COVID and Cancer: A Complete 3D Advanced Radiological CT-Based Analysis to Predict the Outcome
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Procedure
2.3. Methods
2.4. Statistics and Data Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Metastatic Profile of Cancer Patients
3.3. Metastatic Profile of Cancer Patients Post COVID-19 Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Engelhardt, M.; Shoumariyeh, K.; Rösner, A.; Ihorst, G.; Biavasco, F.; Meckel, K.; von Metzler, I.; Theurich, S.; Hebart, H.; Grube, M.; et al. Clinical characteristics and outcome of multiple myeloma patients with concomitant COVID-19 at Comprehensive Cancer Centers in Germany. Haematologica 2020, 105, 2872. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. Jama 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Engelhardt, M.; Cook, G.; Gay, F.; Mateos, M.-V.; Ntanasis-Stathopoulos, I.; van de Donk, N.W.; Avet-Loiseau, H.; Hajek, R.; Vangsted, A.J.; et al. Management of patients with multiple myeloma in the era of COVID-19 pandemic: A consensus paper from the European Myeloma Network (EMN). Leukemia 2020, 34, 2000–2011. [Google Scholar] [CrossRef] [PubMed]
- Mian, H.; Grant, S.J.; Engelhardt, M.; Pawlyn, C.; Bringhen, S.; Zweegman, S.; Stege, C.A.; Rosko, A.E.; von Lilienfeld-Toal, M.; Wildes, T.M. Caring for older adults with multiple myeloma during the COVID-19 Pandemic: Perspective from the International Forum for Optimizing Care of Older Adults with Myeloma. J. Geriatr. Oncol. 2020, 11, 764–768. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA A Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, F.; Xie, L.; Wang, C.; Wang, J.; Chen, R.; Jia, P.; Guan, H.Q.; Peng, L.; Chen, Y.; et al. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020, 31, 894–901. [Google Scholar] [CrossRef]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q.; et al. Patients with cancer appear more vulnerable to SARS-CoV-2: A multicenter study during the COVID-19 outbreakpatients with cancer in SARS-COV-2 infection. Cancer Discov. 2020, 10, 783–791. [Google Scholar] [CrossRef]
- Omarini, C.; Maur, M.; Luppi, G.; Narni, F.; Luppi, M.; Dominici, M.; Longo, G.; Piacentini, F. Cancer treatment during the coronavirus disease 2019 pandemic: Do not postpone, do it! Eur. J. Cancer 2020, 133, 29–32. [Google Scholar] [CrossRef]
- Lee, L.Y.; Cazier, J.B.; Starkey, T.; Turnbull, C.D.; The UK Coronavirus Monitoring Project Team; Kerr, R.; Middleton, G. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet 2020, 395, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Hultcrantz, M.; Richter, J.; Rosenbaum, C.A.; Patel, D.; Smith, E.L.; Korde, N.; Lu, S.X.; Mailankody, S.; Shah, U.A.; Lesokhin, A.M.; et al. COVID-19 infections and clinical outcomes in patients with multiple myeloma in New York City: A cohort study from five academic centers. Blood Cancer Discov. 2020, 1, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020, 21, e181. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Jin, R.; Zhao, J.; Li, W.; Shen, H. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020, 21, e180. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Aguilera, J.V.; Chintakuntlawar, A.; et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: A systematic review and meta-analysis. JAMA Oncol. 2019, 5, 1008–1019. [Google Scholar] [CrossRef]
- Cai, W.; Liu, T.; Xue, X.; Luo, G.; Wang, X.; Shen, Y.; Fang, Q.; Sheng, J.; Chen, F.; Liang, T. CT Quantification and Machine-learning Models for Assessment of Disease Severity and Prognosis of COVID-19 Patients. Acad. Radiol. 2020, 27, 1665–1678. [Google Scholar] [CrossRef]
- von Lilienfeld-Toal, M.; Vehreschild, J.J.; Cornely, O.; Pagano, L.; Compagno, F.; Hirsch, H.H. Frequently asked questions regarding SARS-CoV-2 in cancer patients—Recommendations for clinicians caring for patients with malignant diseases. Leukemia 2020, 34, 1487–1494. [Google Scholar] [CrossRef]
- Wang, B.; Van Oekelen, O.; Mouhieddine, T. A tertiary center experience of multiple myeloma patients with COVID-19: Lessons learned and the path forward. J. Hematol. Oncol. 2020, 13, 94. [Google Scholar] [CrossRef]
- Zheng, R.S.; Sun, K.X.; Zhang, S.W.; Zeng, H.M.; Zou, X.N.; Chen, R.; Gu, X.Y.; Wei, W.W.; He, J. Report of cancer epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi [Chin. J. Oncol.] 2019, 41, 19–28. [Google Scholar] [PubMed]
- Mehta, V.; Goel, S.; Kabarriti, R.; Cole, D.; Goldfinger, M.; Acuna-Villaorduna, A.; Pradhan, K.; Thota, R.; Reissman, S.; Sparano, J.A.; et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020, 10, 935–941. [Google Scholar] [CrossRef]
- Devlin, H. Men are much more likely to die from coronavirus—But why. Guardian 2020, 26. [Google Scholar]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Deakin, C.T.; Webb, K. Sex-bias in COVID-19: A Meta-Analysis and Review of Sex Differences in Disease and Immunity. Available online: https://ssrn.com/abstract=3572881 (accessed on 20 December 2022). [CrossRef]
- Wambier, C.G.; Vaño-Galván, S.; McCoy, J.; Gomez-Zubiaur, A.; Herrera, S.; Hermosa-Gelbard, Á.; Moreno-Arrones, O.M.; Jiménez-Gómez, N.; González-Cantero, A.; Fonda-Pascual, P.; et al. Androgenetic alopecia present in the majority of patients hospitalized with COVID-19: The “Gabrin sign”. J. Am. Acad. Dermatol. 2020, 83, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Scully, E.P.; Haverfield, J.; Ursin, R.L.; Tannenbaum, C.; Klein, S.L. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat. Rev. Immunol. 2020, 20, 442–447. [Google Scholar] [CrossRef]
- Klein, S.L.; Dhakal, S.; Ursin, R.L.; Deshpande, S.; Sandberg, K.; Mauvais-Jarvis, F. Biological sex impacts COVID-19 outcomes. PLoS Pathog. 2020, 16, e1008570. [Google Scholar] [CrossRef]
- Park, R.; Chidharla, A.; Mehta, K.; Sun, W.; Wulff-Burchfield, E.; Kasi, A. Sex-bias in COVID-19-associated illness severity and mortality in cancer patients: A systematic review and meta-analysis. EClinicalMedicine 2020, 26, 100519. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, J.M.; Poon, L.L.; Lee, K.C.; Ng, W.F.; Lai, S.T.; Leung, C.Y.; Chu, C.M.; Hui, P.K.; Mak, K.L.; Lim, W.; et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet 2003, 361, 1773–1778. [Google Scholar] [CrossRef]
- Russell, C.D.; Millar, J.E.; Baillie, J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020, 395, 473–475. [Google Scholar] [CrossRef]
- Passamonti, F.; Romano, A.; Salvini, M.; Merli, F.; Della Porta, M.G.; Bruna, R.; Coviello, E.; Romano, I.; Cairoli, R.; Lemoli, R.; et al. COVID-19 elicits an impaired antibody response against SARS-CoV-2 in patients with haematological malignancies. Br. J. Haematol. 2021, 195, 371–377. [Google Scholar] [CrossRef]
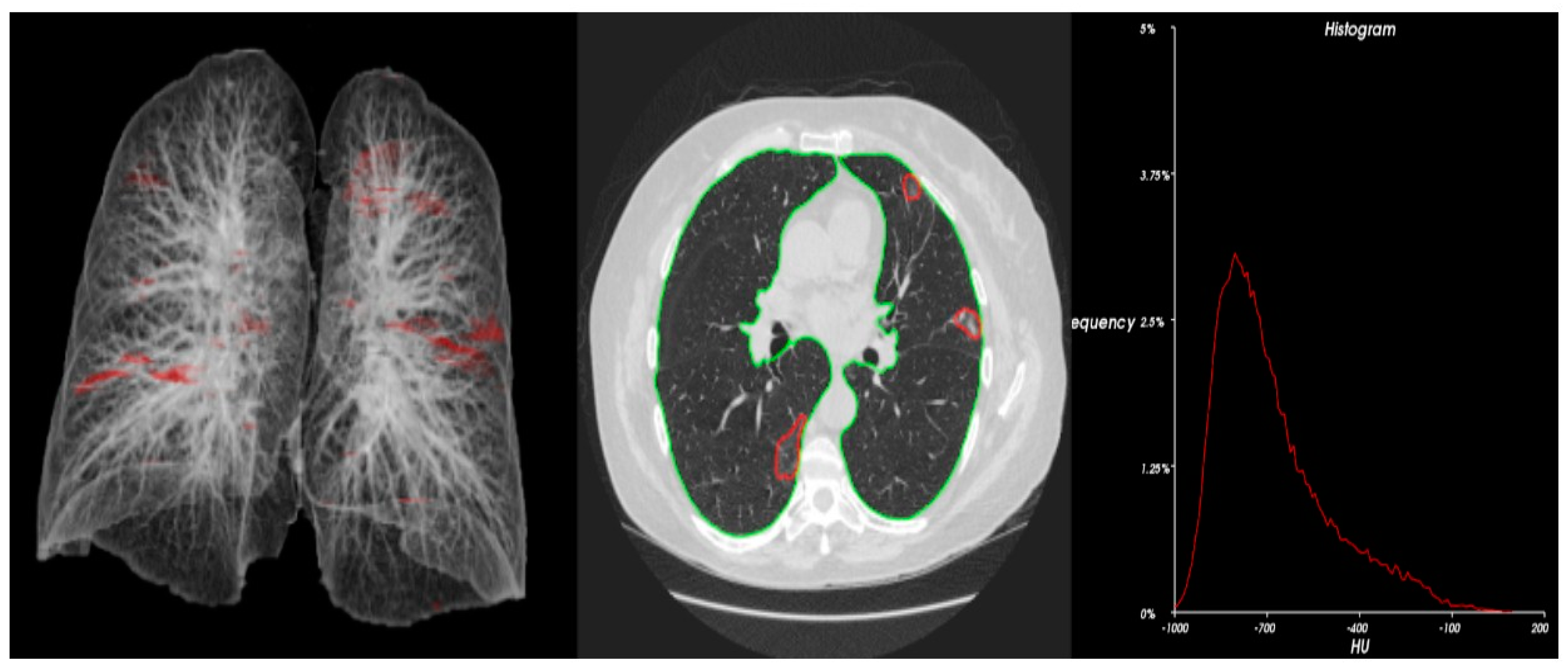


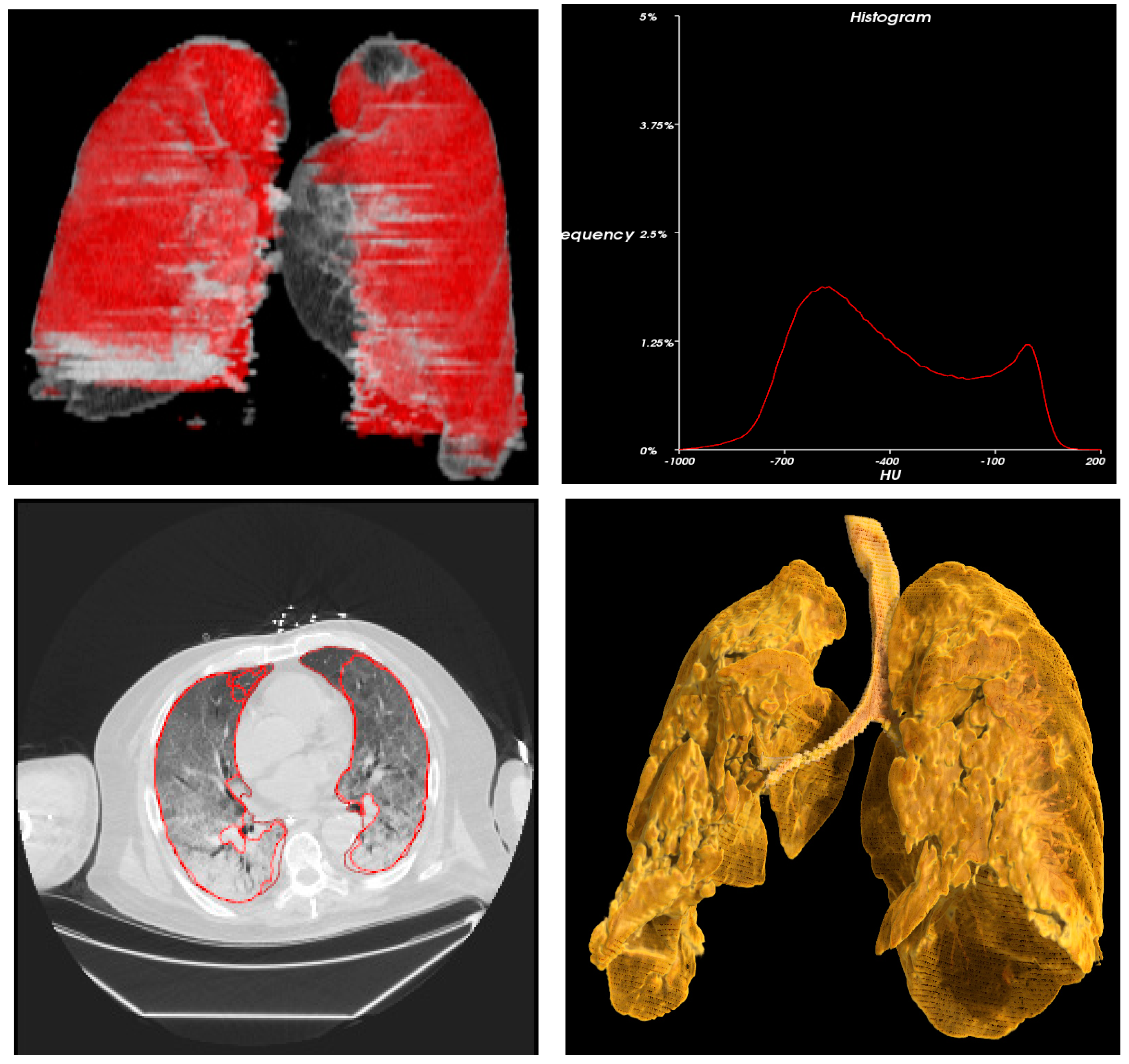

| Demographic Characteristics | Frequency | Percentage | |
|---|---|---|---|
| Age | Mean ± SD | 55.6 ± 18.54 | |
| Gender | Male | 18 | 65.5 |
| Female | 10 | 34.5 | |
| Age (Years) | Gender | Lung (CC) | Lesion (CC) | Lesion (%) | <0.25 (HU) | >0.75 (HU) | Kurtosis | |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 38 | Male | 3417.75 | 251.32 | 7.35 | –668.40 | −232.80 | −1.06 |
| Patient 2 | 64 | Female | 4842.12 | 28.34 | 0.59 | −801.50 | −577.70 | 0.89 |
| Patient 3 | 79 | Male | 3983.27 | 1214.42 | 30.49 | −677.90 | −375.40 | −0.23 |
| Patient 4 | 54 | Female | 3822.75 | 63.32 | 1.66 | −705.60 | −288.80 | −0.76 |
| Patient 5 | 39 | Female | 4759.91 | 3030.76 | 63.67 | −604.70 | −198.80 | −0.96 |
| Patient 6 | 37 | Male | 3646.28 | 535.72 | 14.69 | −679.20 | −372.30 | −0.18 |
| Patient 7 | 73 | Male | 2998.78 | 2395.55 | 79.88 | −590.30 | −207.60 | −1.00 |
| Patient 8 | 59 | Male | 2772.49 | 239.01 | 8.62 | −685.80 | −241.20 | −1.07 |
| Patient 9 | 19 | Male | 4331.45 | 603.58 | 13.93 | −628.40 | −355.90 | −0.59 |
| Patient 10 | 34 | Female | 1625.70 | 725.48 | 44.63 | −603.40 | −209.60 | −1.07 |
| Patient 11 | 68 | Female | 4928.02 | 956.12 | 19.40 | −760.10 | −348.30 | −0.66 |
| Patient 12 | 66 | Male | 5173.26 | 10.36 | 0.20 | −713.00 | −447.40 | −0.30 |
| Patient 13 | 31 | Female | 2550.71 | 16.47 | 0.65 | −763.63 | −766.04 | 0.26 |
| Patient 14 | 18 | Male | 3538.70 | 142.05 | 4.01 | −704.75 | −687.97 | 0.38 |
| Patient 15 | 62 | Male | 2707.00 | 0.00 | 0.00 | −710.00 | −721.00 | −1.06 |
| Patient 16 | 51 | Female | 2387.56 | 313.39 | 13.13 | −699.78 | −722.27 | 0.75 |
| Patient 17 | 83 | Female | 1984.52 | 1487.44 | 74.95 | −413.59 | −307.48 | 0.27 |
| Patient 18 | 23 | Male | 1782.86 | 1031.47 | 57.85 | −398.49 | −450.33 | 1.24 |
| Patient 19 | 83 | Male | 3749.23 | 73.12 | 1.95 | −812.62 | −810.27 | 0.34 |
| Patient 20 | 65 | Male | 3474.40 | 238.29 | 6.86 | −685.86 | −693.05 | 0.51 |
| Patient 21 | 62 | Male | 4233.50 | 146.46 | 3.46 | −776.89 | −784.54 | 0.36 |
| Patient 22 | 73 | Male | 2699.41 | 185.86 | 6.89 | −653.43 | −689.60 | 0.27 |
| Patient 23 | 64 | Female | 4507.96 | 51.79 | 1.15 | −795.79 | −813.74 | 0.27 |
| Patient 24 | 34 | Female | 3341.41 | 0.00 | 0.00 | −797.91 | −810.44 | 0.26 |
| Patient 25 | 74 | Male | 4202.10 | 230.08 | 5.48 | −719.93 | −700.45 | 0.45 |
| Patient 26 | 63 | Male | 1842.53 | 70.84 | 3.84 | −750.79 | −787.42 | 0.24 |
| Patient 27 | 63 | Male | 4486.89 | 351.70 | 7.84 | −664.92 | −691.91 | 0.57 |
| Patient 28 | 70 | Male | 3090.97 | 1716.68 | 55.54 | −493.92 | −466.78 | 0.92 |
| Lung (CC) | Lesion (CC) | Lesion (%) | ||
|---|---|---|---|---|
| Patient 2 | Pre | 4842.12 | 28.34 | 0.59 |
| Post | 4609.06 | 164.81 | 3.58 | |
| Patient 3 | Pre | 3983.27 | 1214.42 | 30.49 |
| Post | 5821.05 | 457.45 | 7.86 | |
| Patient 6 | Pre | 3646.28 | 535.72 | 14.69 |
| Post | 4399.97 | 242.51 | 5.51 | |
| Patient 7 | Pre | 2998.78 | 2395.55 | 79.88 |
| Post | 3390.13 | 2970.58 | 87.62 | |
| Patient 15 | Pre | 2707.00 | 0.00 | 0.00 |
| Post | 1455.00 | 0.00 | 0.00 | |
| Patient 16 | Pre | 2387.56 | 313.39 | 13.13 |
| Post | 2598.47 | 243.58 | 9.37 | |
| Patient 18 | Pre | 1782.86 | 1031.47 | 57.85 |
| Post | 2733.87 | 0.00 | 0.00 | |
| Patient 19 | Pre | 3749.23 | 73.12 | 1.95 |
| Post | 3578.28 | 524.87 | 14.67 | |
| Patient 20 | Pre | 3474.40 | 238.29 | 6.86 |
| Post | 2942.11 | 133.54 | 4.54 | |
| Patient 22 | Pre | 2699.41 | 185.86 | 6.89 |
| Post | 3964.46 | 48.37 | 1.22 | |
| Patient 23 | Pre | 4507.96 | 51.79 | 1.15 |
| Post | 4634.40 | 247.88 | 5.35 | |
| Patient 24 | Pre | 3341.41 | 0.00 | 0.00 |
| Post | 1749.26 | 856.68 | 48.97 | |
| Patient 26 | Pre | 1842.53 | 70.84 | 3.84 |
| Post | 3025.89 | 1271.02 | 42.00 | |
| Patient 27 | Pre | 4486.89 | 351.70 | 7.84 |
| Post | 5492.09 | 7.94 | 0.14 | |
| Patient 28 | Pre | 3090.97 | 1716.68 | 55.54 |
| Post | 4482.71 | 18.68 | 0.42 | |
| n = 15 | ΔLung Vol % (Post-Pre)/Pre | ΔLesion Vol (cc) (Post-Pre)/Pre | ΔLesion % (Post-Pre) |
|---|---|---|---|
| Mean | 13.65% | −67.95 | −3.92 |
| Standard Error | 34.23% | 729.77 | 28.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmanuddin, S.; Jamil, A.; Chaudhry, A.; Seto, T.; Brase, J.; Motarjem, P.; Khan, M.; Tomasetti, C.; Farwa, U.; Boswell, W.; et al. COVID and Cancer: A Complete 3D Advanced Radiological CT-Based Analysis to Predict the Outcome. Cancers 2023, 15, 651. https://doi.org/10.3390/cancers15030651
Rahmanuddin S, Jamil A, Chaudhry A, Seto T, Brase J, Motarjem P, Khan M, Tomasetti C, Farwa U, Boswell W, et al. COVID and Cancer: A Complete 3D Advanced Radiological CT-Based Analysis to Predict the Outcome. Cancers. 2023; 15(3):651. https://doi.org/10.3390/cancers15030651
Chicago/Turabian StyleRahmanuddin, Syed, Asma Jamil, Ammar Chaudhry, Tyler Seto, Jordyn Brase, Pejman Motarjem, Marjaan Khan, Cristian Tomasetti, Umme Farwa, William Boswell, and et al. 2023. "COVID and Cancer: A Complete 3D Advanced Radiological CT-Based Analysis to Predict the Outcome" Cancers 15, no. 3: 651. https://doi.org/10.3390/cancers15030651
APA StyleRahmanuddin, S., Jamil, A., Chaudhry, A., Seto, T., Brase, J., Motarjem, P., Khan, M., Tomasetti, C., Farwa, U., Boswell, W., Ali, H., Guidaben, D., Haseeb, R., Luo, G., Marcucci, G., Rosen, S. T., & Cai, W. (2023). COVID and Cancer: A Complete 3D Advanced Radiological CT-Based Analysis to Predict the Outcome. Cancers, 15(3), 651. https://doi.org/10.3390/cancers15030651









