Enhancing Cervical Cancer Prevention in South African Women: Primary HPV mRNA Screening with Different Genotype Combinations
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
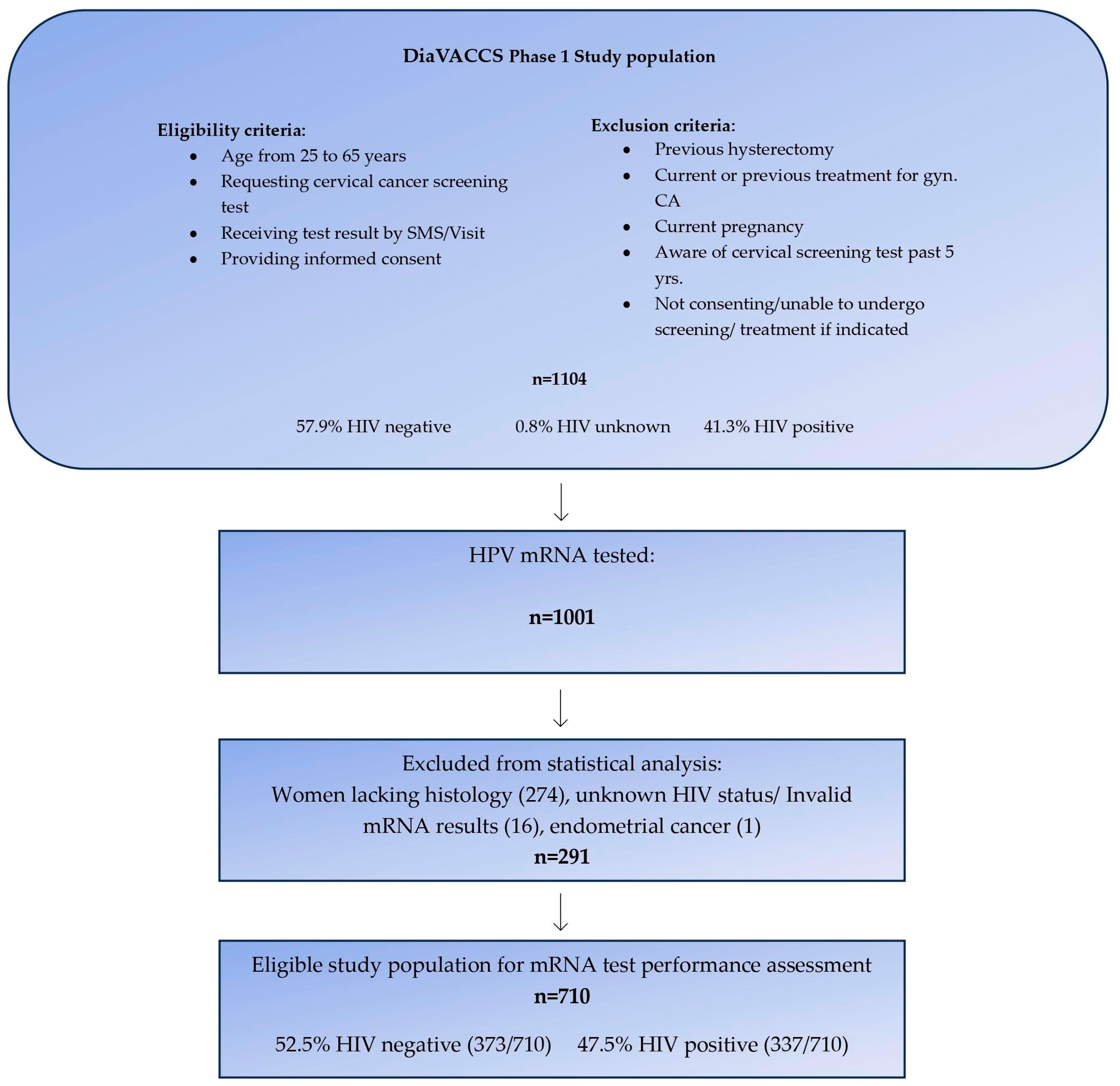
2.1. Study Population and Recruitment
2.2. Study Protocol and Procedures
2.3. Diagnostic Evaluation and Classification
2.4. HIV Status and Categorization
2.5. HPV mRNA E6/E7 Testing
2.6. Analysis of mRNA Assays and Cervical Cancer Prevention Potential
2.7. Statistical Analysis
2.8. Ethical Considerations
3. Results
3.1. Characteristics and Positivity Rate
3.2. HPV Prevalence and Histological Diagnoses
3.3. Performance of HPV mRNA Assays for CIN3+ Detection
3.4. Impact on Cervical Cancer Prevention
4. Discussion
4.1. HPV Prevalence and Testing Approaches
4.2. Sensitivity, Specificity, and Predictive Values
4.3. Cervical Cancer Prevention Potential
4.4. Considerations and Future Directions
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Safaeian, M.; Solomon, D. Cervical cancer prevention—Cervical screening: Science in evolution. Obstet. Gynecol. Clin. N. Am. 2007, 34, 739–760. [Google Scholar] [CrossRef] [PubMed]
- Franco, E.L.; Mahmud, S.M.; Tota, J.; Ferenczy, A.; Coutlee, F. The expected impact of HPV vaccination on the accuracy of cervical cancer screening: The need for a paradigm change. Arch. Med. Res. 2009, 40, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Herzog, T.J.; Huh, W.K.; Einstein, M.H. How does public policy impact cervical screening and vaccination strategies? Gynecol. Oncol. 2010, 119, 175–180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Petersen, Z.; Jaca, A.; Ginindza, T.G.; Maseko, G.; Takatshana, S.; Ndlovu, P.; Zondi, N.; Zungu, N.; Varghese, C.; Hunting, G.; et al. Barriers to uptake of cervical cancer screening services in low-and-middle-income countries: A systematic review. BMC Womens Health 2022, 22, 486. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy towards the Elimination of Cervical Cancer as a Public Health Problem. 2020. Available online: https:/ijgc.bmj.com/content/ijgc/early/2020/03/02/ijgc-2020-0011285.full.pdf (accessed on 17 March 2022).
- Zhang, X.; Zeng, Q.; Cai, W.; Ruan, W. Trends of cervical cancer at global, regional, and national level: Data from the Global Burden of Disease study 2019. BMC Global Health 2021, 21, 894. [Google Scholar] [CrossRef]
- Bruni, L.; Diaz, M.; Barrionuevo-Rosas, L.; Herrero, R.; Bray, F.; Bosch, F.X.; de Sanjosé, S.; Castellsagué, X. Global estimates of human papillomavirus vaccination coverage by region and income level: A pooled analysis. Lancet Glob. Health 2016, 4, e453–e463. [Google Scholar] [CrossRef]
- Botha, M.H.; Dreyer, G. Guidelines for cervical cancer screening in South Africa. S. Afr. J. Gynaecol. Oncol. 2017, 9, 8–12. [Google Scholar]
- Jordaan, S.; Michelow, P.; Richter, K.; Simoens, C.; Bogers, J. A Review of Cervical Cancer in South Africa: Previous, Current and Future. Health Care Curr. Rev. 2016, 4, 180. [Google Scholar] [CrossRef]
- Richter, K.L. Understanding and incorporating human papillomavirus testing in cervical cancer screening: A South African perspective. S. Afr. J. Gynaecol. Oncol. 2011, 3, 9–14. [Google Scholar] [CrossRef][Green Version]
- Mbulawa, Z.Z.A.; Phohlo, K.; Garcia-Jardon, M.; Williamson, A.-L.; Businge, C.B. High human papillomavirus (HPV)-35 prevalence among South African women with cervical intraepithelial neoplasia warrants attention. PLoS ONE 2022, 17, e0264498. [Google Scholar] [CrossRef]
- World Health Organization. South Africa. Human Papillomavirus (HPV) Vaccination Coverage. Available online: https://immunizationdata.who.int/pages/coverage/hpv.html?CODE=ZAF&ANTIGEN=&YEAR= (accessed on 31 January 2023).
- Amponsah-Dacosta, E.; Blose, N.; Nkwinika, V.V.; Chepkurui, V. Human Papillomavirus Vaccination in South Africa: Programmatic Challenges and Opportunities for Integration with Other Adolescent Health Services? Front. Public. Health 2020, 10, 799984. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.J.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in South Africa. Summary Report 10 March 2023. Available online: https://hpvcentre.net/statistics/reports/ZAF.pdf?t=1692013166708 (accessed on 1 November 2023).
- Lekoane, K.M.B.; Kuupiel, D.; Mashamba-Thompson, T.P.; Ginindza, T.G. The interplay of HIV and human papillomavirus-related cancers in sub-Saharan Africa: Scoping review. Syst. Rev. 2020, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Country Factsheets. South Africa 2020. HIV and AIDS Estimates. UNAIDS. Available online: https://www.unaids.org/en/regionscountries/countries/southafrica (accessed on 17 March 2022).
- Stelzle, D.; Tanaka, L.F.; Lee, K.K.; Khalil, A.I.; Baussano, I.; Shah, A.S.V.; McAllister, D.A.; Gottlieb, S.L.; Klug, S.J.; Winkler, A.S.; et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob. Health 2021, 9, e161–e169. [Google Scholar] [CrossRef] [PubMed]
- So, K.A.; Lee, I.H.; Lee, K.H.; Hong, S.R.; Kim, Y.J.; Seo, H.H.; Kim, T.J. Human papillomavirus genotype-specific risk in cervical carcinogenesis. J. Gynecol. Oncol. 2019, 30, e52. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.; Howell-Jones, R.; Li, N.; Bruni, L.; de Sanjosé, S.; Franceschi, S.; Clifford, G.M. Human papillomavirus types in 115,789 HPV-positive women: A meta-analysis from cervical infection to cancer. Int. J. Cancer 2012, 131, 2349–2359. [Google Scholar] [CrossRef]
- Bhatla, N.; Singhal, S. Primary HPV screening for cervical cancer. Best. Pract. Res. Clin. Obstet. Gynaecol. 2020, 65, 98–108. [Google Scholar] [CrossRef]
- zur Hausen, H. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef]
- de Sanjose, S.; Brotons, M.; Pavon, M.A. The natural history of human papillomavirus infection. Best. Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 2–13. [Google Scholar] [CrossRef]
- Sroczynski, G.; Esteban, E.; Widschwendter, A.; Oberaigner, W.; Borena, W.; von Laer, D.; Hackl, M.; Endel, G.; Siebert, U. Reducing overtreatment associated with overdiagnosis in cervical cancer screening—A model-based benefit-harm analysis for Austria. Int. J. Cancer 2020, 147, 1131–1142. [Google Scholar] [CrossRef]
- Derbie, A.; Mekonnen, D.; Woldeamanuel, Y.; van Ostade, X.; Abebe, T. HPV E6/E7 mRNA test for the detection of high grade cervical intraepithelial neoplasia (CIN2+): A systematic review. Infect. Agents Cancer 2020, 15, 9. [Google Scholar] [CrossRef]
- Sorbye, S.W.; Fismen, S.; Gutteberg, T.J.; Mortensen, E.S.; Skjeldestad, F.E. HPV mRNA is more specific than HPV DNA in triage of women with minor cervical lesions. PLoS ONE 2014, 9, e112934. [Google Scholar] [CrossRef]
- Rijkaart, D.C.; Heideman, D.A.; Coupe, V.M.; Brink, A.A.; Verheijen, R.H.; Skomedal, H.; Karlsen, F.; Morland, E.; Snijders, P.J.; Meijer, C.J. High-risk human papillomavirus (hrHPV) E6/E7 mRNA testing by PreTect HPV-Proofer for detection of cervical high-grade intraepithelial neoplasia and cancer among hrHPV DNA-positive women with normal cytology. J. Clin. Microbiol. 2012, 50, 2390–2396. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Benevolo, M.; Vocaturo, A.; Caraceni, D.; French, D.; Rosini, S.; Zappacosta, R.; Terrenato, I.; Ciccocioppo, L.; Frega, A.; Giorgi Rossi, P. Sensitivity, specificity, and clinical value of human papillomavirus (HPV) E6/E7 mRNA assay as a triage test for cervical cytology and HPV DNA test. J. Clin. Microbiol. 2011, 49, 2643–2650. [Google Scholar] [CrossRef] [PubMed]
- Origoni, M.; Cristoforoni, P.; Carminati, G.; Stefani, C.; Costa, S.; Sandri, M.T.; Mariani, L.; Preti, M. E6/E7 mRNA testing for human papilloma virus-induced high-grade cervical intraepithelial disease (CIN2/CIN3): A promising perspective. Ecancermedicalscience 2015, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Westre, B.; Giske, A.; Guttormsen, H.; Sørbye, S.W.; Skjeldestad, F.E. 5-type HPV mRNA versus 14-type HPV DNA test: Test performance, over-diagnosis and overtreatment in triage of women with minor cervical lesions. BMC Clin. Pathol. 2016, 16, 9. [Google Scholar] [CrossRef]
- Sørbye, S.W.; Fismen, S.; Gutteberg, T.; Mortensen, E.S. Triage of women with minor cervical lesions: Data suggesting a “test and treat” approach for HPV E6/E7 mRNA testing. PLoS ONE 2010, 5, e12724. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cuzick, J.; Cadman, L.; Mesher, D.; Austin, J.; Ashdown-Barr, L.; Ho, L.; Terry, G.; Liddle, S.; Wright, C.; Lyons, D.; et al. Comparing the performance of six human papillomavirus tests in a screening population. Br. J. Cancer 2013, 108, 908–913. [Google Scholar] [CrossRef]
- Verdoodt, F.; Szarewski, A.; Halfon, P.; Cuschieri, K.; Arbyn, M. Triage of women with minor abnormal cervical cytology: Meta-analysis of the accuracy of an assay targeting messenger ribonucleic acid of 5 high-risk human papillomavirus types. Cancer Cytopathol. 2013, 121, 675–687. [Google Scholar] [CrossRef]
- Sørbye, S.W.; Falang, B.M.; Antonsen, M. Performance of a 7-Type HPV mRNA Test in Triage of HPV DNA Primary Screen Positive Women Compared to Liquid-Based Cytology. J. Mol. Pathol. 2023, 4, 69–80. [Google Scholar] [CrossRef]
- Duvlis, S.; Popovska-Jankovic, K.; Arsova, Z.S.; Memeti, S.; Popeska, Z.; Plaseska-Karanfilska, D. HPV E6/E7 mRNA versus HPV DNA biomarker in cervical cancer screening of a group of Macedonian women. J. Med. Virol. 2015, 87, 1578–1586. [Google Scholar] [CrossRef]
- Cuschieri, K.; Wentzensen, N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2536–2545. [Google Scholar] [CrossRef]
- Dreyer, G.; Snyman, L.C.; Van der Merwe, F.H.; Richter, K.L.; Dreyer, G.J.; Visser, C.; Botha, M.H. Phase I of the DiaVACCS screening trial: Study design, methods, population demographics and baseline results. S. Afr. Med. J. 2022, 112, 478–486. [Google Scholar] [PubMed]
- Richart, R.M. Cervical intraepithelial neoplasia. Pathol. Annu. 1973, 8, 301–328. [Google Scholar]
- Rad, A.; Sørbye, S.W.; Dreyer, G.; Hovland, S.; Falang, B.M.; Louw, M.; Skjeldestad, F.E. HPV types in cervical cancer tissue in South Africa: A head-to-head comparison by mRNA and DNA tests. Medicine 2017, 96, e8752. [Google Scholar]
- Clifford, G.M.; Smith, J.S.; Plummer, M.; Muñoz, N.; Franceschi, S. Human papillomavirus types in invasive cervical cancer worldwide: A meta-analysis. Br. J. Cancer 2003, 88, 63–73. [Google Scholar] [PubMed]
- IARC. Cervical cancer screening. In IARC Handbooks of Cancer Prevention; IARC: Lyon, France, 2022; Volume 18, pp. 1–456. Available online: https://publications.iarc.fr/604 (accessed on 1 November 2023).
- Mbulawa, Z.Z.A.; Somdyala, N.I.; Mabunda, S.A.; Williamson, A.-L. High human papillomavirus prevalence among females attending high school in the Eastern Cape Province of South Africa. PLoS ONE 2021, 16, e0253074. [Google Scholar]
- McDonald, A.C.; Tergas, A.I.; Kuhn, L.; Denny, L.; Wright, T.C., Jr. Distribution of Human Papillomavirus Genotypes among HIV-Positive and HIV-Negative Women in Cape Town, South Africa. Front. Oncol. 2014, 4, 48. [Google Scholar]
- Taku, O.; Businge, C.B.; Mdaka, M.L.; Phohlo, K.; Basera, W.; Garcia-Jardon, M.; Meiring, T.L.; Gyllensten, U.; Williamson, A.-L.; Mbulawa, Z.Z.A. Human papillomavirus prevalence and risk factors among HIV-negative and HIV-positive women residing in rural Eastern Cape, South Africa. Int. J. Infect. Dis. 2020, 95, 176–182. [Google Scholar]
- Snyman, L.C.; Richter, K.L.; Lukhwareni, A.; Dreyer, G.; Botha, M.H.; Van Der Merwe, F.H.; Visser, C.; Dreyer, G. Cytology compared with Hybrid Capture 2 human papilloma virus cervical cancer screening in HIV positive and HIV negative South African women. Int. J. Gynecol. Cancer 2023, 33, 669–675. [Google Scholar] [CrossRef]
- Pal, A.; Kundu, R. Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy. Front. Microbiol. 2020, 10, 3116. [Google Scholar] [CrossRef]
- Moodley, M.; Moodley, J.; Kleinschmidt, I. Invasive cervical cancer and human immunodeficiency virus (HIV) infection: A South African perspective. Int. J. Gynecol. Cancer 2001, 11, 194–197. [Google Scholar] [CrossRef]
- McCredie, M.R.E.; Sharples, K.J.; Paul, C.; Baranyai, J.; Medley, G.; Jones, R.W.; Skegg, D.C.G. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: A retrospective cohort study. Lancet Oncol. 2008, 9, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Gustafsen, L.; Adami, H.O. Natural history of cervical neoplasia: Consistent results obtained by an identification technique. Br. J. Cancer 1989, 60, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Austin, R.M.; Onisko, A.; Zhao, C. Are CIN3 risk or CIN3+ risk measures reliable surrogate estimates for invasive cervical cancer risk? J. Am. Soc. Cytopath. 2020, 9, 602–606. [Google Scholar] [CrossRef] [PubMed]
- WHO; International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risk to Humans: Some Industrial Chemicals; IARC: Lyon, France, 1994. [Google Scholar]
- Schiffman, M.; Clifford, G.; Buonaguro, F.M. Classification of weakly carcinogenic human papillomavirus types: Addressing the limits of epidemiology at the borderline. Infect. Agents Cancer 2009, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention, 2nd ed.; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- WHO. WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention: Use of mRNA Tests for Human Papillomavirus (HPV); World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
| mRNA HPV Type | (n) | (%) |
|---|---|---|
| 16 | 48 | 6.8 |
| 45 | 33 | 4.6 |
| 58 | 29 | 4.1 |
| 18 | 27 | 3.8 |
| 52 | 27 | 3.8 |
| 31 | 24 | 3.3 |
| 35 | 21 | 3.0 |
| 33 | 15 | 2.1 |
| Negative | 486 | 68.5 |
| Overall positive | 224 | 31.5 |
| Characteristics | HIV Neg. | HIV Pos. | Total | p-Value |
|---|---|---|---|---|
| Women (n) | 373 | 337 | 710 | |
| Age n (years) | ||||
| 25–39 | 181 (48.5) | 171 (50.7) | 352 (49.6) | p = 0.60 |
| 40–65 | 192 (51.5) | 166 (49.3) | 358 (50.4) | |
| mRNA HPV types n (%) | ||||
| 16, 18, 45 | 33 (8.8) | 75 (22.3) | 108 (15.2) | p < 0.01 |
| 16, 18, 45, 35 | 40 (10.7) | 101 (30.0) | 141 (19.9) | |
| 16, 18, 45, 31, 33 | 46 (12.3) | 101 (30.0) | 147 (20.7) | |
| 16, 18, 45, 31, 33, 35 | 52 (13.9) | 123 (36.5) | 175 (24.6) | |
| 16, 18, 45, 31, 33, 52, 58 | 67 (18.0) | 136 (40.4) | 203 (28.6) | |
| 16, 18, 45, 31, 33, 52, 58, 35 | 71 (19.0) | 153 (45.4) | 224 (31.5) | |
| Most severe histology n (%) | ||||
| Normal | 139 (37.3) | 98 (29.1) | 237 (33.4) | |
| CIN1 | 123 (33.0) | 72 (21.4) | 195 (27.5) | |
| CIN2 | 63 (16.9) | 78 (23.1) | 141 (19.9) | p < 0.01 * |
| CIN3 | 44 (11.8) | 81 (24.0) | 125 (17.6) | p < 0.01 ** |
| ICC | 4 (1.1) | 8 (2.4) | 12 (1.7) |
| Histology | Normal | CIN1 | CIN2 | CIN3 | ICC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HIV Status | Neg. | Pos. | Neg. | Pos. | Neg. | Pos. | Neg. | Pos. | Neg. | Pos. |
| Women (N) | 139 | 98 | 123 | 72 | 63 | 78 | 44 | 81 | 4 | 8 |
| mRNA HPV+ (%) | ||||||||||
| 16, 18, 45 | 2.2 | 6.1 | 4.1 | 2.8 | 15.9 | 33.3 | 27.3 | 44.4 | 75 | 62.5 |
| 16, 18, 45, 35 | 2.9 | 9.2 | 4.1 | 4.2 | 20.6 | 47.4 | 34.1 | 56.8 | 75 | 75 |
| 16, 18, 45, 31, 33 | 3.6 | 7.1 | 5.7 | 12.5 | 17.5 | 42.3 | 45.5 | 54.3 | 75 | 100 |
| 16, 18, 45, 31, 33, 35 | 4.3 | 10.2 | 5.7 | 13.9 | 22.2 | 55.1 | 50 | 64.2 | 75 | 100 |
| 16, 18, 45, 31, 33, 52, 58 | 6.5 | 10.2 | 9.8 | 18.1 | 23.8 | 56.4 | 63.6 | 75.3 | 75 | 100 |
| 16, 18, 45, 31, 33, 52, 58, 35 | 7.2 | 13.3 | 9.8 | 19.4 | 27 | 65.4 | 65.9 | 82.7 | 75 | 100 |
| Sensitivity | Specificity | PPV | NPV | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV Status | Neg. | Pos. | Total | Neg. | Pos. | Total | Neg. | Pos. | Total | Neg. | Pos. | Total |
| mRNA HPV+ | % | % | % | % | % | % | % | % | % | % | % | % |
| 16, 18, 45 | 31.3 | 46.1 | 40.9 | 94.5 | 86.3 | 90.9 | 45.5 | 54.7 | 51.9 | 90.3 | 81.7 | 86.5 |
| 16, 18, 45, 35 | 37.5 | 58.4 | 41.1 | 93.2 | 80.2 | 87.6 | 45.0 | 51.5 | 49.6 | 91.0 | 84.3 | 88.2 |
| 16, 18, 45, 31, 33 | 47.5 | 58.4 | 54.7 | 92.9 | 80.2 | 87.4 | 50.0 | 51.5 | 51.0 | 92.4 | 84.3 | 89.0 |
| 16, 18, 45, 31, 33, 35 | 52.1 | 67.4 | 62.0 | 91.7 | 74.6 | 84.3 | 48.1 | 48.8 | 48.6 | 92.8 | 86.4 | 90.3 |
| 16, 18, 45, 31, 33, 52, 58 | 64.6 | 77.5 | 73.0 | 88.9 | 73.0 | 82.0 | 46.3 | 50.7 | 49.3 | 94.4 | 90.0 | 92.7 |
| 16, 18, 45, 31, 33, 52, 58, 35 | 66.6 | 84.3 | 78.1 | 88.0 | 68.5 | 79.6 | 45.1 | 49.0 | 47.8 | 94.7 | 92.4 | 93.8 |
| Positivity Rate mRNA | Sensitivity CIN3+ | NNT * to Address One Case CIN3+ | Estimated % CC Prevented ** [38] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV Status | Neg. | Pos. | Total | Neg. | Pos. | Total | Neg. | Pos. | Total | Neg. | Pos. | Total |
| Women (N) | 373 | 337 | 710 | 48 | 89 | 137 | NA | NA | NA | 96 | 65 | 161 |
| mRNA HPV+ | % | % | % | % | % | % | % | % | % | % | % | % |
| 16, 18, 45 | 8.8 | 22.3 | 15.2 | 31.3 | 46.1 | 40.9 | 2.2 | 1.8 | 1.9 | 66.7 | 70.8 | 68.3 |
| 16, 18, 45, 35 | 10.7 | 30.0 | 19.9 | 37.5 | 58.4 | 41.1 | 2.2 | 1.9 | 2.0 | 78.1 | 76.9 | 77.6 |
| 16, 18, 45, 31, 33 | 12.3 | 30.0 | 20.7 | 47.5 | 58.4 | 54.7 | 2.0 | 1.9 | 2.0 | 72.9 | 80.0 | 75.8 |
| 16, 18, 45, 31, 33, 35 | 13.9 | 36.5 | 24.6 | 52.1 | 67.4 | 62.0 | 2.1 | 2.1 | 2.1 | 84.4 | 86.2 | 85.1 |
| 16, 18, 45, 31, 33, 52, 58 | 18.0 | 40.4 | 28.6 | 64.6 | 77.5 | 73.0 | 2.2 | 2.0 | 2.0 | 78.1 | 83.1 | 80.1 |
| 16, 18, 45, 31, 33, 52, 58, 35 | 19.0 | 45.4 | 31.5 | 66.6 | 84.3 | 78.1 | 2.2 | 2.0 | 2.1 | 89.6 | 89.2 | 89.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sørbye, S.W.; Falang, B.M.; Botha, M.H.; Snyman, L.C.; van der Merwe, H.; Visser, C.; Richter, K.; Dreyer, G. Enhancing Cervical Cancer Prevention in South African Women: Primary HPV mRNA Screening with Different Genotype Combinations. Cancers 2023, 15, 5453. https://doi.org/10.3390/cancers15225453
Sørbye SW, Falang BM, Botha MH, Snyman LC, van der Merwe H, Visser C, Richter K, Dreyer G. Enhancing Cervical Cancer Prevention in South African Women: Primary HPV mRNA Screening with Different Genotype Combinations. Cancers. 2023; 15(22):5453. https://doi.org/10.3390/cancers15225453
Chicago/Turabian StyleSørbye, Sveinung Wergeland, Bente Marie Falang, Matthys H. Botha, Leon Cornelius Snyman, Haynes van der Merwe, Cathy Visser, Karin Richter, and Greta Dreyer. 2023. "Enhancing Cervical Cancer Prevention in South African Women: Primary HPV mRNA Screening with Different Genotype Combinations" Cancers 15, no. 22: 5453. https://doi.org/10.3390/cancers15225453
APA StyleSørbye, S. W., Falang, B. M., Botha, M. H., Snyman, L. C., van der Merwe, H., Visser, C., Richter, K., & Dreyer, G. (2023). Enhancing Cervical Cancer Prevention in South African Women: Primary HPV mRNA Screening with Different Genotype Combinations. Cancers, 15(22), 5453. https://doi.org/10.3390/cancers15225453








