Hyaluronan in the Cancer Cells Microenvironment
Abstract
Simple Summary
Abstract
1. Introduction
2. HA in Cancers: Proliferation and Neoangiogenesis
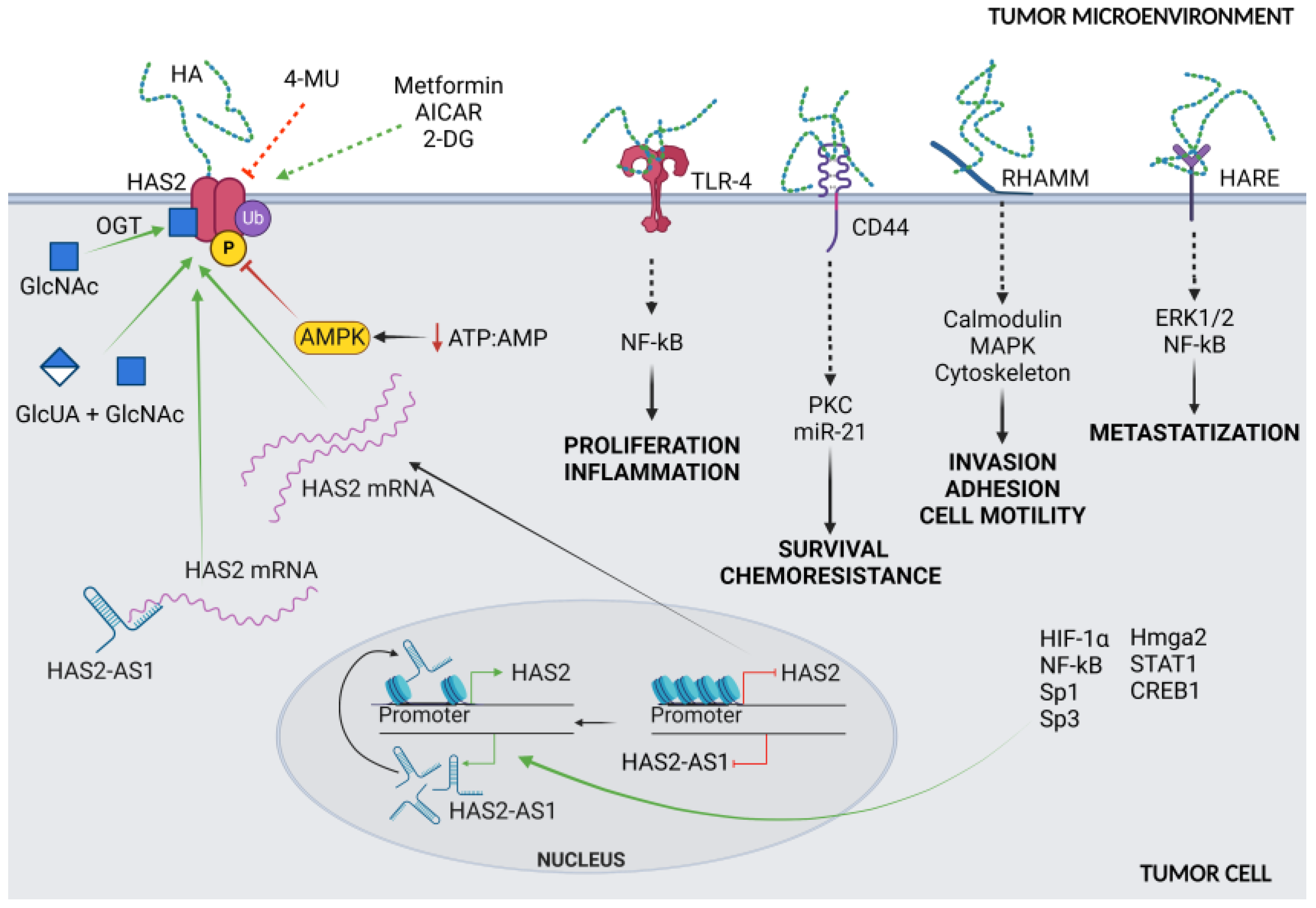
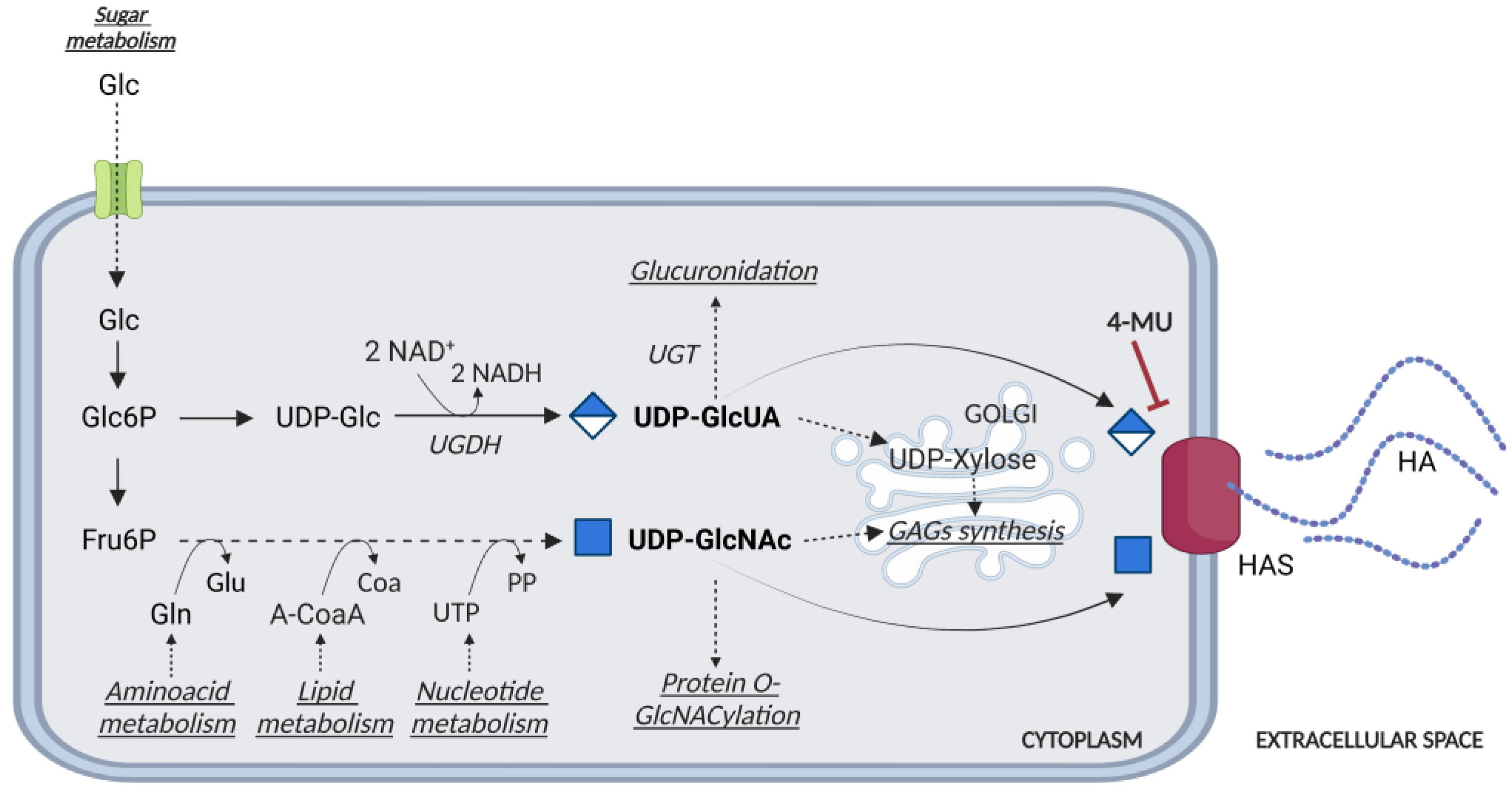
3. Controlling HASes to Control HA
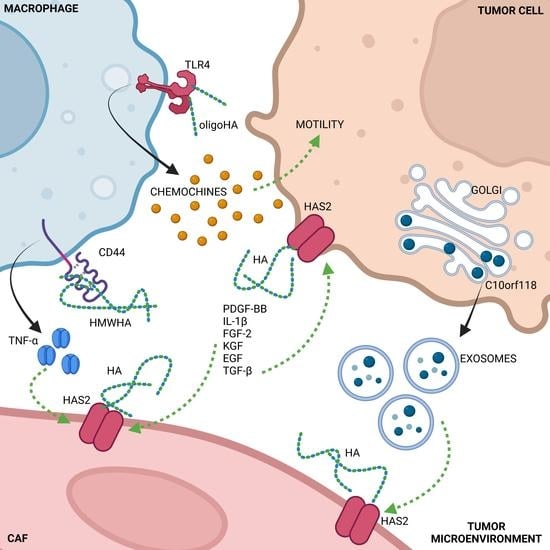
4. Cells Crosstalk Affecting HA Synthesis
5. HA Fragmentation
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marozzi, M.; Parnigoni, A.; Negri, A.; Viola, M.; Vigetti, D.; Passi, A. Inflammation, Extracellular Matrix Remodeling, and Proteostasis in Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 8102. [Google Scholar] [CrossRef] [PubMed]
- Caon, I.; Bartolini, B.; Parnigoni, A.; Caravà, E.; Moretto, P.; Viola, M. Revisiting the hallmarks of cancer: The role of hyaluronan. Semin. Cancer Biol. 2020, 62, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O.; Naba, A. Overview of the Matrisome-An Inventory of Extracellular Matrix Constituents and Functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Ferrer, L.; Schmalfeldt, B.; Dietl, J.; Bartmann, C.; Schumacher, U.; Stürken, C. Ovarian Cancer-Cell Pericellular Hyaluronan Deposition Negatively Impacts Prognosis of Ovarian Cancer Patients. Biomedicines 2022, 10, 2944. [Google Scholar] [CrossRef]
- Toole, B.P.; Biswas, C.; Gross, J. Hyaluronate and invasiveness of the rabbit V2 carcinoma. Proc. Natl. Acad. Sci. USA 1979, 76, 6299–6303. [Google Scholar] [CrossRef]
- Kimata, K.; Honma, Y.; Okayama, M.; Oguri, K.; Hozumi, M.; Suzuki, S. Increased synthesis of hyaluronic acid by mouse mammary carcinoma cell variants with high metastatic potential. Cancer Res 1983, 43, 1347–1354. [Google Scholar]
- Knudson, W.; Biswas, C.; Toole, B.P. Interactions between human tumor cells and fibroblasts stimulate hyaluronate synthesis. Proc. Natl. Acad. Sci. USA 1984, 81, 6767–6771. [Google Scholar] [CrossRef]
- Asplund, T.; Versnel, M.A.; Laurent, T.C.; Heldin, P. Human mesothelioma cells produce factors that stimulate the production of hyaluronan by mesothelial cells and fibroblasts. Cancer Res. 1993, 53, 388–392. [Google Scholar]
- Anttila, M.A.; Tammi, R.H.; Tammi, M.I.; Syrjänen, K.J.; Saarikoski, S.V.; Kosma, V.M. High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res. 2000, 60, 150–155. [Google Scholar]
- Auvinen, P.; Tammi, R.; Parkkinen, J.; Tammi, M.; Agren, U.; Johansson, R. Hyaluronan in Peritumoral Stroma and Malignant Cells Associates with Breast Cancer Spreading and Predicts Survival. Am. J. Pathol. 2000, 156, 529–536. [Google Scholar] [CrossRef]
- Tammi, R.H.; Kultti, A.; Kosma, V.M.; Pirinen, R.; Auvinen, P.; Tammi, M.I. Hyaluronan in human tumors: Pathobiological and prognostic messages from cell-associated and stromal hyaluronan. Semin. Cancer Biol. 2008, 18, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Toole, B.P. Hyaluronan: From extracellular glue to pericellular cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Caon, I.; D’Angelo, M.L.; Bartolini, B.; Caravà, E.; Parnigoni, A.; Contino, F. The Secreted Protein C10orf118 Is a New Regulator of Hyaluronan Synthesis Involved in Tumour-Stroma Cross-Talk. Cancers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Vitale, D.L.; Icardi, A.; Rosales, P.; Spinelli, F.M.; Sevic, I.; Alaniz, L.D. Targeting the Tumor Extracellular Matrix by the Natural Molecule 4-Methylumbelliferone: A Complementary and Alternative Cancer Therapeutic Strategy. Front. Oncol. 2021, 11, 710061. [Google Scholar] [CrossRef] [PubMed]
- Hundhausen, C.; Schneckmann, R.; Ostendorf, Y.; Rimpler, J.; von Glinski, A.; Kohlmorgen, C. Endothelial hyaluronan synthase 3 aggravates acute colitis in an experimental model of inflammatory bowel disease. Matrix Biol. 2021, 102, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.; Freudenberger, T.; Melchior-Becker, A.; Rock, K.; Ter Braak, M.; Jastrow, H. Inhibition of hyaluronan synthesis accelerates murine atherosclerosis: Novel insights into the role of hyaluronan synthesis. Circulation 2010, 122, 2313–2322. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.; Spevak, C.C.; Wong, G.; Xia, W.; Gilad, E. Hyaluronan-CD44 interaction with protein kinase C(epsilon) promotes oncogenic signaling by the stem cell marker Nanog and the Production of microRNA-21, leading to down-regulation of the tumor suppressor protein PDCD4, anti-apoptosis, and chemotherapy resistance in breast tumor cells. J. Biol. Chem. 2009, 284, 26533–26546. [Google Scholar]
- Messam, B.J.; Tolg, C.; McCarthy, J.B.; Nelson, A.C.; Turley, E.A. RHAMM Is a Multifunctional Protein That Regulates Cancer Progression. Int. J. Mol. Sci. 2021, 22, 10313. [Google Scholar] [CrossRef]
- Simpson, M.A.; Weigel, J.A.; Weigel, P.H. Systemic blockade of the hyaluronan receptor for endocytosis prevents lymph node metastasis of prostate cancer. Int. J. Cancer 2012, 131, E836–E840. [Google Scholar] [CrossRef]
- Nikitovic, D.; Tzardi, M.; Berdiaki, A.; Tsatsakis, A.; Tzanakakis, G.N. Cancer Microenvironment and Inflammation: Role of Hyaluronan. Front. Immunol. 2015, 6, 169. [Google Scholar] [CrossRef] [PubMed]
- Price, Z.K.; Lokman, N.A.; Ricciardelli, C. Differing Roles of Hyaluronan Molecular Weight on Cancer Cell Behavior and Chemotherapy Resistance. Cancers 2018, 10, 482. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.N.; Baker, E. Role of the Hyaluronan Receptor, Stabilin-2/HARE, in Health and Disease. Int. J. Mol. Sci. 2020, 21, 3504. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, F.; Wei, F.; Ren, X. The role of toll-like receptor 4 in tumor microenvironment. Oncotarget 2017, 8, 66656–66667. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Carrer, A.; Moimas, S.; Zandonà, L.; Bussani, R.; Casagranda, B. VEGF121 and VEGF165 differentially promote vessel maturation and tumor growth in mice and humans. Cancer Gene Ther. 2016, 23, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Koehler, L.; Ruiz-Gómez, G.; Balamurugan, K.; Rother, S.; Freyse, J.; Möller, S. Dual Action of Sulfated Hyaluronan on Angiogenic Processes in Relation to Vascular Endothelial Growth Factor-A. Sci. Rep. 2019, 9, 18143. [Google Scholar] [CrossRef]
- Mo, W.; Yang, C.; Liu, Y.; He, Y.; Wang, Y.; Gao, F. The influence of hyaluronic acid on vascular endothelial cell proliferation and the relationship with ezrin/merlin expression. Acta Biochim. Biophys. Sin. 2011, 43, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Bono, P.; Cordero, E.; Johnson, K.; Borowsky, M.; Ramesh, V.; Jacks, T. Layilin, a cell surface hyaluronan receptor, interacts with merlin and radixin. Exp. Cell Res. 2005, 308, 177–187. [Google Scholar] [CrossRef]
- Wolny, P.M.; Banerji, S.; Gounou, C.; Brisson, A.R.; Day, A.J.; Jackson, D.G. Analysis of CD44-Hyaluronan Interactions in an Artificial Membrane System Insights into the Distinct Binding Properties of High and Low Molecular Weight Hyaluronan. J. Biol. Chem. 2010, 285, 30170–30180. [Google Scholar] [CrossRef]
- Caravà, E.; Moretto, P.; Caon, I.; Parnigoni, A.; Passi, A.; Karousou, E. HA and HS Changes in Endothelial Inflammatory Activation. Biomolecules 2021, 11, 809. [Google Scholar] [CrossRef]
- Itano, N.; Kimata, K. Mammalian Hyaluronan Synthases. IUBMB Life 2002, 54, 195–199. [Google Scholar] [CrossRef]
- Wang, J.; Jordan, A.R.; Zhu, H.; Hasanali, S.L.; Thomas, E.; Lokeshwar, S.D. Targeting hyaluronic acid synthase-3 (HAS3) for the treatment of advanced renal cell carcinoma. Cancer Cell Int. 2022, 22, 421. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.Z.; Fang, W.Y.; Huang, C.C.; Tsai, S.T.; Wang, Y.C.; Yang, C.L. Hyaluronan synthase 3 mediated oncogenic action through forming inter-regulation loop with tumor necrosis factor alpha in oral cancer. Oncotarget 2017, 8, 15563–15583. [Google Scholar] [CrossRef] [PubMed]
- Capra, J.; Härkönen, K.; Kyykallio, H.; Vihinen, H.; Jokitalo, E.; Rilla, K. Microscopic characterization reveals the diversity of EVs secreted by GFP-HAS3 expressing MCF7 cells. Eur. J. Cell Biol. 2022, 101, 151235. [Google Scholar] [CrossRef]
- Mongiat, M.; Sweeney, S.M.; San Antonio, J.D.; Fu, J.; Iozzo, R.V. Endorepellin, a Novel Inhibitor of Angiogenesis Derived from the C Terminus of Perlecan. J. Biol. Chem. 2002, 278, 4238–4249. [Google Scholar] [CrossRef]
- Chen, C.G.; Gubbiotti, M.A.; Kapoor, A.; Han, X.; Yu, Y.; Linhardt, R.J. Autophagic degradation of HAS2 in endothelial cells: A novel mechanism to regulate angiogenesis. Matrix Biol. 2020, 90, 1–19. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, Y.; He, L.; Wang, J.; Xi, Q.; Gong, C. Extended use of rh-endostatin improves prognosis in patients with advanced non-small cell lung cancer: An analysis of retrospective study. J. Thorac. Dis. 2022, 14, 4416–4426. [Google Scholar] [CrossRef]
- Caon, I.; Parnigoni, A.; Viola, M.; Karousou, E.; Passi, A.; Vigetti, D. Cell Energy Metabolism and Hyaluronan Synthesis. J. Histochem. Cytochem. 2020, 69, 35–47. [Google Scholar] [CrossRef]
- Vitale, D.L.; Caon, I.; Parnigoni, A.; Sevic, I.; Spinelli, F.M.; Icardi, A. Initial Identification of UDP-Glucose Dehydrogenase as a Prognostic Marker in Breast Cancer Patients, Which Facilitates Epirubicin Resistance and Regulates Hyaluronan Synthesis in MDA-MB-231 Cells. Biomolecules 2021, 11, 246. [Google Scholar] [CrossRef]
- Nagy, N.; Kuipers, H.F.; Frymoyer, A.R.; Ishak, H.D.; Bollyky, J.B.; Wight, T.N. 4-Methylumbelliferone Treatment and Hyaluronan Inhibition as a Therapeutic Strategy in Inflammation, Autoimmunity, and Cancer. Front. Immunol. 2015, 6, 123. [Google Scholar] [CrossRef]
- Vigetti, D.; Rizzi, M.; Viola, M.; Karousou, E.; Genasetti, A.; Clerici, M. The effects of 4-methylumbelliferone on hyaluronan synthesis, MMP2 activity, proliferation, and motility of human aortic smooth muscle cells. Glycobiology 2009, 19, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Berninsone, P.; Hwang, H.Y.; Zemtseva, I.; Horvitz, H.R.; Hirschberg, C.B. SQV-7, a protein involved in Caenorhabditis elegans epithelial invagination and early embryogenesis, transports UDP-glucuronic acid, UDP-N- acetylgalactosamine, and UDP-galactose. Proc. Natl. Acad. Sci. USA 2001, 98, 3738–3743. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.; Kaber, G.; Johnson, P.Y.; Gebe, J.A.; Preisinger, A.; Falk, B.A. Inhibition of hyaluronan synthesis restores immune tolerance during autoimmune insulitis. J. Clin. Investig. 2015, 125, 3928–3940. [Google Scholar] [CrossRef] [PubMed]
- Rilla, K.; Pasonen-Seppänen, S.; Rieppo, J.; Tammi, M.; Tammi, R. The Hyaluronan Synthesis Inhibitor 4-Methylumbelliferone Prevents Keratinocyte Activation and Epidermal Hyperproliferation Induced by Epidermal Growth Factor. J. Investig. Dermatol. 2004, 123, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Vigetti, D.; Rizzi, M.; Moretto, P.; Deleonibus, S.; Dreyfuss, J.M.; Karousou, E. Glycosaminoglycans and Glucose Prevent Apoptosis in 4-Methylumbelliferone-treated Human Aortic Smooth Muscle Cells. J. Biol. Chem. 2011, 286, 34497–34503. [Google Scholar] [CrossRef]
- Bistoletti, M.; Bosi, A.; Caon, I.; Chiaravalli, A.M.; Moretto, P.; Genoni, A. Involvement of hyaluronan in the adaptive changes of the rat small intestine neuromuscular function after ischemia/reperfusion injury. Sci. Rep. 2020, 10, 11521. [Google Scholar] [CrossRef]
- Bosi, A.; Banfi, D.; Bistoletti, M.; Catizzone, L.M.; Chiaravalli, A.M.; Moretto, P. Hyaluronan Regulates Neuronal and Immune Function in the Rat Small Intestine and Colonic Microbiota after Ischemic/Reperfusion Injury. Cells 2022, 11, 3370. [Google Scholar] [CrossRef]
- García-Vilas, J.A.; Quesada, A.R.; Medina, M. 4-Methylumbelliferone Inhibits Angiogenesis in Vitro and in Vivo. J. Agric. Food Chem. 2013, 61, 4063–4071. [Google Scholar] [CrossRef]
- Dubisova, J.; Burianova, J.S.; Svobodova, L.; Makovicky, P.; Martinez-Varea, N.; Cimpean, A. Oral treatment of 4-methylumbelliferone reduced perineuronal nets and improved recognition memory in mice. Brain Res. Bull. 2022, 181, 144–156. [Google Scholar] [CrossRef]
- Li, W.; Yang, S.; Xu, P.; Zhang, D.; Tong, Y.; Chen, L. SARS-CoV-2 RNA elements share human sequence identity and upregulate hyaluronan via NamiRNA-enhancer network. EBioMedicine 2022, 76, 103861. [Google Scholar] [CrossRef]
- Díaz, M.; Pibuel, M.; Paglilla, N.; Poodts, D.; Álvarez, E.; Papademetrio, D.L. 4-Methylumbelliferone induces antitumor effects independently of hyaluronan synthesis inhibition in human acute leukemia cell lines. Life Sci. 2021, 287, 120065. [Google Scholar] [CrossRef]
- Pibuel, M.A.; Díaz, M.; Molinari, Y.; Poodts, D.; Silvestroff, L.; Lompardía, S.L. 4-Methylumbelliferone as a potent and selective antitumor drug on a glioblastoma model. Glycobiology 2021, 31, 29–43. [Google Scholar] [CrossRef]
- Andreichenko, I.N.; Tsitrina, A.A.; Fokin, A.V.; Gabdulkhakova, A.I.; Maltsev, D.I.; Perelman, G.S. 4-methylumbelliferone Prevents Liver Fibrosis by Affecting Hyaluronan Deposition, FSTL1 Expression and Cell Localization. Int. J. Mol. Sci. 2019, 20, 6301. [Google Scholar] [CrossRef]
- Vigetti, D.; Clerici, M.; Deleonibus, S.; Karousou, E.; Viola, M.; Moretto, P. Hyaluronan Synthesis Is Inhibited by Adenosine Monophosphate-activated Protein Kinase through the Regulation of HAS2 Activity in Human Aortic Smooth Muscle Cells. J. Biol. Chem. 2011, 286, 7917–7924. [Google Scholar] [CrossRef]
- Vigetti, D.; Deleonibus, S.; Moretto, P.; Karousou, E.; Viola, M.; Bartolini, B. Role of UDP-N-Acetylglucosamine (GlcNAc) and O-GlcNAcylation of Hyaluronan Synthase 2 in the Control of Chondroitin Sulfate and Hyaluronan Synthesis. J. Biol. Chem. 2012, 287, 35544–35555. [Google Scholar] [CrossRef]
- Hascall, V.C.; Wang, A.; Tammi, M.; Oikari, S.; Tammi, R.; Passi, A. The dynamic metabolism of hyaluronan regulates the cytosolic concentration of UDP-GlcNAc. Matrix Biol. 2014, 35, 14–17. [Google Scholar] [CrossRef]
- Raman, P.; Krukovets, I.; Marinic, T.E.; Bornstein, P.; Stenina, O.I. Glycosylation Mediates Up-regulation of a Potent Antiangiogenic and Proatherogenic Protein, Thrombospondin-1, by Glucose in Vascular Smooth Muscle Cells. J. Biol. Chem. 2007, 282, 5704–5714. [Google Scholar] [CrossRef]
- Vigetti, D.; Deleonibus, S.; Moretto, P.; Bowen, T.; Fischer, J.W.; Grandoch, M. Natural antisense transcript for hyaluronan synthase 2 (HAS2-AS1) induces transcription of HAS2 via protein O-GlcNAcylation. J. Biol. Chem. 2014, 289, 28816–28826. [Google Scholar] [CrossRef]
- Kuroda, Y.; Higashi, H. Regulation of hyaluronan production by β2 adrenergic receptor signaling. Biochem. Biophys. Res. Commun. 2021, 575, 50–55. [Google Scholar] [CrossRef]
- Day, A.J.; Milner, C.M. TSG-6: A multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. 2018, 78-79, 60–83. [Google Scholar] [CrossRef]
- Heldin, P.; Lin, C.Y.; Kolliopoulos, C.; Chen, Y.H.; Skandalis, S.S. Regulation of hyaluronan biosynthesis and clinical impact of excessive hyaluronan production. Matrix Biol. 2019, 78-79, 100–117. [Google Scholar] [CrossRef]
- Viola, M.; Vigetti, D.; Karousou, E.; D’Angelo, M.L.; Caon, I.; Moretto, P. Biology and biotechnology of hyaluronan. Glycoconj. J. 2015, 32, 93–103. [Google Scholar] [CrossRef]
- Vigetti, D.; Genasetti, A.; Karousou, E.; Viola, M.; Clerici, M.; Bartolini, B. Modulation of Hyaluronan Synthase Activity in Cellular Membrane Fractions. J. Biol. Chem. 2009, 284, 30684–30694. [Google Scholar] [CrossRef]
- Melero-Fernandez de Mera, R.M.; Arasu, U.T.; Kärnä, R.; Oikari, S.; Rilla, K.; Vigetti, D. Effects of mutations in the post-translational modification sites on the trafficking of hyaluronan synthase 2 (HAS2). Matrix Biol. 2018, 80, 85–103. [Google Scholar] [CrossRef]
- Karousou, E.; Kamiryo, M.; Skandalis, S.S.; Ruusala, A.; Asteriou, T.; Passi, A. The Activity of Hyaluronan Synthase 2 Is Regulated by Dimerization and Ubiquitination. J. Biol. Chem. 2010, 285, 23647–23654. [Google Scholar] [CrossRef]
- Kasai, K.; Kuroda, Y.; Takabuchi, Y.; Nitta, A.; Kobayashi, T.; Nozaka, H. Phosphorylation of Thr328 in hyaluronan synthase 2 is essential for hyaluronan synthesis. Biochem. Biophys. Res. Commun. 2020, 533, 732–738. [Google Scholar] [CrossRef]
- Khorkova, O.; Myers, A.J.; Hsiao, J.; Wahlestedt, C. Natural antisense transcripts. Hum. Mol. Genet 2014, 23, R54–R63. [Google Scholar] [CrossRef]
- Khorkova, O.; Stahl, J.; Joji, A.; Volmar, C.H.; Zeier, Z.; Wahlestedt, C. Natural antisense transcripts as drug targets. Front. Mol. Biosci. 2022, 9, 978375. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, S.; Chen, J.; Wang, Z.; Liang, X.; Wang, X. Long noncoding RNA HAS2-AS1 mediates hypoxia-induced invasiveness of oral squamous cell carcinoma. Mol. Carcinog. 2017, 56, 2210–2222. [Google Scholar] [CrossRef]
- Parnigoni, A.; Caon, I.; Teo, W.X.; Hua, S.H.; Moretto, P.; Bartolini, B. The natural antisense transcript HAS2-AS1 regulates breast cancer cells aggressiveness independently from hyaluronan metabolism. Matrix Biol. 2022, 109, 140–161. [Google Scholar] [CrossRef]
- Tong, L.; Wang, Y.; Ao, Y.; Sun, X. CREB1 induced lncRNA HAS2-AS1 promotes epithelial ovarian cancer proliferation and invasion via the miR-466/RUNX2 axis. Biomed. Pharmacother. 2019, 115, 108891. [Google Scholar] [CrossRef]
- Parnigoni, A.; Caon, I.; Moretto, P.; Viola, M.; Karousou, E.; Passi, A. The role of the multifaceted long non-coding RNAs: A nuclear-cytosolic interplay to regulate hyaluronan metabolism. Matrix Biol. Plus 2021, 11, 100060. [Google Scholar] [CrossRef]
- Cable, J.; Heard, E.; Hirose, T.; Prasanth, K.V.; Chen, L.L.; Henninger, J.E. Noncoding RNAs: Biology and applications—a Keystone Symposia report. Ann. N. Y. Acad. Sci. 2021, 1506, 118–141. [Google Scholar] [CrossRef]
- Bart, G.; Vico, N.O.; Hassinen, A.; Pujol, F.M.; Deen, A.J.; Ruusala, A. Fluorescence Resonance Energy Transfer (FRET) and Proximity Ligation Assays Reveal Functionally Relevant Homo- and Heteromeric Complexes among Hyaluronan Synthases HAS1, HAS2, and HAS3. J. Biol. Chem. 2015, 290, 11479–11490. [Google Scholar] [CrossRef]
- Viola, M.; Bartolini, B.; Vigetti, D.; Karousou, E.; Moretto, P.; Deleonibus, S. Oxidized Low Density Lipoprotein (LDL) Affects Hyaluronan Synthesis in Human Aortic Smooth Muscle Cells. J. Biol. Chem. 2013, 288, 29595–29603. [Google Scholar] [CrossRef]
- Chen, C.G.; Iozzo, R.V. Angiostatic cues from the matrix: Endothelial cell autophagy meets hyaluronan biology. J. Biol. Chem. 2020, 295, 16797–16812. [Google Scholar] [CrossRef]
- Kapoor, A.; Chen, C.G.; Iozzo, R.V. Endorepellin evokes an angiostatic stress signaling cascade in endothelial cells. J. Biol. Chem. 2020, 295, 6344–6356. [Google Scholar] [CrossRef]
- Li, L.; Asteriou, T.; Bernert, B.; Heldin, C.H.; Heldin, P. Growth factor regulation of hyaluronan synthesis and degradation in human dermal fibroblasts: Importance of hyaluronan for the mitogenic response of PDGF-BB. Biochem. J. 2007, 404, 327–336. [Google Scholar] [CrossRef]
- Hellman, U.; Malm, L.; Ma, L.P.; Larsson, G.; Mörner, S.; Fu, M. Growth Factor PDGF-BB Stimulates Cultured Cardiomyocytes to Synthesize the Extracellular Matrix Component Hyaluronan. PLoS ONE 2010, 5, e14393. [Google Scholar] [CrossRef]
- Vigetti, D.; Genasetti, A.; Karousou, E.; Viola, M.; Moretto, P.; Clerici, M. Proinflammatory Cytokines Induce Hyaluronan Synthesis and Monocyte Adhesion in Human Endothelial Cells through Hyaluronan Synthase 2 (HAS2) and the Nuclear Factor-kappa B (NF-kappa B) Pathway. J. Biol. Chem. 2010, 285, 24639–24645. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Sapudom, J.; Müller, C.D.; Nguyen, K.T.; Martin, S.; Anderegg, U.; Pompe, T. Matrix Remodeling and Hyaluronan Production by Myofibroblasts and Cancer-Associated Fibroblasts in 3D Collagen Matrices. Gels 2020, 6, 33. [Google Scholar] [CrossRef]
- Lowe, M. The Physiological Functions of the Golgin Vesicle Tethering Proteins. Front. Cell Dev. Biol. 2019, 7, 94. [Google Scholar] [CrossRef]
- Noble, P.W. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002, 21, 25–29. [Google Scholar] [CrossRef] [PubMed]
- David-Raoudi, M.; Tranchepain, F.; Deschrevel, B.; Vincent, J.C.; Bogdanowicz, P.; Boumediene, K. Differential effects of hyaluronan and its fragments on fibroblasts: Relation to wound healing. Wound Repair Regen. 2008, 16, 274–287. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Fan, J.; Yu, S.; Chen, S.; Luo, Y. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat. Med. 2005, 11, 1173–1179. [Google Scholar] [CrossRef]
- Noble, P.W.; Lake, F.R.; Henson, P.M.; Riches, D.W. Hyaluronate activation of CD44 induces insulin-like growth factor-1 expression by a tumor necrosis factor-alpha-dependent mechanism in murine macrophages. J. Clin. Investig. 1993, 91, 2368–2377. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an Immune Regulator in Human Diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef]
- Irie, F.; Tobisawa, Y.; Murao, A.; Yamamoto, H.; Ohyama, C.; Yamaguchi, Y. The cell surface hyaluronidase TMEM2 regulates cell adhesion and migration via degradation of hyaluronan at focal adhesion sites. J. Biol. Chem. 2021, 296, 100481. [Google Scholar] [CrossRef]
- Kudo, Y.; Sato, N.; Adachi, Y.; Amaike, T.; Koga, A.; Kohi, S. Overexpression of transmembrane protein 2 (TMEM2), a novel hyaluronidase, predicts poor prognosis in pancreatic ductal adenocarcinoma. Pancreatology 2020, 20, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Soltés, L.; Mendichi, R.; Kogan, G.; Schiller, J.; Stankovska, M.; Arnhold, J. Degradative Action of Reactive Oxygen Species on Hyaluronan. Biomacromolecules 2006, 7, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Shi, Q.; Zhang, L.; Zhao, H. High molecular weight hyaluronan attenuates fine particulate matter-induced acute lung injury through inhibition of ROS-ASK1-p38/JNK-mediated epithelial apoptosis. Environ. Toxicol. Pharmacol. 2018, 59, 190–198. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karousou, E.; Parnigoni, A.; Moretto, P.; Passi, A.; Viola, M.; Vigetti, D. Hyaluronan in the Cancer Cells Microenvironment. Cancers 2023, 15, 798. https://doi.org/10.3390/cancers15030798
Karousou E, Parnigoni A, Moretto P, Passi A, Viola M, Vigetti D. Hyaluronan in the Cancer Cells Microenvironment. Cancers. 2023; 15(3):798. https://doi.org/10.3390/cancers15030798
Chicago/Turabian StyleKarousou, Evgenia, Arianna Parnigoni, Paola Moretto, Alberto Passi, Manuela Viola, and Davide Vigetti. 2023. "Hyaluronan in the Cancer Cells Microenvironment" Cancers 15, no. 3: 798. https://doi.org/10.3390/cancers15030798
APA StyleKarousou, E., Parnigoni, A., Moretto, P., Passi, A., Viola, M., & Vigetti, D. (2023). Hyaluronan in the Cancer Cells Microenvironment. Cancers, 15(3), 798. https://doi.org/10.3390/cancers15030798