Urinary Eubacterium sp. CAG:581 Promotes Non-Muscle Invasive Bladder Cancer (NMIBC) Development through the ECM1/MMP9 Pathway
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Cohort Designs and Specimens
2.2. Metagenome Sequencing
2.3. NMIBC Organoids Were Cocultured with Urinary Bacterium in the 2-Chamber Culture System
2.4. RNA-seq and Data Processing
2.5. Western Blotting
2.6. Quantitative Real-Time PCR
2.7. Adenoviral shRNA Infection of NMIBC Organoids
2.8. Statistical Analysis
3. Results
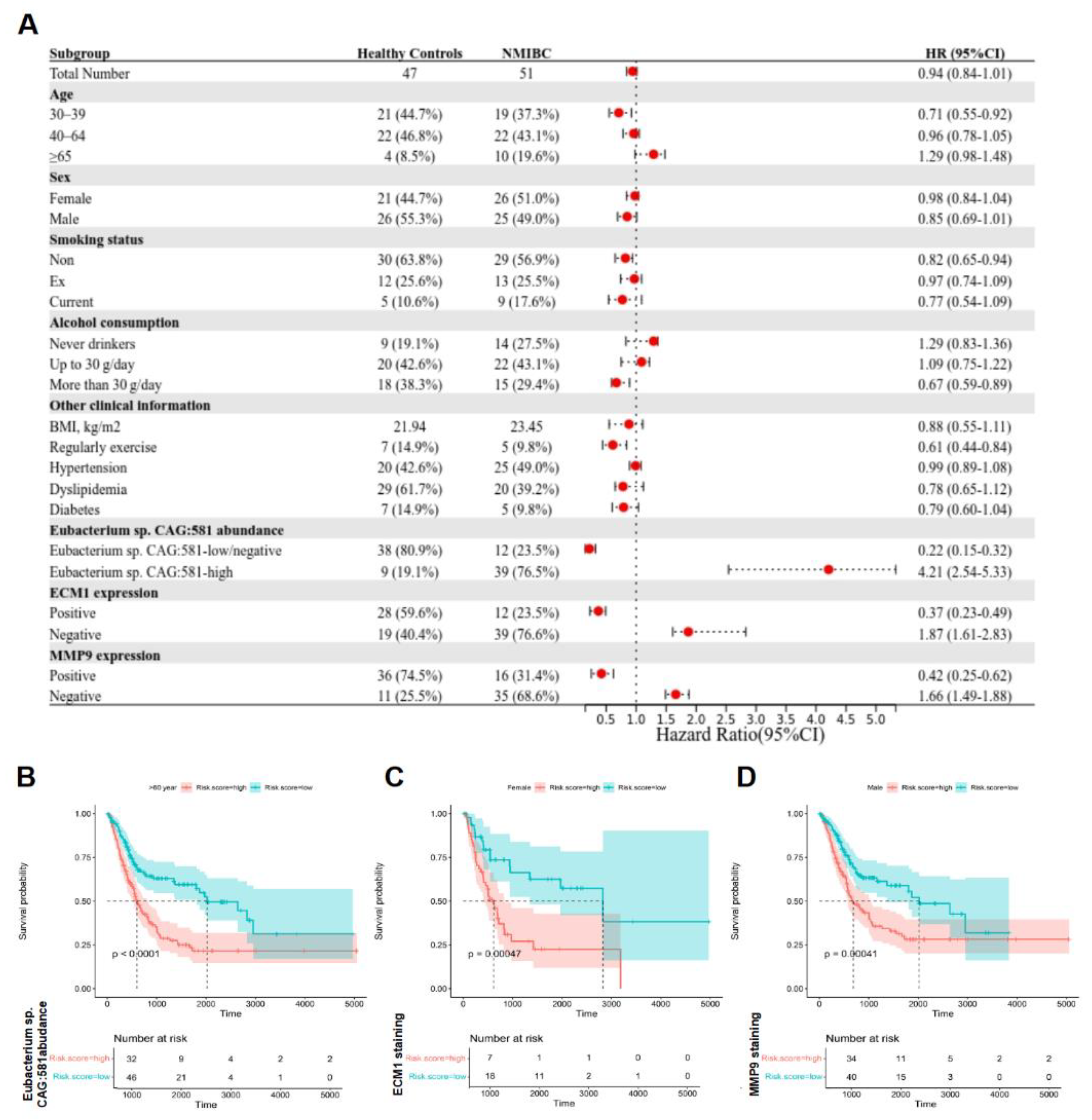
3.1. Eubacterium sp. CAG:581 Is Clinically Associated with NMIBC Occurrence
3.2. Coculture of Eubacterium sp. CAG:581 Promoted the Growth of NMIBC Organoids
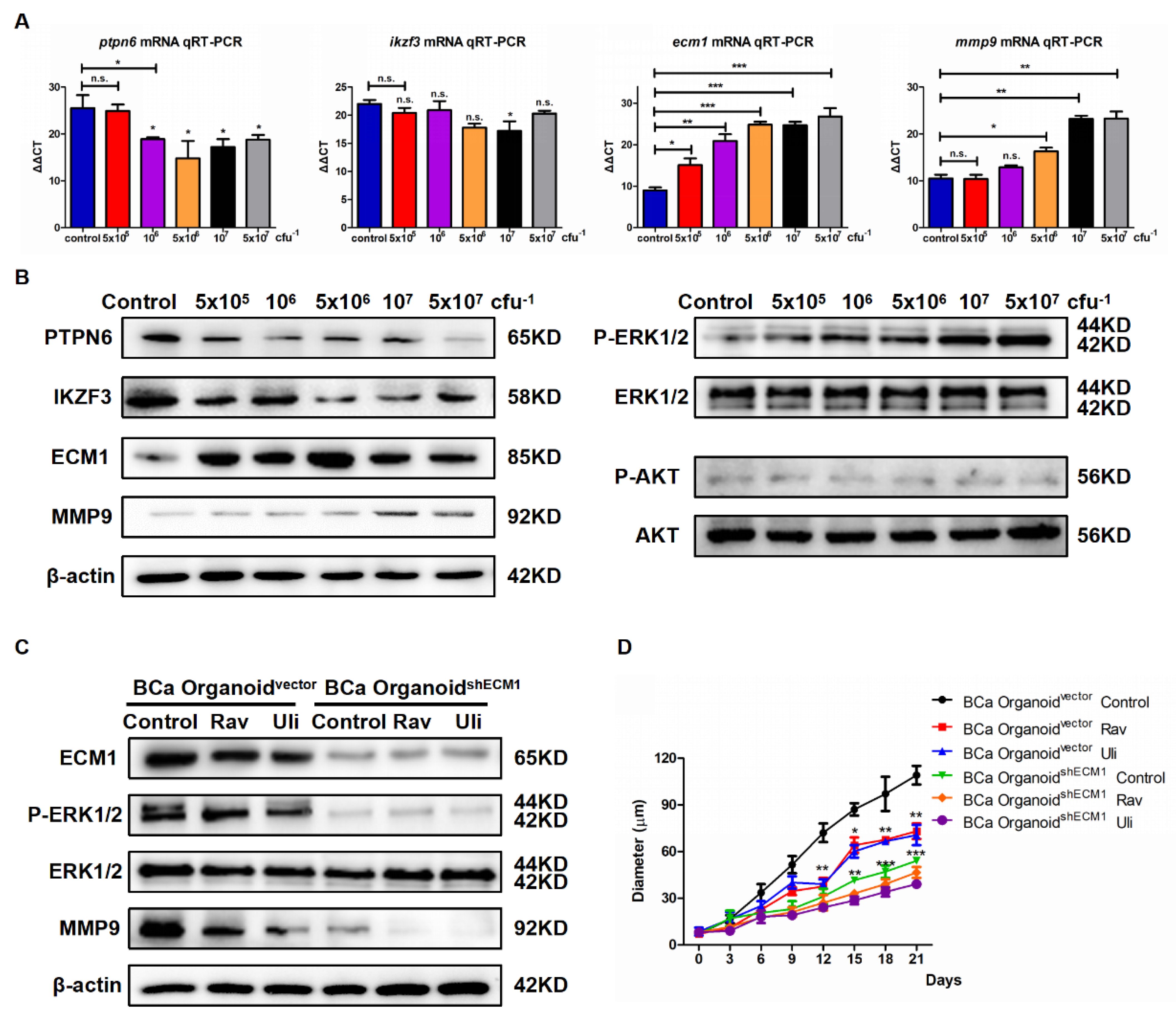
3.3. Eubacterium sp. CAG:581 Activated ECM1/ERK1/2 Phosphorylation/MMP9 of NMIBC Organoids
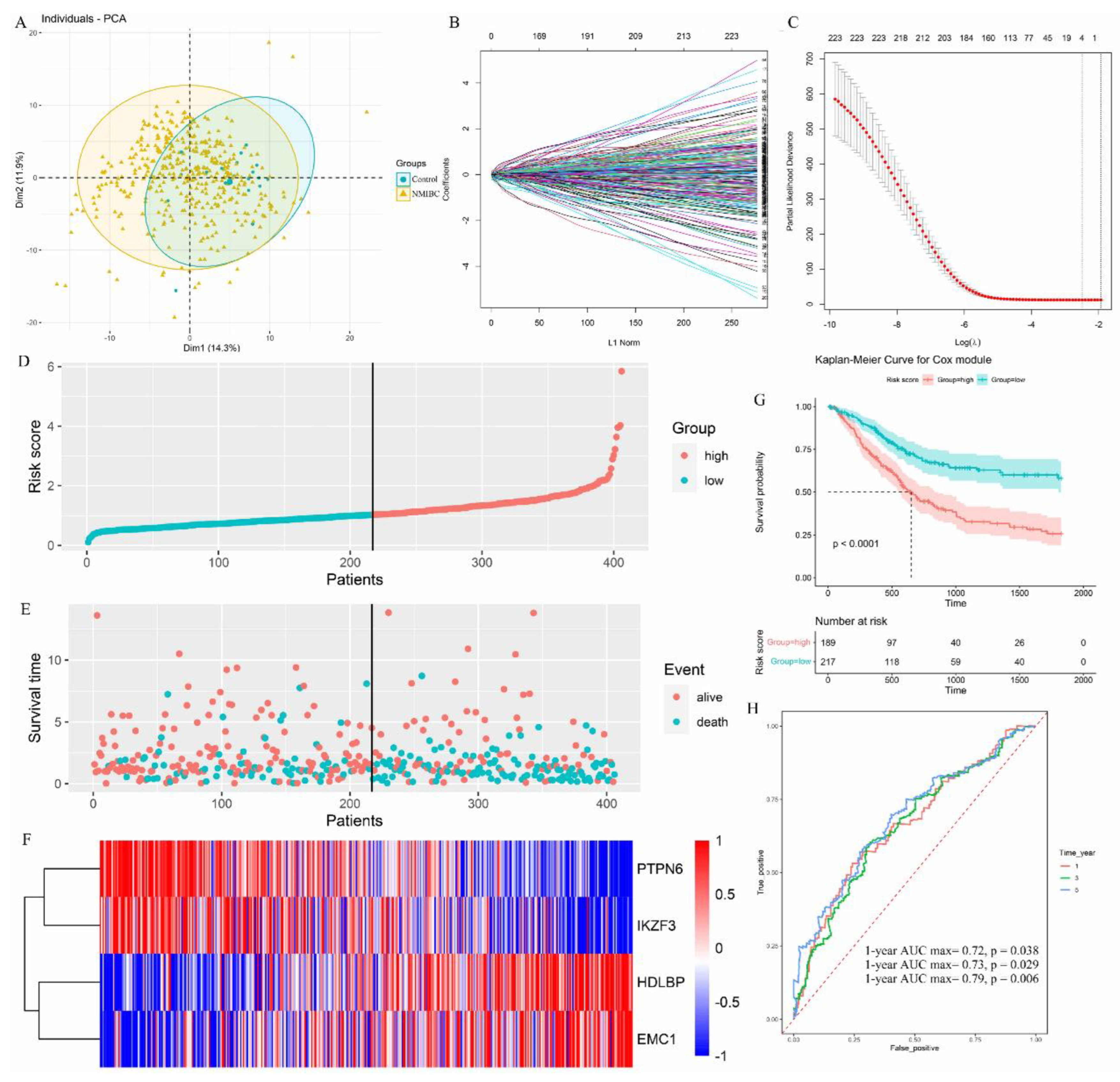
3.4. Eubacterium sp. CAG:581 Was Endowed with the Diagnostic Predictor for NMIBC
3.5. Identification of NMIBC Occurrence-Associated Eubacterium sp. CAG:581 in the Larger Population
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Comperat, E.; Amin, M.B.; Cathomas, R.; Choudhury, A.; De Santis, M.; Kamat, A.; Stenzl, A.; Thoeny, H.C.; Witjes, J.A. Current best practice for bladder cancer: A narrative review of diagnostics and treatments. Lancet 2022, 400, 1712–1721. [Google Scholar] [CrossRef]
- Lindskrog, S.V.; Prip, F.; Lamy, P.; Taber, A.; Groeneveld, C.S.; Birkenkamp-Demtroder, K.; Jensen, J.B.; Strandgaard, T.; Nordentoft, I.; Christensen, E.; et al. An integrated multi-omics analysis identifies prognostic molecular subtypes of non-muscle-invasive bladder cancer. Nat. Commun. 2021, 12, 2301. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Sanli, O.; Dobruch, J.; Knowles, M.A.; Burger, M.; Alemozaffar, M.; Nielsen, M.E.; Lotan, Y. Bladder cancer. Nat. Rev. Dis. Primers 2017, 3, 17022. [Google Scholar] [CrossRef]
- Jobczyk, M.; Stawiski, K.; Fendler, W.; Rozanski, W. Validation of EORTC, CUETO, and EAU risk stratification in prediction of recurrence, progression, and death of patients with initially non-muscle-invasive bladder cancer (NMIBC): A cohort analysis. Cancer Med. 2020, 9, 4014–4025. [Google Scholar] [CrossRef] [PubMed]
- Ba, M.; Cui, S.; Wang, B.; Long, H.; Yan, Z.; Wang, S.; Wu, Y.; Gong, Y. Bladder intracavitary hyperthermic perfusion chemotherapy for the prevention of recurrence of non-muscle invasive bladder cancer after transurethral resection. Oncol. Rep. 2017, 37, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Comperat, E.M.; Dominguez, E.J.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European association of urology guidelines on non-muscle-invasive bladder cancer (Ta, t1, and carcinoma in situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Cheng, X.; Liu, Q.; Luo, W.; Liu, M.; Zhang, M.; Miao, J.; Ji, Z.; Lin, G.N.; Song, W.; et al. Single-cell RNA sequencing reveals the epithelial cell heterogeneity and invasive subpopulation in human bladder cancer. Int. J. Cancer 2021, 149, 2099–2115. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, R.; Zhang, S.; Zhao, P.; Li, G.; Wu, L.; He, J. Report of incidence and mortality in China cancer registries, 2009. Chin. J. Cancer Res. 2013, 25, 10–21. [Google Scholar]
- Knowles, M.A.; Hurst, C.D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 2015, 15, 25–41. [Google Scholar] [CrossRef]
- Andolfi, C.; Bloodworth, J.C.; Papachristos, A.; Sweis, R.F. The urinary microbiome and bladder cancer: Susceptibility and immune responsiveness. Bladder Cancer 2020, 6, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, A.; Lu, X.; Zhang, Z.; Xue, Y.; Xu, J.; Zeng, S.; Xiong, Q.; Tan, H.; He, X.; et al. Dysbiosis signatures of the microbial profile in tissue from bladder cancer. Cancer Med. 2019, 8, 6904–6914. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, Q.; Li, A.; Huang, W.; Cai, Z.; Chen, W. Extracellular matrix protein 1 (ECM1) is associated with carcinogenesis potential of human bladder cancer. OncoTargets Ther. 2019, 12, 1423–1432. [Google Scholar] [CrossRef]
- Wang, J.; Guo, M.; Zhou, X.; Ding, Z.; Chen, X.; Jiao, Y.; Ying, W.; Wu, S.; Zhang, X.; Geng, N. Angiogenesis related gene expression significantly associated with the prognostic role of an urothelial bladder carcinoma. Transl. Androl. Urol. 2020, 9, 2200–2210. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Yuan, L.; Ma, B.; Wang, G.; Qiu, W.; Tian, Y. An EMT-related gene signature for the prognosis of human bladder cancer. J. Cell. Mol. Med. 2020, 24, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Xiong, G.P.; Zhang, J.X.; Gu, S.P.; Wu, Y.B.; Liu, J.F. Overexpression of ECM1 contributes to migration and invasion in cholangiocarcinoma cell. Neoplasma 2012, 59, 409–415. [Google Scholar] [CrossRef]
- Fujimoto, N.; Terlizzi, J.; Aho, S.; Brittingham, R.; Fertala, A.; Oyama, N.; Mcgrath, J.A.; Uitto, J. Extracellular matrix protein 1 inhibits the activity of matrix metalloproteinase 9 through high-affinity protein/protein interactions. Exp. Dermatol. 2006, 15, 300–307. [Google Scholar] [CrossRef]
- Lee, K.M.; Nam, K.; Oh, S.; Lim, J.; Kim, Y.P.; Lee, J.W.; Yu, J.H.; Ahn, S.H.; Kim, S.B.; Noh, D.Y.; et al. Extracellular matrix protein 1 regulates cell proliferation and trastuzumab resistance through activation of epidermal growth factor signaling. Breast Cancer Res. 2014, 16, 479. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Wu, S.; Zhang, Z.; Li, Y. Global geographic diversity and distribution of the myxobacteria. Microbiol. Spectr. 2021, 9, e1221. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Lohinai, Z.; Heshiki, Y.; Dome, B.; Moldvay, J.; Dulka, E.; Galffy, G.; Berta, J.; Weiss, G.J.; Sommer, M.; et al. Distinct composition and metabolic functions of human gut microbiota are associated with cachexia in lung cancer patients. ISME J. 2021, 15, 3207–3220. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Godet, I.; Doctorman, S.; Wu, F.; Gilkes, D.M. Detection of hypoxia in cancer models: Significance, challenges, and advances. Cells 2022, 11, 686. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Nam, K.; Oh, S.; Lim, J.; Kim, R.K.; Shim, D.; Choi, J.H.; Lee, S.J.; Yu, J.H.; Lee, J.W.; et al. ECM1 regulates tumor metastasis and CSC-like property through stabilization of beta-catenin. Oncogene 2015, 34, 6055–6065. [Google Scholar] [CrossRef]
- Mendzelevski, B.; Ferber, G.; Janku, F.; Li, B.T.; Sullivan, R.J.; Welsch, D.; Chi, W.; Jackson, J.; Weng, O.; Sager, P.T. Effect of ulixertinib, a novel ERK1/2 inhibitor, on the QT/QTc interval in patients with advanced solid tumor malignancies. Cancer Chemother. Pharmacol. 2018, 81, 1129–1141. [Google Scholar] [CrossRef]
- Kidger, A.M.; Sipthorp, J.; Cook, S.J. ERK1/2 inhibitors: New weapons to inhibit the RAS-regulated RAF-MEK1/2-ERK1/2 pathway. Pharmacol. Ther. 2018, 187, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Yacouba, A.; Tidjani, A.M.; Lagier, J.C.; Dubourg, G.; Raoult, D. Urinary microbiota and bladder cancer: A systematic review and a focus on uropathogens. Semin. Cancer Biol. 2022, 86, 875–884. [Google Scholar] [CrossRef]
- Cui, Y.; Zhou, Z.; Chai, Y.; Che, X.; Zhang, Y. Identification of a nomogram from Ferroptosis-Related long noncoding RNAs signature to analyze overall survival in patients with bladder cancer. J. Oncol. 2021, 2021, 8533464. [Google Scholar] [CrossRef]
- Witjes, J.A.; Palou, J.; Soloway, M.; Lamm, D.; Kamat, A.M.; Brausi, M.; Persad, R.; Buckley, R.; Colombel, M.; Bohle, A. Current clinical practice gaps in the treatment of intermediate- and high-risk non-muscle-invasive bladder cancer (NMIBC) with emphasis on the use of bacillus Calmette-Guerin (BCG): Results of an international individual patient data survey (IPDS). BJU Int. 2013, 112, 742–750. [Google Scholar] [CrossRef]
- Kamat, A.M.; Shore, N.; Hahn, N.; Alanee, S.; Nishiyama, H.; Shariat, S.; Nam, K.; Kapadia, E.; Frenkl, T.; Steinberg, G. KEYNOTE-676: Phase III study of BCG and pembrolizumab for persistent/recurrent high-risk NMIBC. Future Oncol. 2020, 16, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Mari, A.; Campi, R.; Tellini, R.; Gandaglia, G.; Albisinni, S.; Abufaraj, M.; Hatzichristodoulou, G.; Montorsi, F.; van Velthoven, R.; Carini, M.; et al. Patterns and predictors of recurrence after open radical cystectomy for bladder cancer: A comprehensive review of the literature. World J. Urol. 2018, 36, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.E.; Smelser, W.W.; Chang, S.S. Side effects of intravesical BCG and chemotherapy for bladder cancer: What they are and how to manage them. Urology 2021, 149, 11–20. [Google Scholar] [CrossRef]
- Datovo, J.; Neto, W.A.; Mendonca, G.B.; Andrade, D.L.; Reis, L.O. Prognostic impact of non-adherence to follow-up cystoscopy in non-muscle-invasive bladder cancer (NMIBC). World J. Urol. 2019, 37, 2067–2071. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.E.; Lynch, K.E.; Li, Z.; Mackenzie, T.A.; Seigne, J.D.; Robertson, D.J.; Sirovich, B.; Goodney, P.P.; Schroeck, F.R. The impact of low- versus high-intensity surveillance cystoscopy on surgical care and cancer outcomes in patients with high-risk non-muscle-invasive bladder cancer (NMIBC). PLoS ONE 2020, 15, e230417. [Google Scholar] [CrossRef]
- Lotan, Y.; Bivalacqua, T.J.; Downs, T.; Huang, W.; Jones, J.; Kamat, A.M.; Konety, B.; Malmstrom, P.U.; Mckiernan, J.; O’Donnell, M.; et al. Blue light flexible cystoscopy with hexaminolevulinate in non-muscle-invasive bladder cancer: Review of the clinical evidence and consensus statement on optimal use in the USA-update 2018. Nat. Rev. Urol. 2019, 16, 377–386. [Google Scholar] [CrossRef]
- Reis, L.O. Non-muscle invasive bladder cancer (NMIBC): Boiling arena and promissory future. World J. Urol. 2019, 37, 1999–2000. [Google Scholar] [CrossRef]
- Roupret, M.; Neuzillet, Y.; Pignot, G.; Comperat, E.; Audenet, F.; Houede, N.; Larre, S.; Masson-Lecomte, A.; Colin, P.; Brunelle, S.; et al. French ccAFU guidelines-Update 2018–2020: Bladder cancer. Prog. Urol. 2019, 28, R48–R80. [Google Scholar]
- Tipirneni, K.E.; Rosenthal, E.L.; Moore, L.S.; Haskins, A.D.; Udayakumar, N.; Jani, A.H.; Carroll, W.R.; Morlandt, A.B.; Bogyo, M.; Rao, J.; et al. Fluorescence imaging for cancer screening and surveillance. Mol. Imaging Biol. 2017, 19, 645–655. [Google Scholar] [CrossRef]
- Wolfs, J.; Hermans, T.; Koldewijn, E.L.; van de Kerkhof, D. Novel urinary biomarkers ADXBLADDER and bladder EpiCheck for diagnostics of bladder cancer: A review. Urol. Oncol.-Semin. Orig. Investig. 2021, 39, 161–170. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, S.J.; Kim, S.; Cho, S.C.; Choi, Y.H.; Kim, W.J.; Moon, S.K. Interleukin-5 enhances the migration and invasion of bladder cancer cells via ERK1/2-mediated MMP-9/NF-kappaB/AP-1 pathway: Involvement of the p21WAF1 expression. Cell. Signal. 2013, 25, 2025–2038. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 2019, 59, S130–S152. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Cheng, L.; Zeng, X.; Zhang, X.; Wu, Z.; Weng, P. The modulatory effect of plant polysaccharides on gut flora and the implication for neurodegenerative diseases from the perspective of the microbiota-gut-brain axis. Int. J. Biol. Macromol. 2020, 164, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Yang, Q.; Hu, H.; Ding, C.; Wang, H.; Lin, X. Colorectal cancer occurrence and treatment based on changes in intestinal flora. Semin. Cancer Biol. 2021, 70, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, G.; Zhao, J.; Chen, J.; Chen, Y.; Huang, W.; Zhong, J.; Zeng, J. Profiling the urinary microbiota in male patients with bladder cancer in China. Front. Cell. Infect. Microbiol. 2018, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Thomas-White, K.J.; Kliethermes, S.; Rickey, L.; Lukacz, E.S.; Richter, H.E.; Moalli, P.; Zimmern, P.; Norton, P.; Kusek, J.W.; Wolfe, A.J.; et al. Evaluation of the urinary microbiota of women with uncomplicated stress urinary incontinence. Am. J. Obstet. Gynecol. 2017, 216, 51–55. [Google Scholar] [CrossRef]
- Qiu, Y.; Gao, Y.; Chen, C.; Xie, M.; Huang, P.; Sun, Q.; Zhou, Z.; Li, B.; Zhao, J.; Wu, P. Deciphering the influence of urinary microbiota on FoxP3+ regulatory T cell infiltration and prognosis in Chinese patients with non-muscle-invasive bladder cancer. Hum. Cell 2022, 35, 511–521. [Google Scholar] [CrossRef]
- Yan, H.; Li, J.; Ying, Y.; Xie, H.; Chen, H.; Xu, X.; Zheng, X. MIR-300 in the imprinted DLK1-DIO3 domain suppresses the migration of bladder cancer by regulating the SP1/MMP9 pathway. Cell Cycle 2018, 17, 2790–2801. [Google Scholar] [CrossRef]
- Mondal, S.; Adhikari, N.; Banerjee, S.; Amin, S.A.; Jha, T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur. J. Med. Chem. 2020, 194, 112260. [Google Scholar] [CrossRef]
- Luo, K.W.; Wei, C.; Lung, W.Y.; Wei, X.Y.; Cheng, B.H.; Cai, Z.M.; Huang, W.R. EGCG inhibited bladder cancer SW780 cell proliferation and migration both in vitro and in vivo via down-regulation of NF-kappaB and MMP-9. J. Nutr. Biochem. 2017, 41, 56–64. [Google Scholar] [CrossRef]
- Shin, S.S.; Hwang, B.; Muhammad, K.; Gho, Y.; Song, J.H.; Kim, W.J.; Kim, G.; Moon, S.K. Nimbolide represses the proliferation, migration, and invasion of bladder carcinoma cells via Chk2-Mediated G2/M phase cell cycle arrest, altered signaling pathways, and reduced transcription Factors-Associated MMP-9 expression. Evid.-Based Complement. Altern. Med. 2019, 2019, 3753587. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, W.; Zhou, H.; Cui, Y. Urinary Eubacterium sp. CAG:581 Promotes Non-Muscle Invasive Bladder Cancer (NMIBC) Development through the ECM1/MMP9 Pathway. Cancers 2023, 15, 809. https://doi.org/10.3390/cancers15030809
Zhang Y, Wang W, Zhou H, Cui Y. Urinary Eubacterium sp. CAG:581 Promotes Non-Muscle Invasive Bladder Cancer (NMIBC) Development through the ECM1/MMP9 Pathway. Cancers. 2023; 15(3):809. https://doi.org/10.3390/cancers15030809
Chicago/Turabian StyleZhang, Yuhang, Wenyu Wang, Hang Zhou, and Yimin Cui. 2023. "Urinary Eubacterium sp. CAG:581 Promotes Non-Muscle Invasive Bladder Cancer (NMIBC) Development through the ECM1/MMP9 Pathway" Cancers 15, no. 3: 809. https://doi.org/10.3390/cancers15030809
APA StyleZhang, Y., Wang, W., Zhou, H., & Cui, Y. (2023). Urinary Eubacterium sp. CAG:581 Promotes Non-Muscle Invasive Bladder Cancer (NMIBC) Development through the ECM1/MMP9 Pathway. Cancers, 15(3), 809. https://doi.org/10.3390/cancers15030809






