M.L.; Nadin, S.B. Heat shock proteins and DNA repair mechanisms: An updated overview. Cell Stress Chaperones 2018, 23, 303–315. [Google Scholar] [CrossRef]
- Chao, H.X.; Poovey, C.E.; Privette, A.A.; Grant, G.D.; Chao, H.Y.; Cook, J.G.; Purvis, J.E. Orchestration of DNA Damage Checkpoint Dynamics across the Human Cell Cycle. Cell Syst. 2017, 5, 445–459.e5. [Google Scholar] [CrossRef]
- Che, Y.; Best, O.G.; Zhong, L.; Kaufman, K.L.; MacTier, S.; Raftery, M.; Graves, L.M.; Mulligan, S.P.; Christopherson, R.I. Hsp90 inhibitor SNX-7081 dysregulates proteins involved with DNA repair and replication and the cell cycle in human chronic lymphocytic leukemia (CLL) cells. J. Proteome Res. 2013, 12, 1710–1722. [Google Scholar] [CrossRef]
- Best, O.G.; Che, Y.; Singh, N.; Forsyth, C.; Christopherson, R.I.; Mulligan, S.P. The Hsp90 inhibitor SNX-7081 synergizes with and restores sensitivity to fludarabine in chronic lymphocytic leukemia cells with lesions in the TP53 pathway: A potential treatment strategy for fludarabine refractory disease. Leuk. Lymphoma 2012, 53, 1367–1375. [Google Scholar] [CrossRef]
- Kaufman, K.L.; Jenkins, Y.; Alomari, M.; Mirzaei, M.; Giles Best, O.; Pascovici, D.; Mactier, S.; Mulligan, S.P.; Haynes, P.A.; Christopherson, R.I. The Hsp90 inhibitor SNX-7081 is synergistic with fludarabine nucleoside via DNA damage and repair mechanisms in human, p53-negative chronic lymphocytic leukemia. Oncotarget 2015, 6, 40981–40997. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.H.; Mitchell, S.; Wu, P.J.; Orwick, S.; Liu, C.; Ravikrishnan, J.; Woyach, J.; Mims, A.; Plunkett, W.; Puduvalli, V.K.; et al. HSP90 inhibition depletes DNA repair proteins to sensitize acute myelogenous leukemia to nucleoside analog chemotherapeutics. Leuk. Lymphoma 2019, 60, 2308–2311. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Song, T.; Wang, Z.; Lin, D.; Cao, K.; Liu, P.; Feng, Y.; Zhang, X.; Wang, P.; Yin, F.; et al. The chaperone Hsp70 is a BH3 receptor activated by the pro-apoptotic Bim to stabilize anti-apoptotic clients. J. Biol. Chem. 2020, 295, 12900–12909. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Guo, Y.; Xue, Z.; Guo, Z.; Wang, Z.; Lin, D.; Zhang, H.; Pan, H.; Zhang, X.; Yin, F.; et al. Small-molecule inhibitor targeting the Hsp70-Bim protein–protein interaction in CML cells overcomes BCR-ABL-independent TKI resistance. Leukemia 2021, 35, 2862–2874. [Google Scholar] [CrossRef]
- Eyre, T.A.; Walter, H.S.; Iyengar, S.; Follows, G.; Cross, M.; Fox, C.P.; Hodson, A.; Coats, J.; Narat, S.; Morley, N.; et al. Efficacy of venetoclax monotherapy in patients with relapsed, refractory mantle cell lymphoma after Bruton tyrosine kinase inhibitor therapy. Haematologica 2019, 104, e68. [Google Scholar] [CrossRef]
- Che, Y.; Wang, W.; Liu, Y.; Lee, H.-H.; Li, Y.; Chen, Z.; McIntosh, J.; Jiang, C.; Yao, Y.; Wang, M. HSP27 Promotes Mantle Cell Lymphoma Growth and Mediates Resistance to Venetoclax. Blood 2022, 140, 6405–6406. [Google Scholar] [CrossRef]
- Beeharry, N.; Landrette, S.; Grotzke, J.; Gayle, S.; Hernandez, M.; Halene, S.; Young, P.R.; Miller, L.L.; Xu, T.; Rothberg, J.; et al. LAM-003, a Novel Oral Heat Shock Protein 90 Inhibitor for Treatment of Acute Myeloid Leukemia, Including Wild-Type and FMS-like Tyrosine Kinase 3 (FLT3)-Mutant Disease. Blood 2019, 134, 2664. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, Z.; Tu, Z.; Liu, H. HSP90 Inhibitor Ganetespib Enhances the Sensitivity of Mantle Cell Lymphoma to Bruton’s Tyrosine Kinase Inhibitor Ibrutinib. Front. Pharmacol. 2022, 13, 864194. [Google Scholar] [CrossRef]
- Albakova, Z.; Mangasarova, Y.; Albakov, A.; Nikulina, E.; Kravchenko, S.; Sapozhnikov, A. Aberrant HSP90 Expression in Lymphocytes and HSP90 Response to Anti-PD-1 Therapy in Lymphoma Patients. Front. Immunol. 2022, 13, 893137. [Google Scholar] [CrossRef]
- Schmidt, L.; Issa, I.I.; Haraldsdóttir, H.; Hald, J.L.; Schmitz, A.; Due, H.; Dybkær, K. Hsp90 inhibition sensitizes DLBCL cells to cisplatin. Cancer Chemother. Pharmacol. 2022, 89, 431. [Google Scholar] [CrossRef]
- Lancet, J.E.; Gojo, I.; Burton, M.; Quinn, M.; Tighe, S.M.; Kersey, K.; Zhong, Z.; Albitar, M.X.; Bhalla, K.; Hannah, A.L.; et al. Phase I study of the heat shock protein 90 inhibitor alvespimycin (KOS-1022, 17-DMAG) administered intravenously twice weekly to patients with acute myeloid leukemia. Leukemia 2010, 24, 699–705. [Google Scholar] [CrossRef]
- George, P.; Bali, P.; Cohen, P.; Tao, J.; Guo, F.; Sigua, C.; Vishvanath, A.; Fiskus, W.; Scuto, A.; Annavarapu, S.; et al. Cotreatment with 17-allylamino-demethoxygeldanamycin and FLT-3 kinase inhibitor PKC412 is highly effective against human acute myelogenous leukemia cells with mutant FLT-3. Cancer Res. 2004, 64, 3645–3652. [Google Scholar] [CrossRef]
- George, P.; Bali, P.; Annavarapu, S.; Scuto, A.; Fiskus, W.; Guo, F.; Sigua, C.; Sondarva, G.; Moscinski, L.; Atadja, P.; et al. Combination of the histone deacetylase inhibitor LBH589 and the hsp90 inhibitor 17-AAG is highly active against human CML-BC cells and AML cells with activating mutation of FLT-3. Blood 2005, 105, 1768–1776. [Google Scholar] [CrossRef]
- Khajapeer, K.V.; Baskaran, R. Hsp90 Inhibitors for the Treatment of Chronic Myeloid Leukemia. Leuk. Res. Treatment 2015, 2015. [Google Scholar] [CrossRef]
- Qin, Y.; Liang, Y.; Jiang, G.; Peng, Y.; Feng, W. ACY-1215 suppresses the proliferation and induces apoptosis of chronic myeloid leukemia cells via the ROS/PTEN/Akt pathway. Cell Stress Chaperones 2022, 27, 383–396. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Zhang, T.; Shi, M.; Chen, X.; Chen, Y.; Yu, J. Destabilization of ROR1 enhances activity of Ibrutinib against chronic lymphocytic leukemia in vivo. Pharmacol. Res. 2020, 151, 104512. [Google Scholar] [CrossRef]
- Kourtis, N.; Lazaris, C.; Hockemeyer, K.; Balandrán, J.C.; Jimenez, A.R.; Mullenders, J.; Gong, Y.; Trimarchi, T.; Bhatt, K.; Hu, H.; et al. Oncogenic hijacking of the stress response machinery in T cell acute lymphoblastic leukemia. Nat. Med. 2018, 24, 1157. [Google Scholar] [CrossRef]
- Ikebe, E.; Shimosaki, S.; Hasegawa, H.; Iha, H.; Tsukamoto, Y.; Wang, Y.; Sasaki, D.; Imaizumi, Y.; Miyazaki, Y.; Yanagihara, K.; et al. TAS-116 (pimitespib), a heat shock protein 90 inhibitor, shows efficacy in preclinical models of adult T-cell leukemia. Cancer Sci. 2022, 113, 684–696. [Google Scholar] [CrossRef]
- Gvozdenov, Z.; Kolhe, J.; Freeman, B.C. The nuclear and DNA-associated molecular chaperone network. Cold Spring Harb. Perspect. Biol. 2019, 11, a034009. [Google Scholar] [CrossRef]
- Ren, X.; Li, T.; Zhang, W.; Yang, X. Targeting Heat-Shock Protein 90 in Cancer: An Update on Combination Therapy. Cells 2022, 11, 2556. [Google Scholar] [CrossRef]
- Wang, Y.; Koay, Y.C.; McAlpine, S.R. How selective are Hsp90 inhibitors for cancer cells over normal cells? ChemMedChem 2017, 12, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Birbo, B.; Madu, E.E.; Madu, C.O.; Jain, A.; Lu, Y. Role of hsp90 in cancer. Int. J. Mol. Sci. 2021, 22, 10317. [Google Scholar] [CrossRef] [PubMed]
- Mendillo, M.L.; Santagata, S.; Koeva, M.; Bell, G.W.; Hu, R.; Tamimi, R.M.; Fraenkel, E.; Ince, T.A.; Whitesell, L.; Lindquist, S. HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell 2012, 150, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Jego, G.; Lanneau, D.; De Thonel, A.; Berthenet, K.; Hazoumé, A.; Droin, N.; Hamman, A.; Girodon, F.; Bellaye, P.S.; Wettstein, G.; et al. Dual regulation of SPI1/PU.1 transcription factor by heat shock factor 1 (HSF1) during macrophage differentiation of monocytes. Leukemia 2014, 28, 1676–1686. [Google Scholar] [CrossRef]
- Jin, X.; Moskophidis, D.; Mivechi, N.F. Heat Shock Transcription Factor 1 Is a Key Determinant of HCC Development by Regulating Hepatic Steatosis and Metabolic Syndrome. Cell Metab. 2011, 14, 91–103. [Google Scholar] [CrossRef]
- Dong, B.; Jaeger, A.M.; Hughes, P.F.; Loiselle, D.R.; Spencer Hauck, J.; Fu, Y.; Haystead, T.A.; Huang, J.; Thiele, D.J. Targeting therapy-resistant prostate cancer via a direct inhibitor of the human heat shock transcription factor 1. Sci. Transl. Med. 2020, 12, eabb5647. [Google Scholar] [CrossRef]
- Jego, G.; Chiron, D.; Berthenet, K.; Pellat-Deceunynck, C. Modulation of normal and malignant plasma cells function by toll-like receptors. Front. Biosci—Elit. 2012, 4, 2289–2301. [Google Scholar] [CrossRef]
- Chalmin, F.; Ladoire, S.; Mignot, G.; Vincent, J.; Bruchard, M.; Remy-Martin, J.P.; Boireau, W.; Rouleau, A.; Simon, B.; Lanneau, D.; et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Invest. 2010, 120, 457–471. [Google Scholar] [CrossRef]
- Gobbo, J.; Marcion, G.; Cordonnier, M.; Dias, A.M.M.M.; Pernet, N.; Hammann, A.; Richaud, S.; Mjahed, H.; Isambert, N.; Clausse, V.; et al. Restoring Anticancer Immune Response by Targeting Tumor-Derived Exosomes With a HSP70 Peptide Aptamer. J. Natl. Cancer Inst. 2016, 108, djv330. [Google Scholar] [CrossRef]
- Marcion, G.; Hermetet, F.; Neiers, F.; Uyanik, B.; Dondaine, L.; Dias, A.M.M.; Da Costa, L.; Moreau, M.; Bellaye, P.S.; Collin, B.; et al. Nanofitins targeting heat shock protein 110: An innovative immunotherapeutic modality in cancer. Int. J. Cancer 2021, 148, 3019–3031. [Google Scholar] [CrossRef]
- Regimbeau, M.; Abrey, J.; Vautrot, V.; Causse, S.; Gobbo, J.; Garrido, C. Heat shock proteins and exosomes in cancer theranostics. Semin. Cancer Biol. 2022, 86, 46–57. [Google Scholar] [CrossRef]
- Provencio, M.; Rodríguez, M.; Cantos, B.; Sabín, P.; Quero, C.; García-Arroyo, F.R.; Rueda, A.; Maximiano, C.; Rodríguez-Abreu, D.; Sánchez, A.; et al. mRNA in exosomas as a liquid biopsy in non-Hodgkin Lymphoma: A multicentric study by the Spanish lymphoma oncology group. Oncotarget 2017, 8, 50949–50957. [Google Scholar] [CrossRef]
- Cordonnier, M.; Chanteloup, G.; Isambert, N.; Seigneuric, R.; Fumoleau, P.; Garrido, C.; Gobbo, J. Exosomes in cancer theranostic: Diamonds in the rough. Cell Adhes. Migr. 2017, 11, 151–163. [Google Scholar] [CrossRef]
- Chanteloup, G.; Cordonnier, M.; Isambert, N.; Bertaut, A.; Hervieu, A.; Hennequin, A.; Luu, M.; Zanetta, S.; Coudert, B.; Bengrine, L.; et al. Monitoring HSP70 exosomes in cancer patients’ follow up: A clinical prospective pilot study. J. Extracell. Vesicles 2020, 9, 1766192. [Google Scholar] [CrossRef]
- Cordonnier, M.; Nardin, C.; Chanteloup, G.; Derangere, V.; Algros, M.P.; Arnould, L.; Garrido, C.; Aubin, F.; Gobbo, J. Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J. Extracell. Vesicles 2020, 9, 1710899. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).