CANTO-RT: One of the Largest Prospective Multicenter Cohort of Early Breast Cancer Patients Treated with Radiotherapy including Full DICOM RT Data
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
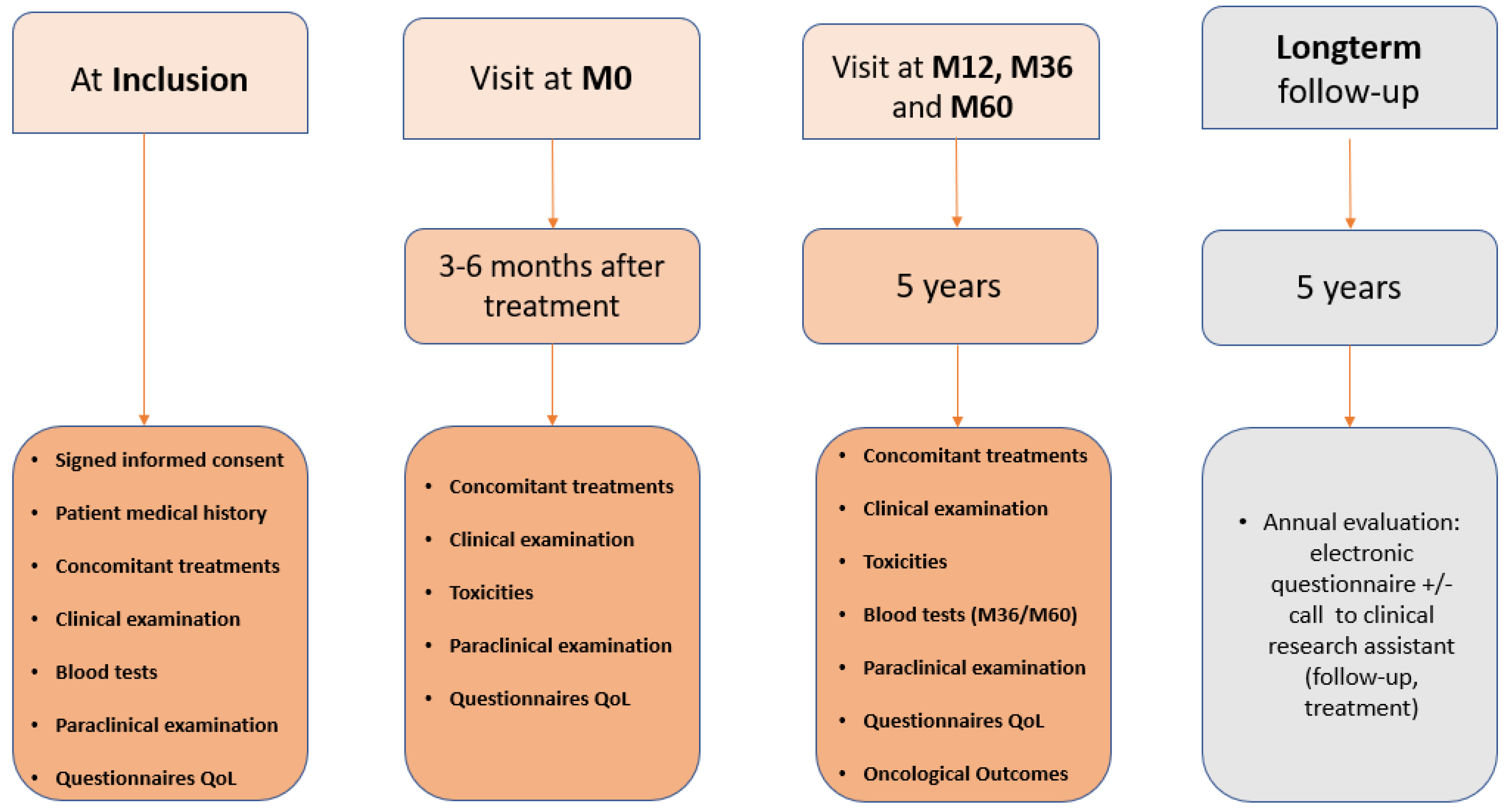
2.1. Study Design
2.2. Study Population
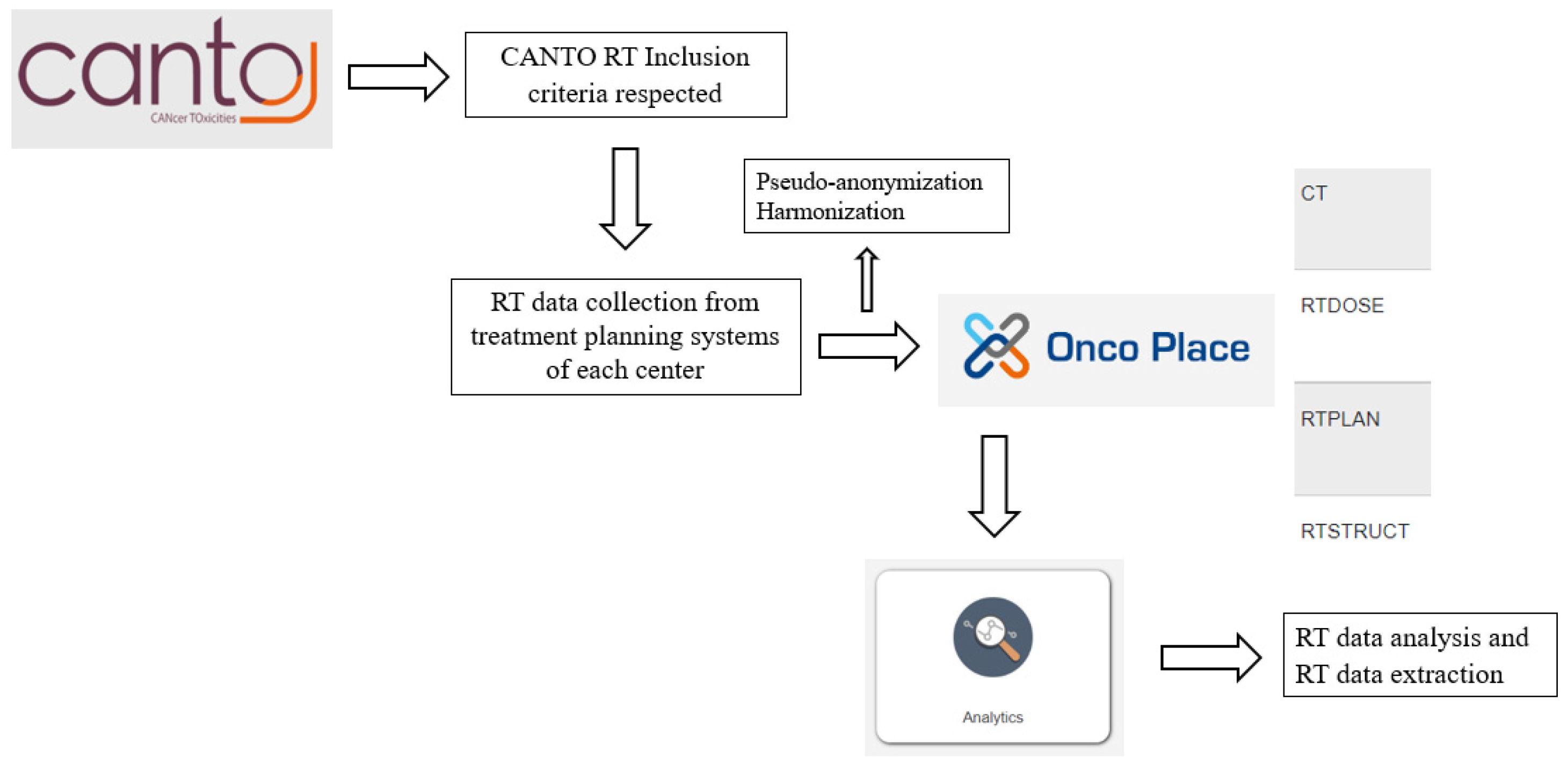
2.3. Data Collection
2.4. Data Management and Quality Control
2.5. Statistical Analysis
3. Results
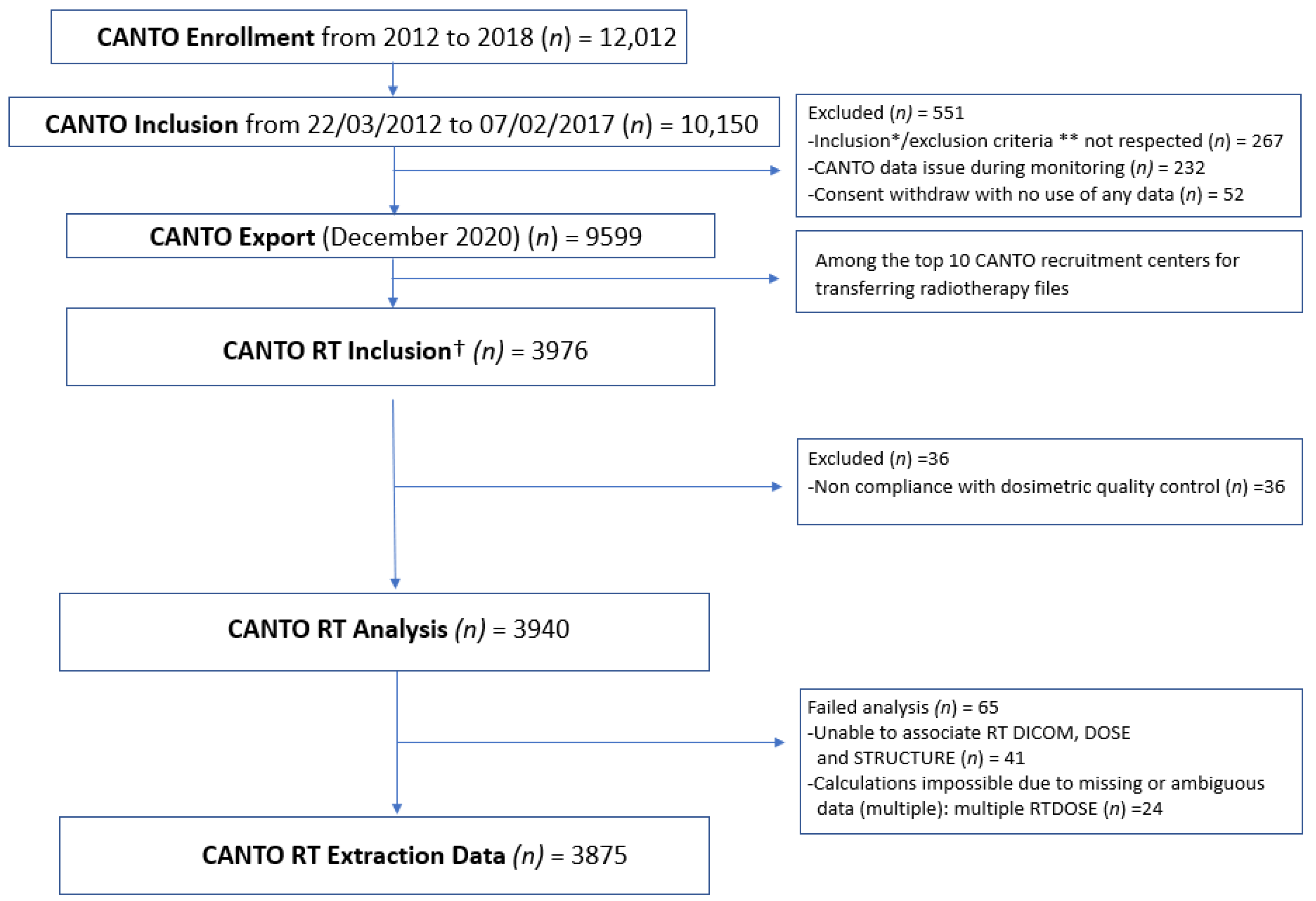
3.1. CANTO-RT Characteristics
3.2. Summary of RT Data Available
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.; Broeders, M.; de Wolf, C.; Törnberg, S.; Holland, R.; von Karsa, L. European guidelines for quality assurance in breast cancer screening and diagnosis. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2008, 19, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- EBCTCG (Early Breast Cancer Trialists’ Collaborative Group); McGale, P.; Taylor, C.; Correa, C.; Cutter, D.; Duane, F.; Ewertz, M.; Gray, R.; Mannu, G.; Peto, R.; et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet Lond Engl. 2014, 383, 2127–2135. [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet Lond Engl. 2011, 378, 1707–1716. [Google Scholar]
- Vaz-Luis, I.; Cottu, P.; Mesleard, C.; Martin, A.L.; Dumas, A.; Dauchy, S.; Tredan, O.; Christelle, L.; Adnet, J.; Rousseau Tsangaris, M.; et al. UNICANCER: French prospective cohort study of treatment-related chronic toxicity in women with localised breast cancer (CANTO). ESMO Open. 2019, 4, e000562. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.M.; Smith, K.L.; Stearns, V. Management of hormone receptor-positive, HER2-negative early breast cancer. Semin. Oncol. 2020, 47, 187–200. [Google Scholar] [CrossRef]
- Lievens, Y.; Dunscombe, P.; Defourny, N.; Gasparotto, C.; Borras, J.M.; Grau, C. HERO (Health Economics in Radiation Oncology): A pan-European project on radiotherapy resources and needs. Clin. Oncol. R. Coll. Radiol. G.B. 2015, 27, 115–124. [Google Scholar] [CrossRef]
- Sardar, P.; Kundu, A.; Chatterjee, S.; Nohria, A.; Nairooz, R.; Bangalore, S.; Mukherjee, D.; Aronow, W.; Lavie, C.J. Long-term cardiovascular mortality after radiotherapy for breast cancer: A systematic review and meta-analysis. Clin. Cardiol. 2017, 40, 73–81. [Google Scholar] [CrossRef]
- Thorsen, L.B.J.; Offersen, B.V.; Danø, H.; Berg, M.; Jensen, I.; Pedersen, A.N.; Zimmermann, S.J.; Brodersen, H.J.; Overgaard, M.; Overgaard, J. DBCG-IMN: A Population-Based Cohort Study on the Effect of Internal Mammary Node Irradiation in Early Node-Positive Breast Cancer. J. Clin. Oncol. Off J. Am. Soc. Clin. Oncol. 2016, 34, 314–320. [Google Scholar] [CrossRef]
- Roche, N.; Reddel, H.; Martin, R.; Brusselle, G.; Papi, A.; Thomas, M.; Postma, D.; Thomas, V.; Rand, C.; Chisholm, A.; et al. Quality standards for real-world research. Focus on observational database studies of comparative effectiveness. Ann. Am. Thorac. Soc. 2014, 11 Suppl. S2, S99–S104. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Kessler, D.; Mackinnon, R.; Chang, T.P.; Nadkarni, V.M.; Hunt, E.A.; Duval-Arnould, J.; Lin, Y.; Cook, D.A.; Pusic, M.; et al. Reporting Guidelines for Health Care Simulation Research: Extensions to the CONSORT and STROBE Statements. Simul. Healthc. J. Soc. Simul. Healthc. 2016, 11, 238–248. [Google Scholar] [CrossRef]
- Seibold, P.; Webb, A.; Aguado-Barrera, M.E.; Azria, D.; Bourgier, C.; Brengues, M.; Briers, E.; Bultijnck, R.; Calvo-Crespo, P.; Carballo, A.; et al. REQUITE: A prospective multicentre cohort study of patients undergoing radiotherapy for breast, lung or prostate cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2019, 138, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef]
- Jacobse, J.N.; Duane, F.K.; Boekel, N.B.; Schaapveld, M.; Hauptmann, M.; Hooning, M.J.; Seynaeve, C.; Baaijens, M.H.A.; Gietema, J.A.; Darby, S.C.; et al. Radiation Dose-Response for Risk of Myocardial Infarction in Breast Cancer Survivors. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 595–604. [Google Scholar] [CrossRef]
- Haviland, J.S.; Owen, J.R.; Dewar, J.A.; Agrawal, R.K.; Barrett, J.; Barrett-Lee, P.J.; Dobbs, H.J.; Hopwood, P.; Lawton, P.A.; Magee, B.J.; et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013, 14, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Shaitelman, S.F.; Schlembach, P.J.; Arzu, I.; Ballo, M.; Bloom, E.S.; Buchholz, D.; Chronowski, G.M.; Dvorak, T.; Grade, E.; Hoffman, K.E.; et al. Acute and Short-term Toxic Effects of Conventionally Fractionated vs Hypofractionated Whole-Breast Irradiation: A Randomized Clinical Trial. JAMA Oncol. 2015, 1, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Swanick, C.W.; Lei, X.; Shaitelman, S.F.; Schlembach, P.J.; Bloom, E.S.; Fingeret, M.C.; Strom, E.A.; Tereffe, W.; Woodward, W.A.; Stauder, M.C.; et al. Longitudinal analysis of patient-reported outcomes and cosmesis in a randomized trial of conventionally fractionated versus hypofractionated whole-breast irradiation. Cancer. 2016, 122, 2886–2894. [Google Scholar] [CrossRef]
- Bartelink, H.; Maingon, P.; Poortmans, P.; Weltens, C.; Fourquet, A.; Jager, J.; Schinagl, D.; Oei, B.; Rodenhius, C.; Horiot, J.C.; et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015, 16, 47–56. [Google Scholar] [CrossRef]
- Choi, K.H.; Ahn, S.J.; Jeong, J.U.; Yu, M.; Kim, J.H.; Jeong, B.K.; Lee, J.H.; Kim, S.H.; Lee, J.H. Postoperative radiotherapy with intensity-modulated radiation therapy versus 3-dimensional conformal radiotherapy in early breast cancer: A randomized clinical trial of KROG 15-03. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2021, 154, 179–186. [Google Scholar] [CrossRef]
- Li, X.A.; Tai, A.; Arthur, D.W.; Buchholz, T.A.; Macdonald, S.; Marks, L.B.; Moran, J.M.; Pierce, L.J.; Rabinovitch, R.; Taghian, A.; et al. Variability of target and normal structure delineation for breast cancer radiotherapy: An RTOG Multi-Institutional and Multiobserver Study. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Besnard, S.; Cutuli, B.; Fourquet, A.; Giard, S.; Hennequin, C.; Leblanc-Onfroy, M.; Mazeau-Woynar, V.; Verdoni, L.; French National Cancer Institute. Radiotherapy of invasive breast cancer: French national guidelines. Cancer Radiother. J. Soc. Francaise Radiother. Oncol. 2012, 16, 503–513. [Google Scholar]
- Hennequin, C.; Belkacémi, Y.; Bourgier, C.; Cowen, D.; Cutuli, B.; Fourquet, A.; Hannoun-Lévi, J.M.; Pasquier, D.; Racadot, S.; Rivera, S. Radiotherapy of breast cancer. Cancer Radiother. J. Soc. Francaise Radiother. Oncol. 2022, 26, 221–230. [Google Scholar] [CrossRef]
- Nielsen, M.H.; Berg, M.; Pedersen, A.N.; Andersen, K.; Glavicic, V.; Jakobsen, E.H.; Jensen, I.; Josipovic, M.; Lorenzen, E.; Nielsen, H.M.; et al. Delineation of target volumes and organs at risk in adjuvant radiotherapy of early breast cancer: National guidelines and contouring atlas by the Danish Breast Cancer Cooperative Group. Acta Oncol Stockh Swed. 2013, 52, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Offersen, B.V.; Boersma, L.J.; Kirkove, C.; Hol, S.; Aznar, M.C.; Biete Sola, A.; Kirova, Y.M.; Pignol, J.P.; Remouchamps, V.; Verhoeven, K.; et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2015, 114, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Salerno, K.E. NCCN Guidelines Update: Evolving Radiation Therapy Recommendations for Breast Cancer. J. Natl. Compr. Cancer Netw. JNCCN. 2017, 15, 682–684. [Google Scholar] [CrossRef]
| Patient’s Characteristics | Breast Cancer Patients {N(%) or Mean (STD) or [Range]} |
|---|---|
| Age at enrolment | |
| Mean (STD), [Range], years | 56.5 (11.2) [23.3–85.8] |
| Smoking status at diagnosis | |
| Current | 650 (16.9) |
| Former | 796 (20.5) |
| Never | 2377 (61.3) |
| Missing | 52 (1.3) |
| Selected comorbidities | |
| Yes * Diabetes | 1566 (40.4) 190 (4.9) |
| Hypertension | 904 (23.3) |
| Dyslipidemia | 500 (12.9) |
| BMI > 30 kg/m² | 768 (19.8) |
| Tumor size (pT) | |
| T0 ** | 37 (1.0) |
| T1 | 2586 (66.7) |
| T2 | 1058 (27.3) |
| T3 | 177 (4.6) |
| Missing | 17 (0.4) |
| Nodal status (pN) | |
| 0 | 2525 (65.2) |
| 1 | 1035 (26.7) |
| 2 | 223 (5.8) |
| 3 | 79 (2.0) |
| Missing | 13 (0.3) |
| Tumor histology | |
| Infiltrating Ductal | 3011 (77.7) |
| Lobular | 473 (12.2) |
| Others (including mixed) | 381 (9.8) |
| Missing | 10 (0.3) |
| Molecular subtype | |
| HR+ HER2+ | 394 (10.2) |
| HR+ HER2- | 2923 (75.4) |
| HR- HER2+ HR- HER2- Missing | 159 (4.1) 381 (9.8) 18 (0.5) |
| SBR Grading | |
| I | 703 (18.1) |
| II | 2019 (52.1) |
| III | 1117 (28.8) |
| Missing | 36 (0.9) |
| Ki67 | |
| No | 1657 (42.8) |
| Yes | 1958 (50.5) |
| <20% | 1154 (58.9) |
| 20–50% | 657 (33.6) |
| >50% | 147 (7.5) |
| Missing | 260 (6.7) |
| Treatment Characteristics | Breast Cancer Patients [N(%) or Mean (Range)] |
|---|---|
| Type of chemotherapy | |
| No chemotherapy | 1788 (46.1) |
| Neoadjuvant chemotherapy | 450 (11.6) |
| Adjuvant chemotherapy | 1629 (42.0) |
| Peri-adjuvant chemotherapy (neo + adjuvant) | 8 (0.2) |
| Hormonal therapy | |
| No | 730 (18.8) |
| Yes | 3138 (81) |
| Missing | 7 (0.2) |
| Trastuzumab treatment | |
| No or Not applicable | 3378 (87.2) |
| Yes | 477 (12.3) |
| Missing | 20 (0.5) |
| Type of breast surgery | |
| Breast-conserving surgery | 3113 (80.3) |
| Right | 1488 (47.8) |
| Left | 1577 (50.7) |
| Bilateral ** | 48 (1.5) |
| Total mastectomy | 734 (18.9) |
| Right | 359 (48.9) |
| Left | 369 (50.3) |
| Bilateral ** | 6 (0.8) |
| Right breast-conserving surgery and left total mastectomy** | 13 (0.3) |
| Right total mastectomy and left breast-conserving ** | 9 (0.2) |
| None | 6 (0.2) |
| Type of lymph node surgery | |
| Sentinel node | 2746 (70.9) |
| Right sentinel node | 1344 (48.9) |
| Left sentinel node | 1368 (49.8) |
| Bilateral sentinel node ** | 34 (1.2) |
| Axillary dissection | 1086 (28.0) |
| Right axillary dissection | 506 (46.6) |
| Left axillary dissection | 574 (52.9) |
| Bilateral axillary dissection ** | 6 (0.6) |
| Right sentinel node, Left axillary dissection ** | 20 (0.5) |
| Right axillary dissection, left sentinel node ** | 12 (0.3) |
| None | 11 (0.3) |
| Radiation therapy | |
| Right Side | 1850 (47.8) |
| Left Side | 1947 (50.2) |
| Bilateral | 78 (2.0) |
| Patients with boost | |
| No or Not applicable | 1217 (31.4) |
| Yes | 2658 (68.6) |
| Right Boost | 1256 (47.3) |
| Left Boost | 1344 (50.6) |
| Bilateral Boost ** | 31 (1.2) |
| Right Boost, no Left Boost ** | 16 (0.6) |
| Left Boost, no Right Boost ** | 11 (0.4) |
| Lymph node levels treated | |
| None | 2519 (65.0) |
| Right | 1222 (48.5) |
| Left | 1258 (49.9) |
| Bilateral ** | 39 (1.5) |
| Yes | 1356 (35.0) |
| CTVn_L1 | 284 (20.9) |
| CTVn_L2 | 340 (25.1) |
| CTVn_L3 | 1072 (79.1) |
| CTVn_L4 | 1348 (99.4) |
| Internal mammary chain | 844 (62.2) |
| Right | 404 (47.9) |
| Left | 415 (49.2) |
| Bilateral ** | 4 (0.5) |
| Right only ** | 7 (0.8) |
| Left only ** | 14 (1.7) |
| Irradiation techniques | |
| 3D | 3691 (95.3) |
| IMRT | 184 (4.7) |
| Fractionation regimens | |
| Normofractionation 25-fractions *¹ | 2707 (69.9) |
| Hypofractionation 15–16 fractions *² | 166 (4.3) |
| Hypofractionation and Partial breast irradiation *³ | 51 (1.3) |
| Unspecified fractionation-CTV breast or chestwall not delineated *** | 951 (24.5) |
| Number Delineated/Number Total | Volume Median (IQR), (cm3) | Dose Delivered, Mean (STD), (Gy) | |
|---|---|---|---|
| Target volumes | |||
| CTV breast | 62.8% (1999/3184) | 598.0 (385.0–871.0) | 53.6 (7.6) |
| Right | 62.0% (922/1488) | 576.5 (371.0–845.0) | 53.8 (7.0) |
| Left | 63.2% (997/1577) | 622.0 (398.0–905.0) | 53.4 (8.3) |
| Bilateral–Right side | 67.2% (41/61) | 585.0 (384.0–866.0) | 52.8 (4.8) |
| Bilateral–Left side | 67.2% (39/58) | 528.0 (387.0–766.0) | 52.9 (4.2) |
| CTV chestwall | 52.3% (399/763) | 314.0 (194.0–484.0) | 48.9 (4.0) |
| Right | 51.0% (183/359) | 314.0 (178.0–478.0) | 49.1 (2.7) |
| Left | 52.6% (194/369) | 309.0 (204.0–489.0) | 48.7 (5.0) |
| Bilateral–Right side | 53.3% (8/15) | 288.5 (205.0–445.5) | 49.1 (3.4) |
| Bilateral–Left side | 70.0% (14/20) | 380.0 (243.0–521.0) | 50.0 (1.2) |
| CTV_tumorbed | 91.4% (2457/2689) | 20.8 (10.7–39.0) | 64.8 (4.6) |
| Right | 91.5% (1149/1256) | 19.6 (9.7–36.7) | 64.8 (4.7) |
| Left | 90.8% (1221/1344) | 21.6 (11.6–40.5) | 64.7 (4.6) |
| Bilateral–Right side | 95.7% (45/47) | 22.0 (15.9–39.7) | 64 (2.8) |
| Bilateral–Left side | 100.0% (42/42) | 25.7 (10.4–45.2) | 64 (3.1) |
| CTVn_Ltot ¹ | 29.9% (408/1364) | 49.4 (33.3–78.2) | 46.8 (7.5) |
| CTVn_L1 ² | 18.6% (53/285) | 53.8 (42.6–80.4) | 46.9 (7.7) |
| CTVn_L2 ² | 15.5% (53/342) | 23.1 (16.9–48.6) | 46.3 (7.7) |
| CTVn_L3 ² | 48.9% (527/1077) | 13.1 (7.8–19.8) | 47.6 (5.4) |
| CTVn_L4 ² | 58.4% (792/1356) | 20.0 (13.5–28.1) | 48.3 (4.8) |
| CTV Internal mammary chain | 73.2% (621/848) | 4.6 (3–7.4) | 46.5 (8.4) |
| Right | 73.0% (295/404) | 4.9 (3.1–6.9) | 46.3 (9.0) |
| Left | 72.5% (301/415) | 4.4 (2.9–7.7) | 46.6 (7.7) |
| Bilateral–Right side | 9.1% (1/11) | 9.7 (9.7–9.7) | 50.3 (.) |
| Bilateral–Left side | 83.3% (15/18) | 4.5 (3.6–6.6) | 49.9 (1.6) |
| Organs at risk | |||
| External Outline | 98.3% (3810/3875) | 20476.5 (17340.0–24588.0) | 5.2 (2.1) |
| Heart | 75.8% (2939/3875) | 609 (534.0–693.0) | 3.4 (3.4) |
| Right | 59,5% (1100/1850) | 612.5 (538.0–697.5) | 2.2 (2.8) |
| Left | 90,1% (1764/1947) | 604.0 (530.0–686.0) | 4.0 (3.4) |
| Bilateral | 96,2% (75/78) | 651.6 (546.0–748.0) | 6.0 (4.7) |
| Lungs * | 25.1% (972/3875) | 2564.0 (2173.0–2979.5) | 5.2 (3.5) |
| Right Lung | 90.1% (3492/3875) | 1428.5 (1226.0–1663) | 5.1 (5.3) |
| Left Lung | 89.5% (3470/3875) | 1143.0 (961.0–1363.0) | 5.3 (5.4) |
| Spinal Cord | 56.7% (2197/3875) | 49.0 (33.7–68.3) | 1.7 (2.2) |
| Esophagus | 17.5% (677/3875) | 27.7 (22.4–33.9) | 5.6 (6.4) |
| Thyroid | 16.4% (635/3875) | 13.0 (9.1–18.9) | 12.9 (11.4) |
| LAD Coronary Artery | 4.9% (188/3875) | 5.3 (3.3–6.3) | 15.0 (9.1) |
| Right Controlateral Breast | 7.6% (147/1947) | 7.6 (4.8–11.7) | 2.7 (2.1) |
| Left Controlateral Breast | 7.4% (137/1850) | 7.8 (4.5–12.4) | 2.9 (4.5) |
| Right Humeral Head | 6.2% (119/1928) | 45.3 (30.7–58.4) | 10.3 (14.5) |
| Left Humeral Head | 5.2% (105/2025) | 47.4 (32.8–54.2) | 6.7 (8.9) |
| Right Brachial Plexus | 2.3% (45/1928) | 8.9 (4.7–13.5) | 28.6 (15.1) |
| Left Brachial Plexus | 2.2% (44/2025) | 7.6 (4.1–13.5) | 24.4 (15.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarrade, T.; Allodji, R.; Ghannam, Y.; Auzac, G.; Everhard, S.; Kirova, Y.; Peignaux, K.; Guilbert, P.; Pasquier, D.; Racadot, S.; et al. CANTO-RT: One of the Largest Prospective Multicenter Cohort of Early Breast Cancer Patients Treated with Radiotherapy including Full DICOM RT Data. Cancers 2023, 15, 751. https://doi.org/10.3390/cancers15030751
Sarrade T, Allodji R, Ghannam Y, Auzac G, Everhard S, Kirova Y, Peignaux K, Guilbert P, Pasquier D, Racadot S, et al. CANTO-RT: One of the Largest Prospective Multicenter Cohort of Early Breast Cancer Patients Treated with Radiotherapy including Full DICOM RT Data. Cancers. 2023; 15(3):751. https://doi.org/10.3390/cancers15030751
Chicago/Turabian StyleSarrade, Thomas, Rodrigue Allodji, Youssef Ghannam, Guillaume Auzac, Sibille Everhard, Youlia Kirova, Karine Peignaux, Philippe Guilbert, David Pasquier, Séverine Racadot, and et al. 2023. "CANTO-RT: One of the Largest Prospective Multicenter Cohort of Early Breast Cancer Patients Treated with Radiotherapy including Full DICOM RT Data" Cancers 15, no. 3: 751. https://doi.org/10.3390/cancers15030751
APA StyleSarrade, T., Allodji, R., Ghannam, Y., Auzac, G., Everhard, S., Kirova, Y., Peignaux, K., Guilbert, P., Pasquier, D., Racadot, S., Bourgier, C., Ducornet, S., André, F., De Vathaire, F., & Rivera, S. (2023). CANTO-RT: One of the Largest Prospective Multicenter Cohort of Early Breast Cancer Patients Treated with Radiotherapy including Full DICOM RT Data. Cancers, 15(3), 751. https://doi.org/10.3390/cancers15030751







