Combining sCD163 with CA 19-9 Increases the Predictiveness of Pancreatic Ductal Adenocarcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Definition of Covariates
2.3. Biological Samples
2.4. sCD163 ELISA
2.5. CA 19-9, CRP, IL-6, and YKL-40 Assays
2.6. Statistical Analysis
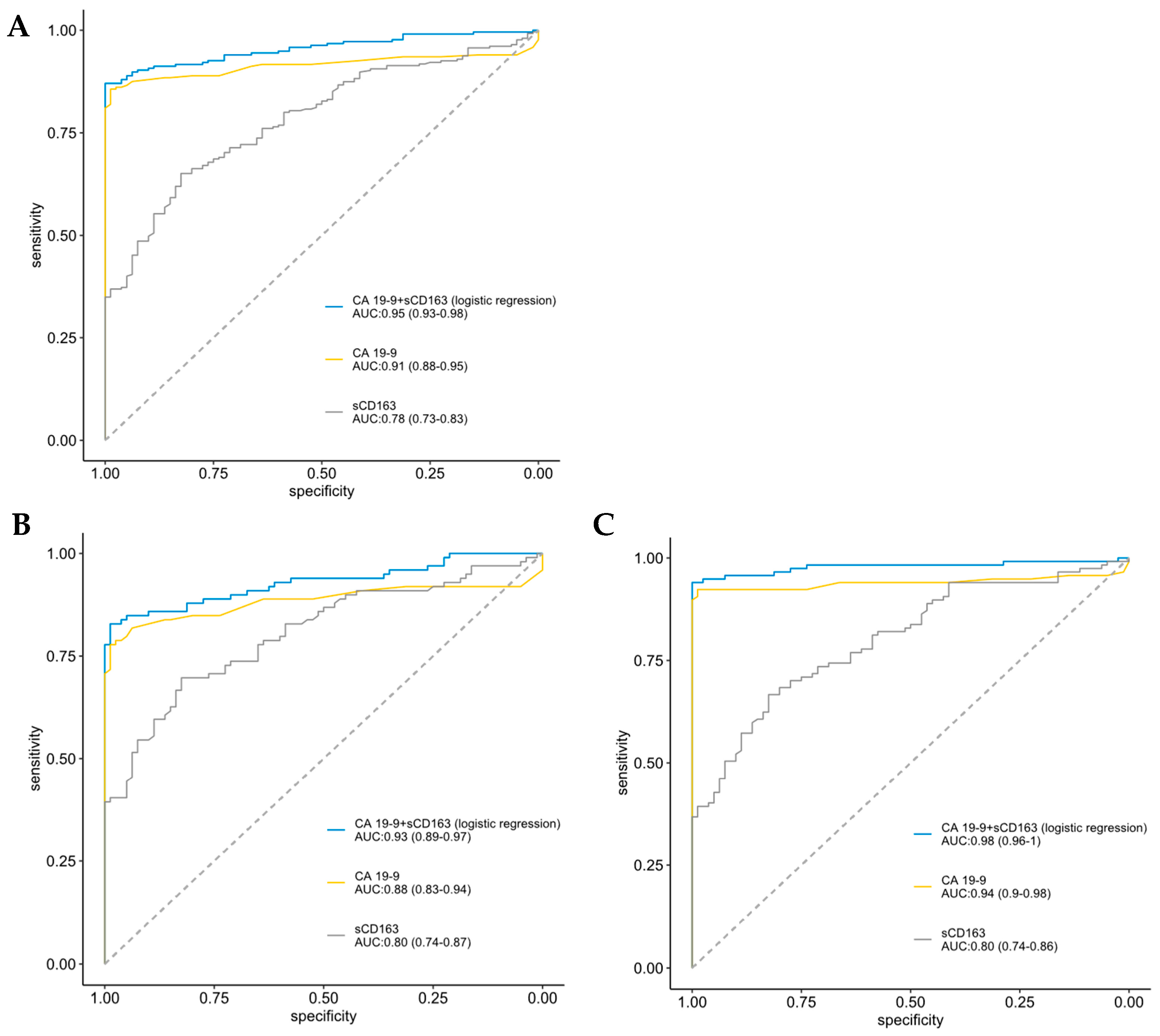
3. Results
3.1. Characteristics of Study Population
3.2. sCD163 According to Clinical and Tumor Characteristics
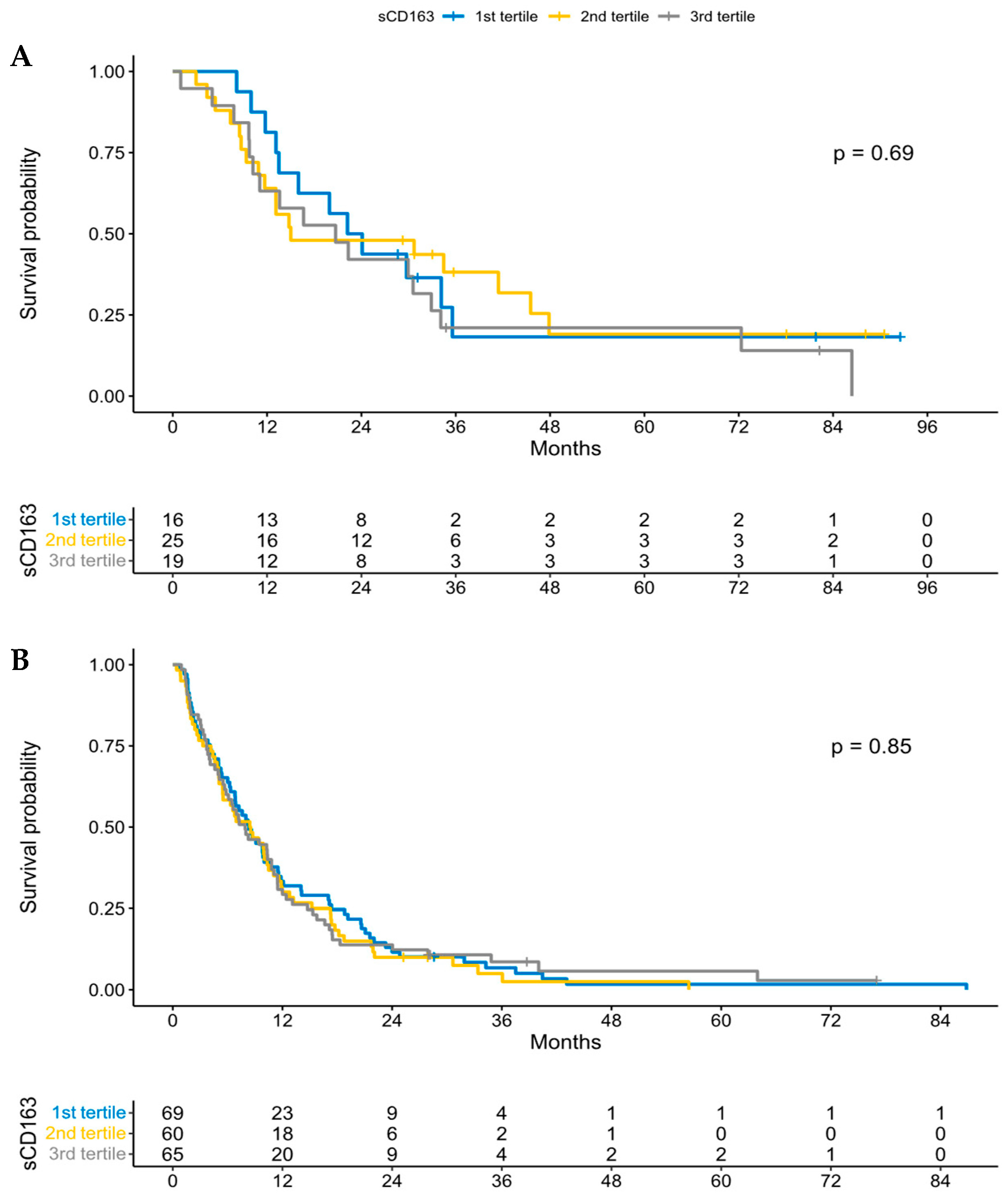
3.3. sCD163 and Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALAT | alanine aminotransferase |
| ALP | alkaline phosphatase |
| ASAT | aspartate aminotransferase |
| AUC | area under curve |
| BIOPAC | BIOmarkers in patients with PAncreatic Cancer |
| CA 19-9 | carbohydrate antigen 19-9 |
| CACI | age-adjusted Charlson comorbidity index |
| CCI | Charlson comorbidity index |
| CI | confidence interval |
| CRP | C-reactive protein |
| CSD | cancer-specific death |
| DFS | disease-free survival |
| ECOG | Eastern Cooperative Oncology Group |
| ELISA | enzyme-linked immunosorbent assay |
| HR | hazard ratio |
| IL-6 | interleukin-6 |
| IL-10 | interleukin-10 |
| LPS | Lipopolysaccharide |
| NR | not reported |
| OD | optical density |
| OS | overall survival |
| PC | pancreatic cancer |
| PDAC | pancreatic ductal adenocarcinoma |
| PFS | progression-free survival |
| PS | performance status |
| REMARK | reporting recommendations for tumor marker prognostic study |
| ROC | receiver operating characteristic |
| sCD163 | soluble CD163 |
| TBT | tumor biomarker test |
| TMA | tumor microarray |
| TME | tumor microenvironment |
| TAMs | tumor-associated macrophages |
| YKL-40 | chitinase 3-like 1 protein (CHI3L1) |
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open 2021, 4, e214708. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef] [PubMed]
- Diaz, C.L.; Cinar, P.; Hwang, J.; Ko, A.H.; Tempero, M.A. CA 19-9 Response: A Surrogate to Predict Survival in Patients with Metastatic Pancreatic Adenocarcinoma. Am. J. Clin. Oncol. 2019, 42, 898–902. [Google Scholar] [CrossRef]
- Chen, I.M.; Johansen, A.Z.; Dehlendorff, C.; Jensen, B.V.; Bojesen, S.E.; Pfeiffer, P.; Bjerregaard, J.K.; Nielsen, S.E.; Andersen, F.; Holländer, N.H.; et al. Prognostic Value of Combined Detection of Serum IL6, YKL-40, and C-reactive Protein in Patients with Unresectable Pancreatic Cancer. Cancer Epidemiol. Biomark. Prev. 2020, 29, 176–184. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Kurahara, H.; Shinchi, H.; Mataki, Y.; Maemura, K.; Noma, H.; Kubo, F.; Sakoda, M.; Ueno, S.; Natsugoe, S.; Takao, S. Significance of M2-Polarized Tumor-Associated Macrophage in Pancreatic Cancer. J. Surg. Res. 2009, 167, e211–e219. [Google Scholar] [CrossRef]
- Moestrup, S.K.; Møller, H.J. CD163: A regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann. Med. 2004, 36, 347–354. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polar-ized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- McGuigan, A.J.; Coleman, H.G.; McCain, R.S.; Kelly, P.J.; Johnston, D.I.; Taylor, M.A.; Turkington, R.C. Immune cell infiltrates as prognostic biomarkers in pancreatic ductal adeno-carcinoma: A systematic review and meta-analysis. J. Pathol. Clin. Res. 2021, 7, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, E.S.; Vail, P.; Balaji, U.; Ngo, H.; Botros, I.W.; Makarov, V.; Riaz, N.; Balachandran, V.; Leach, S.; Thompson, D.M.; et al. Stratification of Pancreatic Ductal Adenocarcinoma: Combinatorial Genetic, Stromal, and Immunologic Markers. Clin. Cancer Res. 2017, 23, 4429–4440. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, Q.; Zeng, L.; Lian, G.; Li, J.; Qian, C.; Chen, Y.; Huang, K. Distribution and Clinical Significance of Tumour-Associated Macrophages in Pancreatic Ductal Adenocarcinoma: A Retrospective Analysis in China. Curr. Oncol. 2015, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, J.; Balaji, U.; Porembka, M.R.; Wachsmann, M.B.; McCue, P.A.; Knudsen, E.S.; Witkiewicz, A.K. Immunologic and Metabolic Features of Pancreatic Ductal Adenocarcinoma Define Prognostic Subtypes of Disease. Clin. Cancer Res. 2016, 22, 3606–3617. [Google Scholar] [CrossRef]
- Shi, B.; Chu, J.; Huang, T.; Wang, X.; Li, Q.; Gao, Q.; Xia, Q.; Luo, S. The Scavenger Receptor MARCO Expressed by Tumor-Associated Macrophages Are Highly Associated With Poor Pancreatic Cancer Prognosis. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Kridel, R.; Xerri, L.; Gelas-Dore, B.; Tan, K.; Feugier, P.; Vawda, A.; Canioni, D.; Farinha, P.; Boussetta, S.; Moccia, A.A.; et al. The Prognostic Impact of CD163-Positive Macrophages in Follicular Lymphoma: A Study from the BC Cancer Agency and the Lymphoma Study Association. Clin. Cancer Res. 2015, 21, 3428–3435. [Google Scholar] [CrossRef]
- Jamiyan, T.; Kuroda, H.; Yamaguchi, R.; Abe, A.; Hayashi, M. CD68- and CD163-positive tumor-associated macrophages in triple negative cancer of the breast. Virchows Arch. 2020, 477, 767–775. [Google Scholar] [CrossRef]
- Minami, K.; Hiwatashi, K.; Ueno, S.; Sakoda, M.; Iino, S.; Okumura, H.; Hashiguchi, M.; Kawasaki, Y.; Kurahara, H.; Mataki, Y.; et al. Prognostic significance of CD68, CD163 and Folate receptor-β positive macrophages in hepatocellular carcinoma. Exp. Ther. Med. 2018, 15, 4465–4476. [Google Scholar] [CrossRef]
- Møller, H.J.; Peterslund, N.A.; Graversen, J.H.; Moestrup, S.K. Identification of the hemoglobin scavenger receptor/CD163 as a natural soluble protein in plasma. Blood 2002, 99, 378–380. [Google Scholar] [CrossRef]
- Andersen, M.N.; Abildgaard, N.; Maniecki, M.B.; Møller, H.J.; Andersen, N.F. Monocyte/macrophage-derived soluble CD163: A novel biomarker in multiple myeloma. Eur. J. Haematol. 2014, 93, 41–47. [Google Scholar] [CrossRef]
- Ding, D.; Song, Y.; Yao, Y.; Zhang, S. Preoperative serum macrophage activated biomarkers soluble mannose receptor (sMR) and soluble haemoglobin scavenger receptor (sCD163), as novel markers for the diagnosis and prognosis of gastric cancer. Oncol. Lett. 2017, 14, 2982–2990. [Google Scholar] [CrossRef] [PubMed]
- Krijgsman, D.; De Vries, N.L.; Andersen, M.N.; Skovbo, A.; Tollenaar, R.A.; Møller, H.J.; Hokland, M.; Kuppen, P.J. CD163 as a Biomarker in Colorectal Cancer: The Expression on Circulating Monocytes and Tumor-Associated Macrophages, and the Soluble Form in the Blood. Int. J. Mol. Sci. 2020, 21, 5925. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.O.; Schmidt, H.; Møller, H.J.; Høyer, M.; Maniecki, M.B.; Sjoegren, P.; Christensen, I.J.; Steiniche, T. Macrophage Markers in Serum and Tumor Have Prognostic Impact in American Joint Committee on Cancer Stage I/II Melanoma. J. Clin. Oncol. 2009, 27, 3330–3337. [Google Scholar] [CrossRef] [PubMed]
- Kazankov, K.; Rode, A.; Simonsen, K.; Villadsen, G.E.; Nicoll, A.; Møller, H.J.; Lim, L.; Angus, P.; Kronborg, I.; Arachchi, N.; et al. Macrophage activation marker soluble CD163 may predict disease progression in hepatocellular carcinoma. Scand. J. Clin. Lab. Investig. 2015, 76, 64–73. [Google Scholar] [CrossRef]
- Waidmann, O.; Köberle, V.; Bettinger, D.; Trojan, J.; Zeuzem, S.; Schultheiß, M.; Kronenberger, B.; Piiper, A. Diagnostic and prognostic significance of cell death and macrophage activation markers in patients with hepatocellular carcinoma. J. Hepatol. 2013, 59, 769–779. [Google Scholar] [CrossRef] [PubMed]
- No, J.H.; Moon, J.M.; Kim, K.; Kim, Y.-B. Prognostic Significance of Serum Soluble CD163 Level in Patients with Epithelial Ovarian Cancer. Gynecol. Obstet. Investig. 2013, 75, 263–267. [Google Scholar] [CrossRef]
- Kanakry, J.A.; Hong, F.; Martinez-Maza, O.; Horning, S.J.; Gordon, L.I.; Gascoyne, R.D.; Gellert, L.L.; Lemas, M.V.; Cheson, B.; Advani, R.H.; et al. Serum Biomarkers Predict Outcomes in Advanced Hodgkin Lymphoma In-dependent of International Prognostic Score (IPS) and Treatment: Correlative Analysis from a Large North American Co-operative Group Trial. Blood 2016, 128, 2992. [Google Scholar] [CrossRef]
- Nederby, L.; Roug, A.S.; Knudsen, S.S.; Skovbo, A.; Kjeldsen, E.; Møller, H.J.; Hokland, M. Soluble CD163 as a prognostic biomarker in B-cell chronic lymphocytic leukemia. Leuk. Lymphoma 2015, 56, 3219–3221. [Google Scholar] [CrossRef]
- Vajavaara, H.; Ekeblad, F.; Holte, H.; Jorgensen, J.; Leivonen, S.-K.; Berglund, M.; Kamper, P.; Moller, H.J.; D’Amore, F.; Molin, D.; et al. Prognostic impact of soluble CD163 in patients with diffuse large B-cell lymphoma. Haematologica 2021, 106, 2502–2506. [Google Scholar] [CrossRef]
- Davidsson, S.; Huotilainen, S.; Carlsson, J.; Sundqvist, P. Soluble Levels of CD163, PD-L1, and IL-10 in Renal Cell Carcinoma Patients. Diagnostics 2022, 12, 336. [Google Scholar] [CrossRef]
- Qian, S.; Zhang, H.; Dai, H.; Ma, B.; Tian, F.; Jiang, P.; Gao, H.; Sha, X.; Sun, X. Is sCD163 a clinical significant prognostic value in cancers? A systematic review and me-ta-analysis. Front Oncol. 2020, 10, 585297. [Google Scholar] [CrossRef] [PubMed]
- The BIOPAC Study. Available online: https://www.herlevhospital.dk/BIOPAC/aboutbiopac/Sider/Ressources.aspx (accessed on 29 May 2022).
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: De-velopment and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.B.; Klee, M.R.; Palshof, T.; Hansen, H.H. Performance status assessment in cancer patients. An inter-observer variability study. Br. J. Cancer 1993, 67, 773–775. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Sauerbrei, W.; Taube, S.E.; McShane, L.M.; Cavenagh, M.M.; Altman, D.G. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): An Abridged Explanation and Elaboration. J. Natl. Cancer Inst. 2018, 110, 803–811. [Google Scholar] [CrossRef]
- Sun, X.; He, X.; Zhang, Y.; Hosaka, K.; Andersson, P.; Wu, J.; Wu, J.; Jing, X.; Du, Q.; Hui, X.; et al. Inflammatory cell-derived CXCL3 promotes pancreatic cancer metastasis through a novel my-ofibroblast-hijacked cancer escape mechanism. Gut 2022, 71, 129–147. [Google Scholar] [CrossRef]
- Weaver, L.K.; Pioli, P.A.; Wardwell, K.; Vogel, S.N.; Guyre, P.M. Up-regulation of human monocyte CD163 upon activation of cell-surface Toll-like receptors. J. Leukoc. Biol. 2006, 81, 663–671. [Google Scholar] [CrossRef]
- Lindgaard, S.C.; Sztupinszki, Z.; Maag, E.; Chen, I.M.; Johansen, A.Z.; Jensen, B.V.; Bojesen, S.E.; Nielsen, D.L.; Hansen, C.P.; Hasselby, J.P.; et al. Circulating Protein Biomarkers for Use in Pancreatic Ductal Adenocarcinoma Identification. Clin. Cancer Res. 2021, 27, 2592–2603. [Google Scholar] [CrossRef]
- Yu, J.; Ploner, A.; Kordes, M.; Löhr, M.; Nilsson, M.; Maturana, M.E.L.; Estudillo, L.; Renz, H.; Carrato, A.; Molero, X.; et al. Plasma protein biomarkers for early detection of pancreatic ductal adenocarcinoma. Int. J. Cancer 2021, 148, 2048–2058. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Yu, J.; Li, Z.; Gao, S.; Wang, H.; Yang, S.; Wu, L.; Lan, C.; Zhao, T.; Gao, C.; et al. Serum insulin-like growth factor binding protein 2 levels as a biomarker for pancreatic ductal ade-nocarcinoma-associated malnutrition and muscle wasting. J. Cachexia Sarcopenia Muscle 2021, 12, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Radhakrishnan, P. Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol. Cancer 2019, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Lindgaard, S.C.; Maag, E.; Sztupinszki, Z.; Chen, I.M.; Johansen, A.Z.; Jensen, B.V.; Bojesen, S.E.; Nielsen, D.L.; Szallasi, Z.; Johansen, J.S. Circulating Protein Biomarkers for Prognostic Use in Patients with Advanced Pancreatic Ductal Adenocarcinoma Undergoing Chemotherapy. Cancers 2022, 14, 3250. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.F. Defining Clinical Utility of Tumor Biomarker Tests: A Clinician’s Viewpoint. J. Clin. Oncol. 2021, 39, 238–248. [Google Scholar] [CrossRef]

| Pancreatic Ductal Adenocarcinoma | N = 255 (%) | sCD163 Median (Range) | p-Value |
|---|---|---|---|
| Age | 0.013 | ||
| <50 years | 14 (5.5) | 886 (441–3407) | |
| 50–70 years | 140 (54.9) | 1046 (350–4196) | |
| >70 years | 101 (39.6) | 1135 (368–5568) | |
| Sex | 0.018 | ||
| Male | 134 (52.5) | 982 (368–3587) | |
| Female | 121 (47.5) | 1157 (350–5568) | |
| Performance status | 0.287 | ||
| 0 | 92 (36.1) | 1108 (441–4196) | |
| 1 | 146 (57.3) | 1024 (350–5568) | |
| ≥2 | 17 (6.7) | 1062 (466–2763) | |
| Diabetes | 0.800 | ||
| Yes | 70 (27.5) | 1059 (350–3802) | |
| No | 185 (72.5) | 1058 (394–5568) | |
| CACI | 0.062 | ||
| 0–1 | 31 (12.2) | 834 (441–3407) | |
| 2–3 | 119 (46.7) | 1126 (419–4196) | |
| ≥4 | 69 (27.1) | 1135 (350–5568) | |
| BMI | 0.790 | ||
| <18.5 | 16 (6.4) | 1036 (394–2872) | |
| 18.5–25 | 135 (54.2) | 1060 (368–5568) | |
| >25 | 98 (39.4) | 1071 (350–4756) | |
| Cachexia | 0.042 | ||
| Yes | 135 (52.9) | 1188 (368–5568) | |
| No | 73 (28.6) | 1037 (350–4196) | |
| Smoking status | 0.243 | ||
| Currently/Previously | 159 (62.4) | 1017 (368–4756) | |
| No | 91 (35.7) | 1084 (350–5568) | |
| Alcohol status | 0.618 | ||
| Abuse/Previous abuse | 60 (23.5) | 1060 (466–3802) | |
| No abuse | 189 (74.1) | 1057 (350–5568) | |
| Stent | 0.010 | ||
| Yes | 88 (34.5) | 1196 (441–5568) | |
| No | 167 (65.5) | 1011 (350–4756) | |
| Stage | 0.319 | ||
| 1 + 2 | 60 | 1138 (419–4196) | |
| 3 + 4 | 195 | 1042 (350–5568) | |
| Tumor size | 0.808 | ||
| >median (3.5 cm) | 120 (47.1) | 1061 (388–4756) | |
| ≤median (3.5 cm) | 122 (47.8) | 1047 (350–5568) | |
| Tumor location | 0.341 | ||
| Caput | 151 (59.2) | 1080 (368–5568) | |
| Corpus | 50 (19.6) | 975 (350–3725) | |
| Cauda | 42 (16.5) | 1192 (531–3043) | |
| Diffuse | 9 (3.5) | 1120 (760–2975) | |
| Papillary | 2 (0.8) | 847 (637–1057) | |
| Metastatic sites | 0.515 | ||
| None | 102 (40.0) | 1126 (368–5568) | |
| Liver Only | 81 (31.8) | 1014 (350–4756) | |
| Liver + Lung | 19 (7.5) | 1105 (637–2528) | |
| Liver + Carcinosis | 16 (6.3) | 1006 (394–3094) | |
| Other | 37 (14.5) | 1018 (388–2763) | |
| Number of metastatic sites | 0.352 | ||
| 0 | 102 (40.0) | 1126 (368–5568) | |
| 1 | 99 (38.8) | 1042 (350–4756) | |
| ≥2 | 54 (21.2) | 1014 (388–3094) |
| Number of Observations | sCD163 Spearman’s (95% CI) | p-Value | |
|---|---|---|---|
| CA19-9 | 216 | 0.03 (−0.10–0.17) | 0.616 |
| YKL-40 | 253 | 0.23 (0.11–0.34) | 0.0002 |
| IL-6 | 253 | 0.20 (0.08–0.32) | 0.001 |
| CRP | 183 | 0.11 (−0.04–0.25) | 0.142 |
| ALP | 168 | 0.20 (0.05–0.34) | 0.008 |
| Bilirubin | 188 | 0.28 (0.14–0.40) | 0.0001 |
| ALAT | 183 | 0.23 (0.09–0.37) | 0.002 |
| ASAT | 91 | −0.03 (−0.23–0.18) | 0.780 |
| Platelets | 189 | 0.17 (0.03–0.31) | 0.019 |
| Leucocytes | 123 | 0.01 (−0.16–0.19) | 0.875 |
| Neutrophils | 95 | −0.01 (−0.21–0.20) | 0.950 |
| Variable | Observations (Events) | HR (95% CI) | p-Value |
|---|---|---|---|
| sCD163 | 60 (47) | 1.19 (0.81–1.76) | 0.373 |
| CRP | 42 (32) | 1.06 (0.90–1.24) | 0.479 |
| CA19-9 | 59 (46) | 1.09 (0.99–1.20) | 0.086 |
| IL-6 | 59 (47) | 1.07 (0.88–1.31) | 0.490 |
| YKL-40 | 59 (47) | 1.20 (0.98–1.48) | 0.080 |
| PS | 60 (47) | ||
| 0 | 26 (19) | Reference | |
| 1 | 32 (26) | 1.25 (0.69–2.26) | 0.463 |
| ≥2 | 2 (2) | 2.36 (0.54–10.30) | 0.252 |
| Age | 60 (47) | ||
| ≤70 years | 39 (31) | Reference | |
| >70 years | 21 (16) | 1.39 (0.74–2.59) | 0.304 |
| Sex | |||
| Male | 29 (24) | Reference | |
| Female | 31 (23) | 0.79 (0.45–1.41) | 0.432 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | Observations (Events) | HR (95% CI) | p-Value | Observations (Events) | HR (95% CI) | p-Value |
| sCD163 | 194 (187) | 0.99 (0.83–1.19) | 0.926 | 139 (133) | 0.92 (0.72–1.18) | 0.518 |
| CRP | 140 (134) | 1.28 (1.17–1.41) | <0.0001 | 139 (133) | 1.17 (1.04–1.31) | 0.011 |
| CA19-9 | 157 (150) | 1.12 (1.07–1.18) | <0.0001 | 139 (133) | 1.12 (1.06–1.19) | <0.0001 |
| IL-6 | 194 (187) | 1.17 (1.09–1.25) | <0.0001 | 139 (133) | 1.01 (0.90–1.13) | 0.863 |
| YKL-40 | 194 (187) | 1.42 (1.24–1.64) | <0.0001 | 139 (133) | 1.22 (1.01–1.53) | 0.037 |
| PS | 194 (187) | 139 (133) | ||||
| 0 | 66 (62) | Reference | 56 (52) | Reference | ||
| 1 | 113 (111) | 1.62 (1.18–2.22) | 0.003 | 74 (73) | 1.24 (0.85–1.81) | 0.256 |
| ≥2 | 15 (14) | 2.73 (1.51–4.92) | 0.0009 | 9 (8) | 2.63 (1.23–5.88) | 0.013 |
| Age | 194 (187) | 139 (133) | ||||
| ≤70 | 115 (110) | Reference | 85 (81) | Reference | ||
| >70 | 79 (77) | 1.74 (1.29–2.35) | 0.0003 | 54 (52) | 2.08 (1.39–3.10) | 0.0004 |
| Sex | 194 (187) | 139 (133) | ||||
| Male | 104 (102) | Reference | 72 (70) | Reference | ||
| Female | 90 (85) | 1.02 (0.76–1.36) | 0.909 | 67 (63) | 1.094 (0.75–1.59) | 0.639 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuhr, L.K.; Madsen, K.; Johansen, A.Z.; Chen, I.M.; Hansen, C.P.; Jensen, L.H.; Hansen, T.F.; Kløve-Mogensen, K.; Nielsen, K.R.; Johansen, J.S. Combining sCD163 with CA 19-9 Increases the Predictiveness of Pancreatic Ductal Adenocarcinoma. Cancers 2023, 15, 897. https://doi.org/10.3390/cancers15030897
Stuhr LK, Madsen K, Johansen AZ, Chen IM, Hansen CP, Jensen LH, Hansen TF, Kløve-Mogensen K, Nielsen KR, Johansen JS. Combining sCD163 with CA 19-9 Increases the Predictiveness of Pancreatic Ductal Adenocarcinoma. Cancers. 2023; 15(3):897. https://doi.org/10.3390/cancers15030897
Chicago/Turabian StyleStuhr, Liva K., Kasper Madsen, Astrid Z. Johansen, Inna M. Chen, Carsten P. Hansen, Lars H. Jensen, Torben F. Hansen, Kirstine Kløve-Mogensen, Kaspar R. Nielsen, and Julia S. Johansen. 2023. "Combining sCD163 with CA 19-9 Increases the Predictiveness of Pancreatic Ductal Adenocarcinoma" Cancers 15, no. 3: 897. https://doi.org/10.3390/cancers15030897
APA StyleStuhr, L. K., Madsen, K., Johansen, A. Z., Chen, I. M., Hansen, C. P., Jensen, L. H., Hansen, T. F., Kløve-Mogensen, K., Nielsen, K. R., & Johansen, J. S. (2023). Combining sCD163 with CA 19-9 Increases the Predictiveness of Pancreatic Ductal Adenocarcinoma. Cancers, 15(3), 897. https://doi.org/10.3390/cancers15030897








