Outcomes of Radiofrequency Ablation for Solitary T1a Renal Cell Carcinoma: A 20-Year Tertiary Cancer Center Experience
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ablation Technique
2.2. Data Collection
2.3. Definitions of Outcomes
2.4. Data Analysis and Statistics
3. Results
3.1. Procedural Outcomes
3.2. Pathologic Outcomes
3.3. Oncologic Outcomes
3.3.1. Residual Disease or Local Recurrence
3.3.2. Disease Recurrence and Distant Metastases
3.4. Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, S.C.; Clark, P.E.; Chang, S.S.; Karam, J.A.; Souter, L.; Uzzo, R.G. Renal Mass and Localized Renal Cancer: Evaluation, Management, and Follow-Up: AUA Guideline: Part I. J. Urol. 2021, 206, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Pierorazio, P.M.; Johnson, M.H.; Patel, H.D.; Sozio, S.; Sharma, R.; Iyoha, E.; Bass, E.; Allaf, M.E. Management of Renal Masses and Localized Renal Cancer: Systematic Review and Meta-Analysis. J. Urol. 2016, 196, 989–999. [Google Scholar] [CrossRef]
- Khondker, A.; Jain, A.; Groff, M.L.; Brzezinski, J.; Lorenzo, A.J.; Zappitelli, M. Late Kidney Effects of Nephron-Sparing vs Radical Nephrectomy for Wilms Tumor: A Systematic Review and Meta-Analysis. J. Urol. 2022, 207, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bensalah, K.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2019 Update. Eur. Urol. 2019, 75, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Finelli, A.; Ismaila, N.; Bro, B.; Durack, J.; Eggener, S.; Evans, A.; Gill, I.; Graham, D.; Huang, W.; Jewett, M.A.; et al. Management of Small Renal Masses: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017, 35, 668–680. [Google Scholar] [CrossRef]
- Kumar, S.K.; Callander, N.S.; Adekola, K.; Anderson, L.D.; Baljevic, M.; Campagnaro, E. Kidney Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 67–81. [Google Scholar] [PubMed]
- Campbell, S.; Uzzo, R.G.; Allaf, M.E.; Bass, E.B.; Cadeddu, J.A.; Chang, A.; Clark, P.E.; Davis, B.J.; Derweesh, I.H.; Giambarresi, L.; et al. Renal Mass and Localized Renal Cancer: AUA Guideline. J. Urol. 2017, 198, 520–529. [Google Scholar] [CrossRef]
- Gervais, D.A.; McGovern, F.J.; Arellano, R.S.; McDougal, W.S.; Mueller, P.R. Renal cell carcinoma: Clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology 2003, 226, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, T.; Hiraki, T.; Tomita, K.; Gobara, H.; Fujiwara, H.; Sakurai, J.; Matsui, Y.; Kanazawa, S. Simultaneous biopsy and radiofrequency ablation of T1a renal cell carcinoma. Diagn. Interv. Imaging 2016, 97, 1159–1164. [Google Scholar] [CrossRef]
- Karam, J.A.; Ahrar, K.; Vikram, R.; Romero, C.A.; Jonasch, E.; Tannir, N.M.; Rao, P.; Wood, C.G.; Matin, S.F. Radiofrequency ablation of renal tumours with clinical, radiographical and pathological results. BJU Int. 2013, 111, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, B.K.; Park, J.J.; Kim, C.K. CT-Guided Radiofrequency Ablation of T1a Renal Cell Carcinoma in Korea: Mid-Term Outcomes. Korean J. Radiol. 2016, 17, 763–770. [Google Scholar] [CrossRef]
- Levinson, A.W.; Su, L.-M.; Agarwal, D.; Sroka, M.; Jarrett, T.W.; Kavoussi, L.R.; Solomon, S.B. Long-term oncological and overall outcomes of percutaneous radio frequency ablation in high risk surgical patients with a solitary small renal mass. J. Urol. 2008, 180, 499–504, discussion. [Google Scholar] [CrossRef] [PubMed]
- Mayo-Smith, W.W.; Dupuy, D.E.; Parikh, P.M.; Pezzullo, J.A.; Cronan, J.J. Imaging-guided percutaneous radiofrequency ablation of solid renal masses: Techniques and outcomes of 38 treatment sessions in 32 consecutive patients. AJR Am. J. Roentgenol. 2003, 180, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- McDougal, W.S.; Gervais, D.A.; McGovern, F.J.; Mueller, P.R. Long-term followup of patients with renal cell carcinoma treated with radio frequency ablation with curative intent. J. Urol. 2005, 174, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Zagoria, R.J. Percutaneous image-guided radiofrequency ablation of renal malignancies. Radiol. Clin. North Am. 2003, 41, 1067–1075. [Google Scholar] [CrossRef]
- Zagoria, R.J.; Pettus, J.A.; Rogers, M.; Werle, D.M.; Childs, D.; Leyendecker, J.R. Long-term outcomes after percutaneous radiofrequency ablation for renal cell carcinoma. Urology 2011, 77, 1393–1397. [Google Scholar] [CrossRef]
- Zagoria, R.J.; Traver, M.A.; Werle, D.M.; Perini, M.; Hayasaka, S.; Clark, P.E. Oncologic efficacy of CT-guided percutaneous radiofrequency ablation of renal cell carcinomas. AJR Am. J. Roentgenol. 2007, 189, 429–436. [Google Scholar] [CrossRef]
- Psutka, S.P.; Feldman, A.S.; McDougal, W.S.; McGovern, F.J.; Mueller, P.; Gervais, D.A. Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur. Urol. 2013, 63, 486–492. [Google Scholar] [CrossRef]
- Wah, T.M.; Irving, H.C.; Gregory, W.; Cartledge, J.; Joyce, A.D.; Selby, P.J. Radiofrequency ablation (RFA) of renal cell carcinoma (RCC): Experience in 200 tumours. BJU Int. 2014, 113, 416–428. [Google Scholar] [CrossRef]
- Johnson, B.A.; Sorokin, I.; Cadeddu, J.A. Ten-Year Outcomes of Renal Tumor Radio Frequency Ablation. J. Urol. 2019, 201, 251–258. [Google Scholar] [CrossRef]
- Ahrar, K.; Matin, S.; Wood, C.G.; Wallace, M.J.; Gupta, S.; Madoff, D.C.; Rao, S.; Tannir, N.M.; Jonasch, E.; Pisters, L.L.; et al. Percutaneous radiofrequency ablation of renal tumors: Technique, complications, and outcomes. J. Vasc. Interv. Radiol. 2005, 16, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, M.E.; Ahrar, K. Ablation of Small Renal Masses. Tech. Vasc. Interv. Radiol. 2020, 23, 100674. [Google Scholar] [CrossRef]
- Ahmed, M.; Solbiati, L.; Brace, C.L.; Breen, D.J.; Callstrom, M.R.; Charboneau, J.W.; Chen, M.-H.; Choi, B.I.; De Baère, T.; Dodd, G.D., III; et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria—A 10-year update. Radiology 2014, 273, 241–260. [Google Scholar] [CrossRef]
- Campbell, S.C.; Novick, A.C.; Belldegrun, A.; Blute, M.L.; Chow, G.K.; Derweesh, I.H.; Faraday, M.M.; Kaouk, J.H.; Leveillee, R.J.; Matin, S.F.; et al. Guideline for management of the clinical T1 renal mass. J. Urol. 2009, 182, 1271–1279. [Google Scholar] [CrossRef]
- Ma, Y.; Bedir, S.; Cadeddu, J.A.; Gahan, J.C. Long-term outcomes in healthy adults after radiofrequency ablation of T1a renal tumours. BJU Int. 2014, 113, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, M.E.; Sabir, S.H.; Karam, J.A.; Matin, S.F.; Wood, C.G.; Ahrar, K. Outcomes of Percutaneous Thermal Ablation for Biopsy-Proven T1a Renal Cell Carcinoma in Patients with Other Primary Malignancies. AJR Am. J. Roentgenol. 2021, 217, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.R.; Atwell, T.; Schmit, G.; Lohse, C.M.; Kurup, A.N.; Weisbrod, A. Oncologic Outcomes Following Partial Nephrectomy and Percutaneous Ablation for cT1 Renal Masses. Eur. Urol. 2019, 76, 244–251. [Google Scholar] [CrossRef]
- Talenfeld, A.D.; Gennarelli, R.L.; Elkin, E.B.; Atoria, C.L.; Durack, J.C.; Huang, W.C. Percutaneous Ablation Versus Partial and Radical Nephrectomy for T1a Renal Cancer: A Population-Based Analysis. Ann. Intern. Med. 2018, 169, 69–77. [Google Scholar] [CrossRef]
- Antonelli, A.; Ficarra, V.; Bertini, R.; Carini, M.; Carmignani, G.; Corti, S.; Longo, N.; Martorana, G.; Minervini, A.; Mirone, V.; et al. Elective partial nephrectomy is equivalent to radical nephrectomy in patients with clinical T1 renal cell carcinoma: Results of a retrospective, comparative, multi-institutional study. BJU Int. 2012, 109, 1013–1018. [Google Scholar] [CrossRef]
- Lane, B.R.; Campbell, S.C.; Gill, I.S. 10-year oncologic outcomes after laparoscopic and open partial nephrectomy. J. Urol. 2013, 190, 44–49. [Google Scholar] [CrossRef]
- Xing, M.; Kokabi, N.; Zhang, D.; Ludwig, J.M.; Kim, H.S. Comparative Effectiveness of Thermal Ablation, Surgical Resection, and Active Surveillance for T1a Renal Cell Carcinoma: A Surveillance, Epidemiology, and End Results (SEER)-Medicare-linked Population Study. Radiology 2018, 288, 81–90. [Google Scholar] [CrossRef] [PubMed]
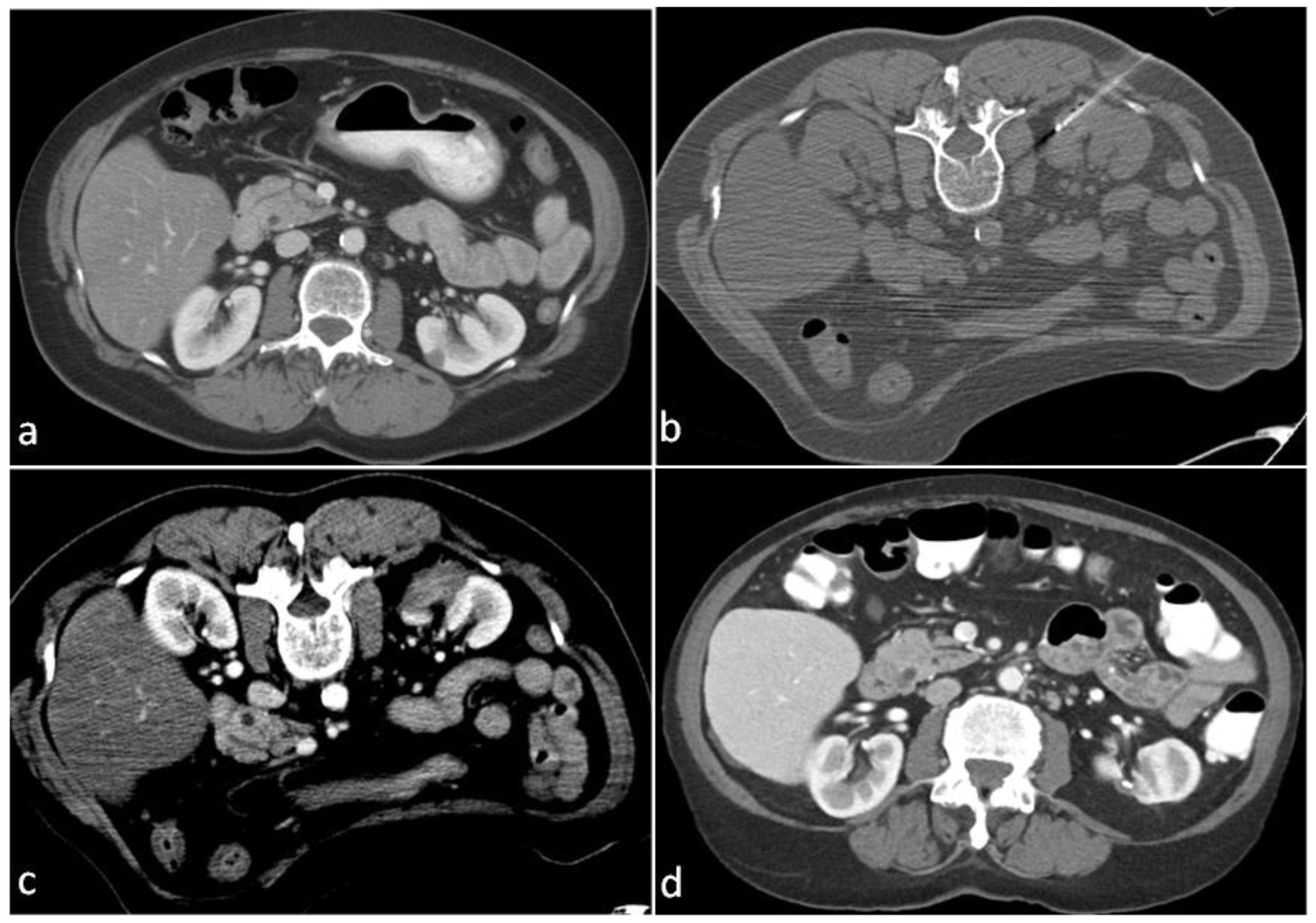

| Characteristic | n | % |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 67.0 (11.4) | |
| Median (range) | 67.7 (37.6–87.4) | |
| Gender | ||
| Female | 83 | 34.2 |
| Male | 160 | 65.8 |
| Another primary malignancy | ||
| Yes | 128 | 52.7 |
| No | 115 | 47.3 |
| Size of lesion (cm) | ||
| Mean (SD) | 2.4 (0.6) | |
| Median (range) | 2.5 (0.9–3.9) | |
| Affected kidney | ||
| Left | 103 | 42.4 |
| Right | 140 | 57.6 |
| Ablation modality | ||
| RFA | 255 | 100 |
| Guidance modality | ||
| CT | 255 | 100 |
| Pathology | ||
| RCC, chromophobe | 11 | 4.5 |
| RCC, clear cell | 175 | 72.0 |
| RCC, papillary | 45 | 18.5 |
| RCC, not otherwise specified | 5 | 2.1 |
| RCC, mucinous and tubular and spindle | 3 | 1.2 |
| RCC, clear cell papillary | 2 | 0.8 |
| RCC, papillary versus clear cell papillary | 1 | 0.4 |
| RCC, papillary versus mucinous and tubular | 1 | 0.4 |
| Grade | N = 204 | |
| 1 | 36 | 17.6 |
| 2 | 155 | 76 |
| 3 | 13 | 6.4 |
| Complication | Management |
|---|---|
| Subcapsular hematoma | Angiography and embolization |
| Hematuria, obstructing clot | Percutaneous nephroureteral catheter |
| Subcapsular hematoma | Angiography (no embolization) |
| Pneumothorax | Chest tube |
| Perirenal hematoma | Angiography (no embolization) |
| Bleeding, obstructing clot | Percutaneous nephroureteral catheter |
| Pneumothorax | Aspiration |
| Subcapsular hematoma | Angiography and embolization |
| Renal infection | Nephrectomy |
| Ureteral stricture | Ureteral stent |
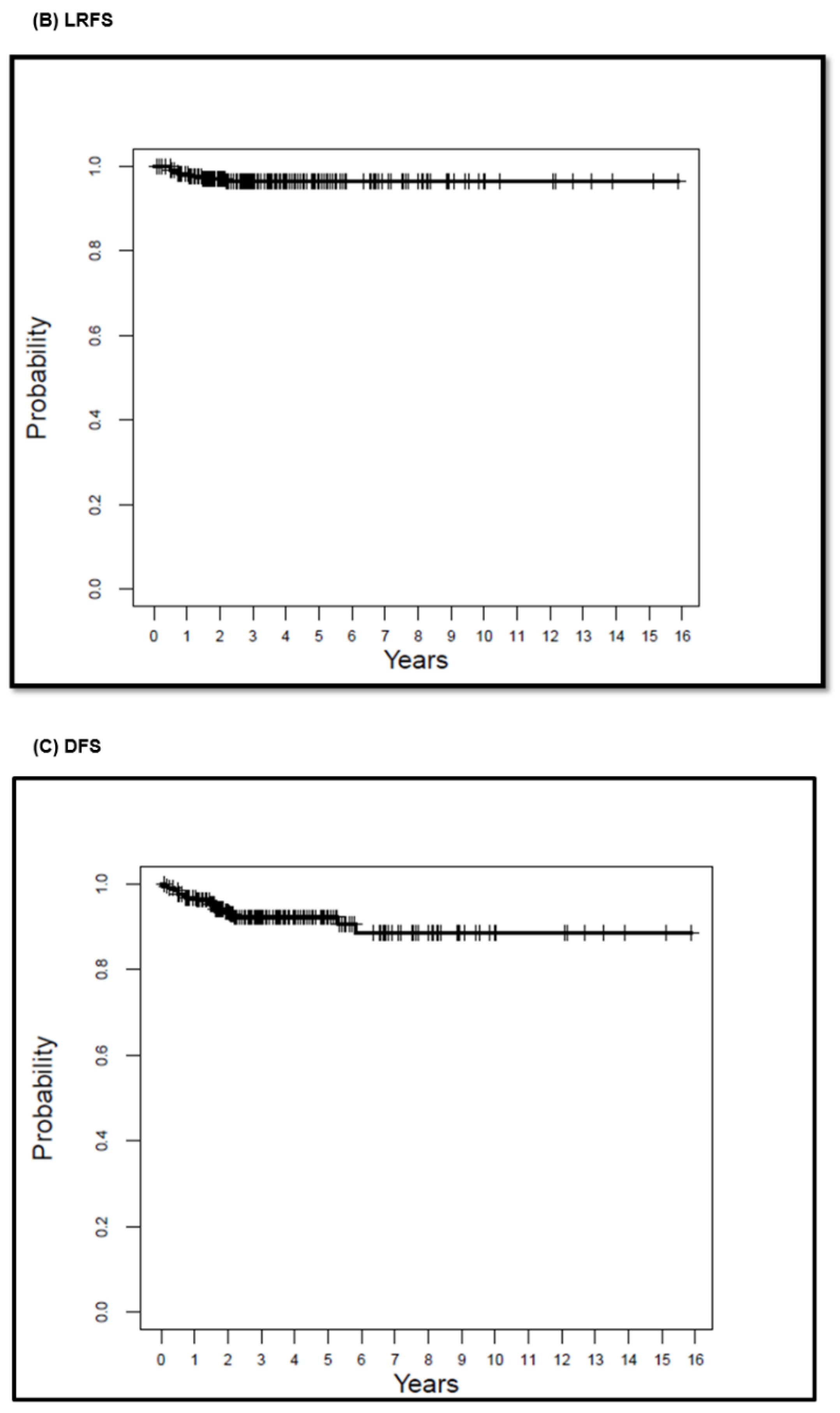
| Median (Years) | 5-Year Survival (%) (95% CI) | 10-Year Survival (%) (95% CI) | 15-Year Survival (%) (95% CI) | |
|---|---|---|---|---|
| OS | 8.88 | 74.7 (67.9–82.3) | 40.7 (30–55.3) | 15.1 (6.5–35) |
| LRFS | 8.39 | 96.5 (94–99.1) | 96.5 (94–99.1) | 96.5 (94–99.1) |
| DFS | 8.24 | 92.3 (88.6–96.2) | 88.6 (82.7–95) | 88.6 (82.7–95) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelsalam, M.E.; Awad, A.; Baiomy, A.; Irwin, D.; Karam, J.A.; Matin, S.F.; Sheth, R.A.; Habibollahi, P.; Odisio, B.C.; Lu, T.; et al. Outcomes of Radiofrequency Ablation for Solitary T1a Renal Cell Carcinoma: A 20-Year Tertiary Cancer Center Experience. Cancers 2023, 15, 909. https://doi.org/10.3390/cancers15030909
Abdelsalam ME, Awad A, Baiomy A, Irwin D, Karam JA, Matin SF, Sheth RA, Habibollahi P, Odisio BC, Lu T, et al. Outcomes of Radiofrequency Ablation for Solitary T1a Renal Cell Carcinoma: A 20-Year Tertiary Cancer Center Experience. Cancers. 2023; 15(3):909. https://doi.org/10.3390/cancers15030909
Chicago/Turabian StyleAbdelsalam, Mohamed E., Ahmed Awad, Ali Baiomy, David Irwin, Jose A. Karam, Surena F. Matin, Rahul A. Sheth, Peiman Habibollahi, Bruno C. Odisio, Thomas Lu, and et al. 2023. "Outcomes of Radiofrequency Ablation for Solitary T1a Renal Cell Carcinoma: A 20-Year Tertiary Cancer Center Experience" Cancers 15, no. 3: 909. https://doi.org/10.3390/cancers15030909
APA StyleAbdelsalam, M. E., Awad, A., Baiomy, A., Irwin, D., Karam, J. A., Matin, S. F., Sheth, R. A., Habibollahi, P., Odisio, B. C., Lu, T., & Ahrar, K. (2023). Outcomes of Radiofrequency Ablation for Solitary T1a Renal Cell Carcinoma: A 20-Year Tertiary Cancer Center Experience. Cancers, 15(3), 909. https://doi.org/10.3390/cancers15030909







