Effect of Selenium and Lycopene on Radiation Sensitivity in Prostate Cancer Patients Relative to Controls
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Collection and Irradiation of Lymphocytes
2.3. Cytokinesis-Block Micronucleus Cytome (CBMN Cyt) Assay for Lymphocytes Using Whole Blood Cultures
2.4. Micronutrient Analysis and PSA Levels
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of the Cohorts
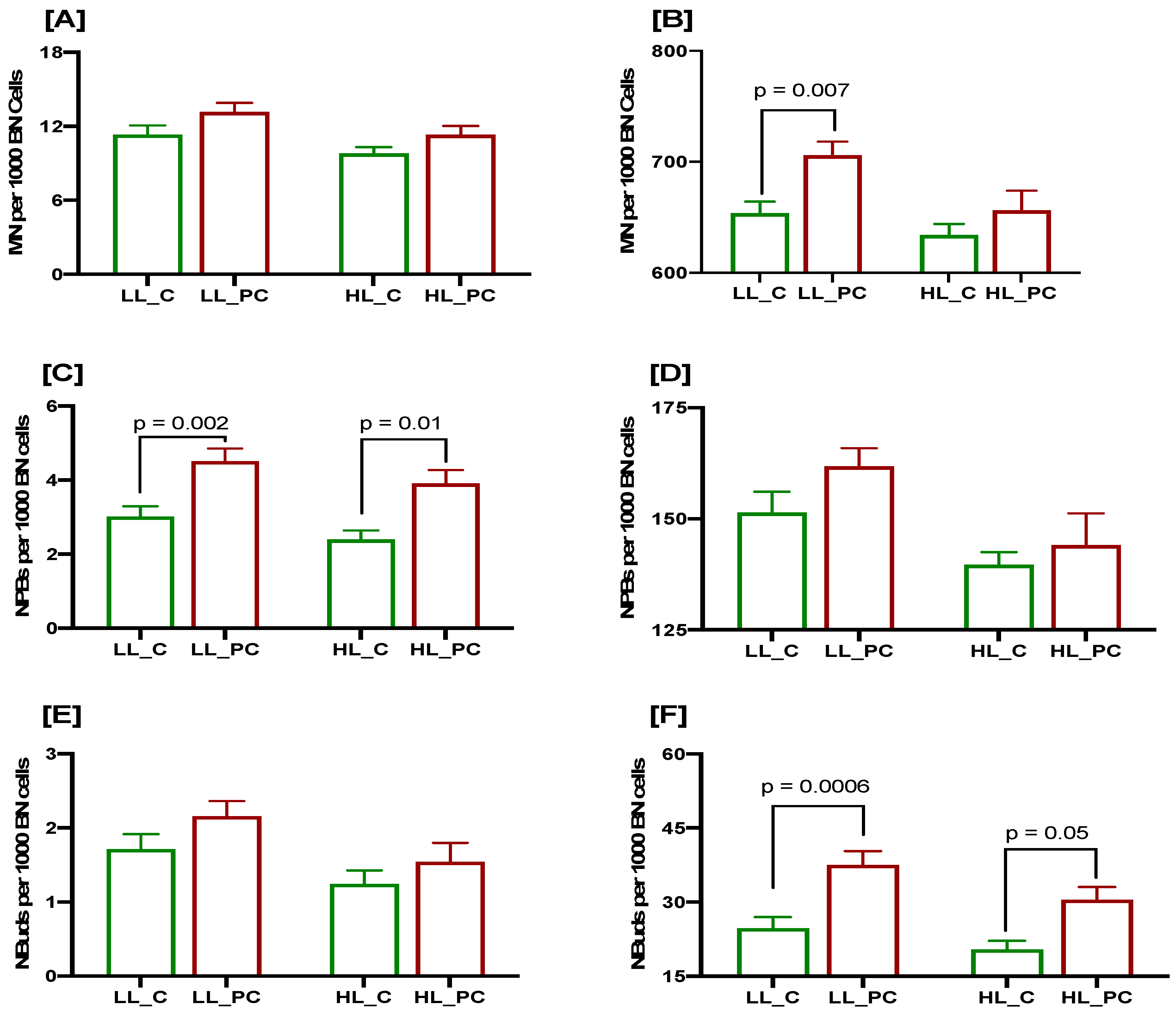
3.2. Association of Low Selenium Concentrations with DNA Damage Biomarkers at Baseline and after a 3 Gy Radiation Challenge in Controls and Patients
3.3. Association of Low Lycopene Concentration with DNA Damage Biomarkers at Baseline and after a 3 Gy Radiation Challenge in Controls and Patients
3.4. Cumulative Effects of Low Selenium and Lycopene on DNA Damage Biomarkers at Baseline and after a 3 Gy Radiation Challenge in Controls and Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries from 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef] [PubMed]
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Rebbeck, T.R. Prostate Cancer Genetics: Variation by Race, Ethnicity, and Geography. Semin. Radiat. Oncol. 2017, 27, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.B.; Eurich, D.T.; Majumdar, S.R.; Johnson, J.A. Risk of prostate cancer across different racial/ethnic groups in men with diabetes: A retrospective cohort study. Diabet. Med. 2018, 35, 107–111. [Google Scholar] [CrossRef]
- Tabassum, A.; Bristow, R.G.; Venkateswaran, V. Ingestion of selenium and other antioxidants during prostate cancer radiotherapy: A good thing? Cancer Treat. Rev. 2010, 36, 230–234. [Google Scholar] [CrossRef]
- Helzlsouer, K.J.; Huang, H.Y.; Alberg, A.J.; Hoffman, S.; Burke, A.; Norkus, E.P.; Morris, J.S.; Comstock, G.W. Association between alpha-tocopherol, gamma-tocopherol, selenium, and subsequent prostate cancer. J. Natl. Cancer Inst. 2000, 92, 2018–2023. [Google Scholar] [CrossRef]
- Peters, U.; Foster, C.B.; Chatterjee, N.; Schatzkin, A.; Reding, D.; Andriole, G.L.; Crawford, E.D.; Sturup, S.; Chanock, S.J.; Hayes, R.B. Serum selenium and risk of prostate cancer-a nested case-control study. Am. J. Clin. Nutr. 2007, 85, 209–217. [Google Scholar] [CrossRef]
- Grundmark, B.; Zethelius, B.; Garmo, H.; Holmberg, L. Serum levels of selenium and smoking habits at age 50 influence long term prostate cancer risk; a 34 year ULSAM follow-up. BMC Cancer 2011, 11, 431. [Google Scholar] [CrossRef]
- Geybels, M.S.; Verhage, B.A.; van Schooten, F.J.; Goldbohm, R.A.; van den Brandt, P.A. Advanced prostate cancer risk in relation to toenail selenium levels. J. Natl. Cancer Inst. 2013, 105, 1394–1401. [Google Scholar] [CrossRef]
- Outzen, M.; Tjønneland, A.; Hughes, D.J.; Jenab, M.; Frederiksen, K.; Schomburg, L.; Morris, S.; Overvad, K.; Olsen, A. Toenail selenium, plasma selenoprotein P and risk of advanced prostate cancer: A nested case-control study. Int. J. Cancer 2021, 148, 876–883. [Google Scholar] [CrossRef]
- Allen, N.E.; Travis, R.C.; Appleby, P.N.; Albanes, D.; Barnett, M.J.; Black, A.; Bueno-de-Mesquita, H.B.; Deschasaux, M.; Galan, P.; Goodman, G.E.; et al. Selenium and Prostate Cancer: Analysis of Individual Participant Data From Fifteen Prospective Studies. J. Natl. Cancer Inst. 2016, 108, djw153. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Takeshima, M.; Nakano, S. Mechanism of the Anticancer Effect of Lycopene (Tetraterpenoids). Enzymes 2015, 37, 139–166. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Rengasamy, K.R.R.; Mahomoodally, F.M.; Keum, Y.S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: An update on epidemiological and mechanistic perspectives. Pharmacol. Res. 2020, 155, 104730. [Google Scholar] [CrossRef] [PubMed]
- Mirahmadi, M.; Azimi-Hashemi, S.; Saburi, E.; Kamali, H.; Pishbin, M.; Hadizadeh, F. Potential inhibitory effect of lycopene on prostate cancer. Biomed. Pharmacother 2020, 129, 110459. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D. Lycopene metabolism and its biological significance. Am. J. Clin. Nutr. 2012, 96, 1214s–1222s. [Google Scholar] [CrossRef]
- Zu, K.; Mucci, L.; Rosner, B.A.; Clinton, S.K.; Loda, M.; Stampfer, M.J.; Giovannucci, E. Dietary lycopene, angiogenesis, and prostate cancer: A prospective study in the prostate-specific antigen era. J. Natl. Cancer Inst. 2014, 106, djt430. [Google Scholar] [CrossRef]
- Kawashima, A.; Madarame, T.; Koike, H.; Komatsu, Y.; Wise, J.A. Four week supplementation with mixed fruit and vegetable juice concentrates increased protective serum antioxidants and folate and decreased plasma homocysteine in Japanese subjects. Asia Pac. J. Clin. Nutr. 2007, 16, 411–421. [Google Scholar]
- Kucuk, O.; Sarkar, F.H.; Sakr, W.; Djuric, Z.; Pollak, M.N.; Khachik, F.; Li, Y.W.; Banerjee, M.; Grignon, D.; Bertram, J.S.; et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol. Biomarkers Prev. 2001, 10, 861–868. [Google Scholar]
- Datta, M.; Taylor, M.L.; Frizzell, B. Dietary and serum lycopene levels in prostate cancer patients undergoing intensity-modulated radiation therapy. J. Med. Food 2013, 16, 1131–1137. [Google Scholar] [CrossRef]
- Meade, A.D.; Maguire, A.; Bryant, J.; Cullen, D.; Medipally, D.; White, L.; McClean, B.; Shields, L.; Armstrong, J.; Dunne, M.; et al. Prediction of DNA damage and G2 chromosomal radio-sensitivity ex vivo in peripheral blood mononuclear cells with label-free Raman micro-spectroscopy. Int. J. Radiat. Biol. 2019, 95, 44–53. [Google Scholar] [CrossRef]
- Herskind, C.; Talbot, C.J.; Kerns, S.L.; Veldwijk, M.R.; Rosenstein, B.S.; West, C.M. Radiogenomics: A systems biology approach to understanding genetic risk factors for radiotherapy toxicity? Cancer Lett. 2016, 382, 95–109. [Google Scholar] [CrossRef]
- Pinkawa, M.; Brzozowska, K.; Kriehuber, R.; Eble, M.J.; Schmitz, S. Prediction of radiation-induced toxicity by in vitro radiosensitivity of lymphocytes in prostate cancer patients. Future Oncol. 2016, 12, 617–624. [Google Scholar] [CrossRef]
- Burgaz, S.; Coskun, E.; Demircigil, G.C.; Kocabas, N.A.; Cetindag, F.; Sunter, O.; Edinsel, H. Micronucleus frequencies in lymphocytes and buccal epithelial cells from patients having head and neck cancer and their first-degree relatives. Mutagenesis 2011, 26, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
- Bonassi, S.; Hagmar, L.; Strömberg, U.; Montagud, A.H.; Tinnerberg, H.; Forni, A.; Heikkilä, P.; Wanders, S.; Wilhardt, P.; Hansteen, I.L.; et al. Chromosomal aberrations in lymphocytes predict human cancer independently of exposure to carcinogens. European Study Group on Cytogenetic Biomarkers and Health. Cancer Res. 2000, 60, 1619–1625. [Google Scholar] [PubMed]
- Fenech, M. In vitro micronucleus technique to predict chemosensitivity. Methods Mol. Med. 2005, 111, 3–32. [Google Scholar] [CrossRef]
- Heaven, C.J.; Wanstall, H.C.; Henthorn, N.T.; Warmenhoven, J.W.; Ingram, S.P.; Chadwick, A.L.; Santina, E.; Honeychurch, J.; Schmidt, C.K.; Kirkby, K.J.; et al. The suitability of micronuclei as markers of relative biological effect. Mutagenesis 2022, 37, 3–12. [Google Scholar] [CrossRef]
- Vral, A.; Fenech, M.; Thierens, H. The micronucleus assay as a biological dosimeter of in vivo ionising radiation exposure. Mutagenesis 2011, 26, 11–17. [Google Scholar] [CrossRef]
- Gleason, D.F. Classification of prostatic carcinomas. Cancer Chemother. Rep. 1966, 50, 125–128. [Google Scholar]
- Fenech, M.; Baghurst, P.; Luderer, W.; Turner, J.; Record, S.; Ceppi, M.; Bonassi, S. Low intake of calcium, folate, nicotinic acid, vitamin E, retinol, beta-carotene and high intake of pantothenic acid, biotin and riboflavin are significantly associated with increased genome instability--results from a dietary intake and micronucleus index survey in South Australia. Carcinogenesis 2005, 26, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Rode, A.; Maass, K.K.; Willmund, K.V.; Lichter, P.; Ernst, A. Chromothripsis in cancer cells: An update. Int. J. Cancer 2016, 138, 2322–2333. [Google Scholar] [CrossRef] [PubMed]
- Podrimaj-Bytyqi, A.; Borovečki, A.; Selimi, Q.; Manxhuka-Kerliu, S.; Gashi, G.; Elezaj, I.R. The frequencies of micronuclei, nucleoplasmic bridges and nuclear buds as biomarkers of genomic instability in patients with urothelial cell carcinoma. Sci. Rep. 2018, 8, 17873. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.S.; Gupta, N.P.; Hemal, A.K. Chemoprevention of carcinoma prostate: A review. Int. Urol. Nephrol. 2002, 34, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Mokbel, K.; Wazir, U.; Mokbel, K. Chemoprevention of Prostate Cancer by Natural Agents: Evidence from Molecular and Epidemiological Studies. Anticancer Res. 2019, 39, 5231–5259. [Google Scholar] [CrossRef]
- Levenson, A.S. Metastasis-associated protein 1-mediated antitumor and anticancer activity of dietary stilbenes for prostate cancer chemoprevention and therapy. Semin. Cancer Biol. 2022, 80, 107–117. [Google Scholar] [CrossRef]
- El-Zein, R.A.; Schabath, M.B.; Etzel, C.J.; Lopez, M.S.; Franklin, J.D.; Spitz, M.R. Cytokinesis-blocked micronucleus assay as a novel biomarker for lung cancer risk. Cancer Res. 2006, 66, 6449–6456. [Google Scholar] [CrossRef]
- Kimura, M.; Umegaki, K.; Higuchi, M.; Thomas, P.; Fenech, M. Methylenetetrahydrofolate reductase C677T polymorphism, folic acid and riboflavin are important determinants of genome stability in cultured human lymphocytes. J. Nutr. 2004, 134, 48–56. [Google Scholar] [CrossRef]
- Iarmarcovai, G.; Bonassi, S.; Botta, A.; Baan, R.A.; Orsière, T. Genetic polymorphisms and micronucleus formation: A review of the literature. Mutat. Res. 2008, 658, 215–233. [Google Scholar] [CrossRef]
- Krupina, K.; Goginashvili, A.; Cleveland, D.W. Causes and consequences of micronuclei. Curr Opin Cell Biol. 2021, 70, 91–99. [Google Scholar] [CrossRef]
- Shoshani, O.; Brunner, S.F.; Yaeger, R.; Ly, P.; Nechemia-Arbely, Y.; Kim, D.H.; Fang, R.; Castillon, G.A.; Yu, M.; Li, J.S.Z.; et al. Chromothripsis drives the evolution of gene amplification in cancer. Nature 2021, 591, 137–141. [Google Scholar] [CrossRef]
- Fenech, M.; Knasmueller, S.; Bolognesi, C.; Holland, N.; Bonassi, S.; Kirsch-Volders, M. Micronuclei as biomarkers of DNA damage, aneuploidy, inducers of chromosomal hypermutation and as sources of pro-inflammatory DNA in humans. Mutat. Res. Rev. Mutat. Res. 2020, 786, 108342. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, V.S.; Yeoh, E.; Salisbury, C.; Butters, J.; Di Matteo, A.; Olver, I.; Fenech, M. Cytokinesis Block Micronucleus Cytome (CBMN Cyt) Assay Biomarkers and Their Association With Radiation Sensitivity Phenotype in Prostate Cancer Cases and DNA Repair Gene hOGG1 (C1245G) Polymorphism. Environ. Mol. Mutagen. 2018, 59, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Allison, R.R.; O’Brien, K.F.; Johnke, R.M.; Christie, K.I.; Naves, J.L.; Kovacs, C.J.; Arastu, H.; Karlsson, U.L. Lymphocyte radiosensitivity correlated with pelvic radiotherapy morbidity. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, E.K.; Krol, R.; Dhillon, V.S.; Botten, R.; Di Matteo, A.; Butters, J.; Brock, A.R.; Esterman, A.; Salisbury, C.; Fenech, M. Predictors of radiation-induced gastrointestinal morbidity: A prospective, longitudinal study following radiotherapy for carcinoma of the prostate. Acta Oncol. 2016, 55, 604–610. [Google Scholar] [CrossRef]
- Kaiser, A.; Haskins, C.; Siddiqui, M.M.; Hussain, A.; D’Adamo, C. The evolving role of diet in prostate cancer risk and progression. Curr. Opin. Oncol. 2019, 31, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, E.; Rudolf, K.; Cervinka, M. Selenium activates p53 and p38 pathways and induces caspase-independent cell death in cervical cancer cells. Cell Biol. Toxicol. 2008, 24, 123–141. [Google Scholar] [CrossRef]
- Mladenova, V.; Mladenov, E.; Stuschke, M.; Iliakis, G. DNA Damage Clustering after Ionizing Radiation and Consequences in the Processing of Chromatin Breaks. Molecules 2022, 27, 1540. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Müller, L.; Caris-Veyrat, C.; Lowe, G.; Böhm, V. Lycopene and Its Antioxidant Role in the Prevention of Cardiovascular Diseases-A Critical Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1868–1879. [Google Scholar] [CrossRef]
- Vasconcelos, A.G.; Amorim, A.; Dos Santos, R.C.; Souza, J.M.T.; de Souza, L.K.M.; Araújo, T.S.L.; Nicolau, L.A.D.; de Lima Carvalho, L.; de Aquino, P.E.A.; da Silva Martins, C.; et al. Lycopene rich extract from red guava (Psidium guajava L.) displays anti-inflammatory and antioxidant profile by reducing suggestive hallmarks of acute inflammatory response in mice. Food Res. Int. 2017, 99, 959–968. [Google Scholar] [CrossRef]
- Friedman, M. Anticarcinogenic, cardioprotective, and other health benefits of tomato compounds lycopene, α-tomatine, and tomatidine in pure form and in fresh and processed tomatoes. J. Agric. Food Chem. 2013, 61, 9534–9550. [Google Scholar] [CrossRef] [PubMed]
- Costa-Rodrigues, J.; Pinho, O.; Monteiro, P.R.R. Can lycopene be considered an effective protection against cardiovascular disease? Food Chem 2018, 245, 1148–1153. [Google Scholar] [CrossRef]
- McBride, W.H.; Schaue, D. Radiation-induced tissue damage and response. J. Pathol 2020, 250, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Schaue, D.; McBride, W.H. Links between innate immunity and normal tissue radiobiology. Radiat. Res. 2010, 173, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, Z.J. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med. 2018, 215, 1287–1299. [Google Scholar] [CrossRef]
- Abuetabh, Y.; Wu, H.H.; Chai, C.; Al Yousef, H.; Persad, S.; Sergi, C.M.; Leng, R. DNA damage response revisited: The p53 family and its regulators provide endless cancer therapy opportunities. Exp. Mol. Med. 2022, 54, 1658–1669. [Google Scholar] [CrossRef]



| Characteristics | Cases | Controls | p Value |
|---|---|---|---|
| Age (years; Mean ± SD) | 71.24 ± 7.18 | 69.07 ± 7.99 | 0.88 |
| Total plasma PSA (ng/mL; mean ± SD) | 9.5 ± 8.5 | 2.4 ± 2.45 | 0.0001 * |
| Gleason score | 6–9 | - | |
| Smoking status | |||
| Current smokers | 9 | 3 | 0.0001 * |
| Ex-smokers | 60 | 39 | |
| Non-smokers | 25 | 54 | |
| Undeclared | 24 | 36 | |
| Selenium (µg/L) | 116.1 ± 1.59 (71.83–157.60) | 125.6 ± 2.56 (79.17 –238.10) | 0.002 * |
| Lycopene (µg/L) | 0.184 ± 0.011 (0.013–0.655) | 0.215 ± 0.009 (0.03–0.58) | 0.008 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhillon, V.S.; Deo, P.; Fenech, M. Effect of Selenium and Lycopene on Radiation Sensitivity in Prostate Cancer Patients Relative to Controls. Cancers 2023, 15, 979. https://doi.org/10.3390/cancers15030979
Dhillon VS, Deo P, Fenech M. Effect of Selenium and Lycopene on Radiation Sensitivity in Prostate Cancer Patients Relative to Controls. Cancers. 2023; 15(3):979. https://doi.org/10.3390/cancers15030979
Chicago/Turabian StyleDhillon, Varinderpal S., Permal Deo, and Michael Fenech. 2023. "Effect of Selenium and Lycopene on Radiation Sensitivity in Prostate Cancer Patients Relative to Controls" Cancers 15, no. 3: 979. https://doi.org/10.3390/cancers15030979
APA StyleDhillon, V. S., Deo, P., & Fenech, M. (2023). Effect of Selenium and Lycopene on Radiation Sensitivity in Prostate Cancer Patients Relative to Controls. Cancers, 15(3), 979. https://doi.org/10.3390/cancers15030979






