Harnessing 3D-CT Simulation and Planning for Enhanced Precision Surgery: A Review of Applications and Advancements in Lung Cancer Treatment
Simple Summary
Abstract
1. Introduction
2. Segmental Anatomy Analysis Using 3D-CT Images for the Lung
3. 3D-CT Simulation and Navigation for Sublobar Lung Cancer Surgery
3.1. 3D-CT Simulation for Clinical Case Series
3.2. 3D Output Technologies from 3D-CT Reconstruction Images
3.3. 3D Simulation-Based Precision Sublobar Resection at Shinshu University
4. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Horn, L.; Johnson, D.H.; Evarts, A. Graham and the first pneumonectomy for lung cancer. J. Clin. Oncol. 2008, 26, 3268–3275. [Google Scholar] [CrossRef] [PubMed]
- Cahan, W.G. Radical lobectomy. J. Thorac. Cardiovasc. Surg. 1960, 39, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, R.J.; Rubinstein, L.V. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann. Thorac. Surg. 1995, 60, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Koike, T.; Asakawa, T.; Kusumoto, M.; Asamura, H.; Nagai, K.; Tada, H.; Mitsudomi, T.; Tsuboi, M.; Shibata, T.; et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J. Thorac. Oncol. 2011, 6, 751–756. [Google Scholar] [CrossRef]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): A multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef]
- Altorki, N.; Wang, X.; Kozono, D.; Watt, C.; Landrenau, R.; Wigle, D.; Port, J.; Jones, D.R.; Conti, M.; Ashrafi, A.S.; et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 388, 489–498. [Google Scholar] [CrossRef]
- Nagashima, T.; Shimizu, K.; Ohtaki, Y.; Obayashi, K.; Kakegawa, S.; Nakazawa, S.; Kamiyoshihara, M.; Igai, H.; Takeyoshi, I. An analysis of variations in the bronchovascular pattern of the right upper lobe using three-dimensional CT angiography and bronchography. Gen. Thorac. Cardiovasc. Surg. 2015, 63, 354–360. [Google Scholar] [CrossRef]
- Shimizu, K.; Nagashima, T.; Ohtaki, Y.; Obayashi, K.; Nakazawa, S.; Kamiyoshihara, M.; Igai, H.; Takeyoshi, I.; Mogi, A.; Kuwano, H. Analysis of the variation pattern in right upper pulmonary veins and establishment of simplified vein models for anatomical segmentectomy. Gen. Thorac. Cardiovasc. Surg. 2016, 64, 604–611. [Google Scholar] [CrossRef]
- Nagashima, T.; Shimizu, K.; Ohtaki, Y.; Obayashi, K.; Nakazawa, S.; Mogi, A.; Kuwano, H. Analysis of variation in bronchovascular pattern of the right middle and lower lobes of the lung using three-dimensional CT angiography and bronchography. Gen. Thorac. Cardiovasc. Surg. 2017, 65, 343–349. [Google Scholar] [CrossRef]
- Isaka, T.; Mitsuboshi, S.; Maeda, H.; Kikkawa, T.; Oyama, K.; Murasugi, M.; Kanzaki, M.; Onuki, T. Anatomical analysis of the left upper lobe of lung on three-dimensional images with focusing the branching pattern of the subsegmental veins. J. Cardiothorac. Surg. 2020, 15, 273. [Google Scholar] [CrossRef]
- Deng, Y.; Cai, S.; Huang, C.; Liu, W.; Du, L.; Wang, C.; Jia, R.; Lin, S.; Yu, X.; Yu, X.; et al. Anatomical variation analysis of left upper pulmonary blood vessels and bronchi based on three-dimensional reconstruction of chest CT. Front. Oncol. 2022, 12, 1028467. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.G.; Landreneau, J.R.; Schuchert, M.J.; Odell, D.D.; Gu, S.; Pu, J.; Luketich, J.D.; Landreneau, R.J. Preoperative (3-dimensional) computed tomography lung reconstruction before anatomic segmentectomy or lobectomy for stage I non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2015, 150, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Gan, F.; Xia, C.; Wang, Z.; Zhao, K.; Li, C.; Mei, J.; Liu, C.; Liao, H.; Pu, Q.; et al. Discovery of lung surface intersegmental landmarks by three-dimensional reconstruction and morphological measurement. Transl. Lung Cancer Res. 2019, 8, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Chu, X.P.; Zhang, J.T.; Fu, R.; Kang, J.; Chen, J.H.; Jiang, B.Y.; Wu, Y.L.; Zhong, W.Z.; Nie, Q. The regularity of anatomical variations of dominant pulmonary segments in the right upper lobe. Thorac. Cancer 2023, 14, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, M.; Shimada, Y.; Kato, Y.; Nawa, K.; Makino, Y.; Furumoto, H.; Akata, S.; Kakihana, M.; Kajiwara, N.; Ohira, T.; et al. High-quality 3-dimensional image simulation for pulmonary lobectomy and segmentectomy: Results of preoperative assessment of pulmonary vessels and short-term surgical outcomes in consecutive patients undergoing video-assisted thoracic surgerydagger. Eur. J. Cardiothorac. Surg. 2014, 46, e120–e126. [Google Scholar] [CrossRef]
- Cui, Z.; Ding, C.; Li, C.; Song, X.; Chen, J.; Chen, T.; Xu, C.; Zhao, J. Preoperative evaluation of the segmental artery by three-dimensional image reconstruction vs. thin-section multi-detector computed tomography. J. Thorac. Dis. 2020, 12, 4196–4204. [Google Scholar] [CrossRef]
- Nakao, M.; Omura, K.; Hashimoto, K.; Ichinose, J.; Matsuura, Y.; Okumura, S.; Mun, M. Novel three-dimensional image simulation for lung segmentectomy developed with surgeons’ perspective. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 1360–1365. [Google Scholar] [CrossRef]
- Chen, X.; Xu, H.; Qi, Q.; Sun, C.; Jin, J.; Zhao, H.; Wang, X.; Weng, W.; Wang, S.; Sui, X.; et al. AI-based chest CT semantic segmentation algorithm enables semi-automated lung cancer surgery planning by recognizing anatomical variants of pulmonary vessels. Front. Oncol. 2022, 12, 1021084. [Google Scholar] [CrossRef]
- Eguchi, T.; Takasuna, K.; Kitazawa, A.; Fukuzawa, Y.; Sakaue, Y.; Yoshida, K.; Matsubara, M. Three-dimensional imaging navigation during a lung segmentectomy using an iPad. Eur. J. Cardiothorac. Surg. 2012, 41, 893–897. [Google Scholar] [CrossRef][Green Version]
- Shimizu, K.; Nakazawa, S.; Nagashima, T.; Kuwano, H.; Mogi, A. 3D-CT anatomy for VATS segmentectomy. J. Vis. Surg. 2017, 3, 88. [Google Scholar] [CrossRef]
- Kato, H.; Oizumi, H.; Inoue, T.; Oba, E.; Nakamura, K.; Hayashi, J.; Watarai, H.; Yasumoto, T.; Sadahiro, M. Port-access thoracoscopic anatomical lung subsegmentectomy. Interact. Cardiovasc. Thorac. Surg. 2013, 16, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Oizumi, H.; Kato, H.; Endoh, M.; Suzuki, J.; Watarai, H.; Suzuki, K.; Sadahiro, M. Port-access thoracoscopic anatomical right anterior segmentectomy. J. Vis. Surg. 2015, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Takamori, S.; Oizumi, H.; Suzuki, J.; Suzuki, K.; Kabasawa, T. Video-Assisted Thoracoscopic Segmentectomy for Deep and Peripheral Small Lung Cancer. Thorac. Cardiovasc. Surg. 2022, 70, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Hamada, A.; Oizumi, H.; Kato, H.; Suzuki, J.; Nakahashi, K.; Takamori, S.; Sho, R.; Sadahiro, M. Outcome of thoracoscopic anatomical sublobar resection under 3-dimensional computed tomography simulation. Surg. Endosc. 2022, 36, 2312–2320. [Google Scholar] [CrossRef] [PubMed]
- She, X.W.; Gu, Y.B.; Xu, C.; Li, C.; Ding, C.; Chen, J.; Zhao, J. Three-dimensional (3D)-computed tomography bronchography and angiography combined with 3D-video-assisted thoracic surgery (VATS) versus conventional 2D-VATS anatomic pulmonary segmentectomy for the treatment of non-small cell lung cancer. Thorac. Cancer 2018, 9, 305–309. [Google Scholar] [CrossRef]
- Xue, L.; Fan, H.; Shi, W.; Ge, D.; Zhang, Y.; Wang, Q.; Yuan, Y. Preoperative 3-dimensional computed tomography lung simulation before video-assisted thoracoscopic anatomic segmentectomy for ground glass opacity in lung. J. Thorac. Dis. 2018, 10, 6598–6605. [Google Scholar] [CrossRef]
- Wang, J.; Xu, X.; Wen, W.; Wu, W.; Zhu, Q.; Chen, L. Modified method for distinguishing the intersegmental border for lung segmentectomy. Thorac. Cancer 2018, 9, 330–333. [Google Scholar] [CrossRef]
- Niu, Z.; Chen, K.; Jin, R.; Zheng, B.; Gong, X.; Nie, Q.; Jiang, B.; Zhong, W.; Chen, C.; Li, H. Three-dimensional computed tomography reconstruction in video-assisted thoracoscopic segmentectomy (DRIVATS): A prospective, multicenter randomized controlled trial. Front. Surg. 2022, 9, 941582. [Google Scholar] [CrossRef]
- Ohtaki, Y.; Yajima, T.; Nagashima, T.; Nakazawa, S.; Kawatani, N.; Obayashi, K.; Yazawa, T.; Shimizu, K.; Shirabe, K. Complex vs. simple segmentectomy: Comparing surgical outcomes in the left upper division. Gen. Thorac. Cardiovasc. Surg. 2022, 70, 962–970. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, T.; Feng, Y.; Chen, Y.; Feng, C.; Qin, D.; Han, C. Clinical application of VATS combined with 3D-CTBA in anatomical basal segmentectomy. Front. Oncol. 2023, 13, 1137620. [Google Scholar] [CrossRef]
- Le Moal, J.; Peillon, C.; Dacher, J.N.; Baste, J.M. Three-dimensional computed tomography reconstruction for operative planning in robotic segmentectomy: A pilot study. J. Thorac. Dis. 2018, 10, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Xu, G.; Fu, X.; Wu, W.; Liang, M.; Zeng, T.; Zhang, S.; Zhu, Y.; Zheng, W.; Chen, C.; et al. Management of the inter-segmental plane using the “Combined Dimensional Reduction Method” is safe and viable in uniport video-assisted thoracoscopic pulmonary segmentectomy. Transl. Lung Cancer Res. 2019, 8, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, N.; Igai, H.; Ohsawa, F.; Yazawa, T.; Kamiyoshihara, M. Safety and feasibility of uniportal video-assisted thoracoscopic uncommon segmentectomy. J. Thorac. Dis. 2021, 13, 3001–3009. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K.; Omori, K.; Nezu, K.; Lung Cancer Project Group of West-Seto Inland Sea, J. Superselective segmentectomy for deep and small pulmonary nodules under the guidance of three-dimensional reconstructed computed tomographic angiography. Ann. Thorac. Surg. 2010, 89, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, M.; Maeda, H.; Wachi, N.; Kikkawa, T.; Komine, H.; Isaka, T.; Oyama, K.; Murasugi, M.; Onuki, T. Complete video-assisted thoracoscopic multi-subsegmentectomy based on patients’ specific virtual 3-D pulmonary models. Asian J. Endosc. Surg. 2013, 6, 110–115. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Zhang, X.; Yin, H.; Fu, Y.; Cao, M.; Zhao, X. Application of three-dimensional (3D) reconstruction in the treatment of video-assisted thoracoscopic complex segmentectomy of the lower lung lobe: A retrospective study. Front. Surg. 2022, 9, 968199. [Google Scholar] [CrossRef]
- Iwano, S.; Yokoi, K.; Taniguchi, T.; Kawaguchi, K.; Fukui, T.; Naganawa, S. Planning of segmentectomy using three-dimensional computed tomography angiography with a virtual safety margin: Technique and initial experience. Lung Cancer 2013, 81, 410–415. [Google Scholar] [CrossRef]
- Wu, Y.J.; Shi, Q.T.; Zhang, Y.; Wang, Y.L. Thoracoscopic segmentectomy and lobectomy assisted by three-dimensional computed-tomography bronchography and angiography for the treatment of primary lung cancer. World J. Clin. Cases 2021, 9, 10494–10506. [Google Scholar] [CrossRef]
- Xu, G.; Du, J.; Chen, C.; Zheng, W.; Chen, H.; Xiao, J.; Wu, W. Intersegmental plane simulation based on the bronchus-vein-artery triad in pulmonary segmentectomy. Transl. Cancer Res. 2021, 10, 4702–4713. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Xuan, Y.; Lan, X.; Zhao, J.; Lan, X.; Han, B.; Jiao, W. Three-dimensional printing in the preoperative planning of thoracoscopic pulmonary segmentectomy. Transl. Lung Cancer Res. 2019, 8, 929–937. [Google Scholar] [CrossRef]
- Qiu, B.; Ji, Y.; He, H.; Zhao, J.; Xue, Q.; Gao, S. Three-dimensional reconstruction/personalized three-dimensional printed model for thoracoscopic anatomical partial-lobectomy in stage I lung cancer: A retrospective study. Transl. Lung Cancer Res. 2020, 9, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Chen, Q.; Li, T.; Chen, K.; Yu, Q.; Lin, X. Three-dimensional printing technology for localised thoracoscopic segmental resection for lung cancer: A quasi-randomised clinical trial. World J. Surg. Oncol. 2020, 18, 223. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, K.; Han, X.; Zhao, J.; Wang, G.; Yuan, S.; He, B. Three-dimensional computed tomography angiography and bronchography combined with three-dimensional printing for thoracoscopic pulmonary segmentectomy in stage IA non-small cell lung cancer. J. Thorac. Dis. 2021, 13, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Tongxin, L.; Jing, X.; Runyuan, W.; Wei, W.; Yu, Z.; Dong, W.; Wang, H.; Yi, W.; Ping, H.; Yong, F. Application Research of Three-Dimensional Printing Technology and Three-Dimensional Computed Tomography in Segmentectomy. Front. Surg. 2022, 9, 881076. [Google Scholar] [CrossRef]
- Kanzaki, M.; Isaka, T.; Kikkawa, T.; Sakamoto, K.; Yoshiya, T.; Mitsuboshi, S.; Oyama, K.; Murasugi, M.; Onuki, T. Binocular stereo-navigation for three-dimensional thoracoscopic lung resection. BMC Surg. 2015, 15, 56. [Google Scholar] [CrossRef]
- Ujiie, H.; Yamaguchi, A.; Gregor, A.; Chan, H.; Kato, T.; Hida, Y.; Kaga, K.; Wakasa, S.; Eitel, C.; Clapp, T.R.; et al. Developing a virtual reality simulation system for preoperative planning of thoracoscopic thoracic surgery. J. Thorac. Dis. 2021, 13, 778–783. [Google Scholar] [CrossRef]
- Chang, S.S.; Okamoto, T.; Tokunaga, Y.; Nakano, T. Intraoperative Computed Tomography Navigation During Thoracoscopic Segmentectomy for Small-sized Lung Tumors. Semin. Thorac. Cardiovasc. Surg. 2018, 30, 96–101. [Google Scholar] [CrossRef]
- Chang, S.S.; Yokomise, H.; Yokota, N.; Yoshida, C.; Katoh, A.; Misaki, N.; Go, T. Dual Image Navigation to Secure Surgical Margins in Thoracoscopic Segmentectomy. Ann. Surg. Oncol. 2023, 30, 843–849. [Google Scholar] [CrossRef]
- Hamanaka, K.; Miura, K.; Eguchi, T.; Shimizu, K. Simulation and Navigation techniques in segmentectomy for lung cancer. Video-Assist. Thorac. Surg. 2023, 8, 4. [Google Scholar] [CrossRef]
- Eguchi, T.; Shimura, M.; Mishima, S.; Hara, D.; Matsuoka, S.; Kumeda, H.; Miura, K.; Hamanaka, K.; Shimizu, K. Tailored Practical Simulation Training in Robotic Surgery: A New Educational Technology. Ann. Thorac. Surg. Short Rep. 2023, 1, 474–478. [Google Scholar] [CrossRef]
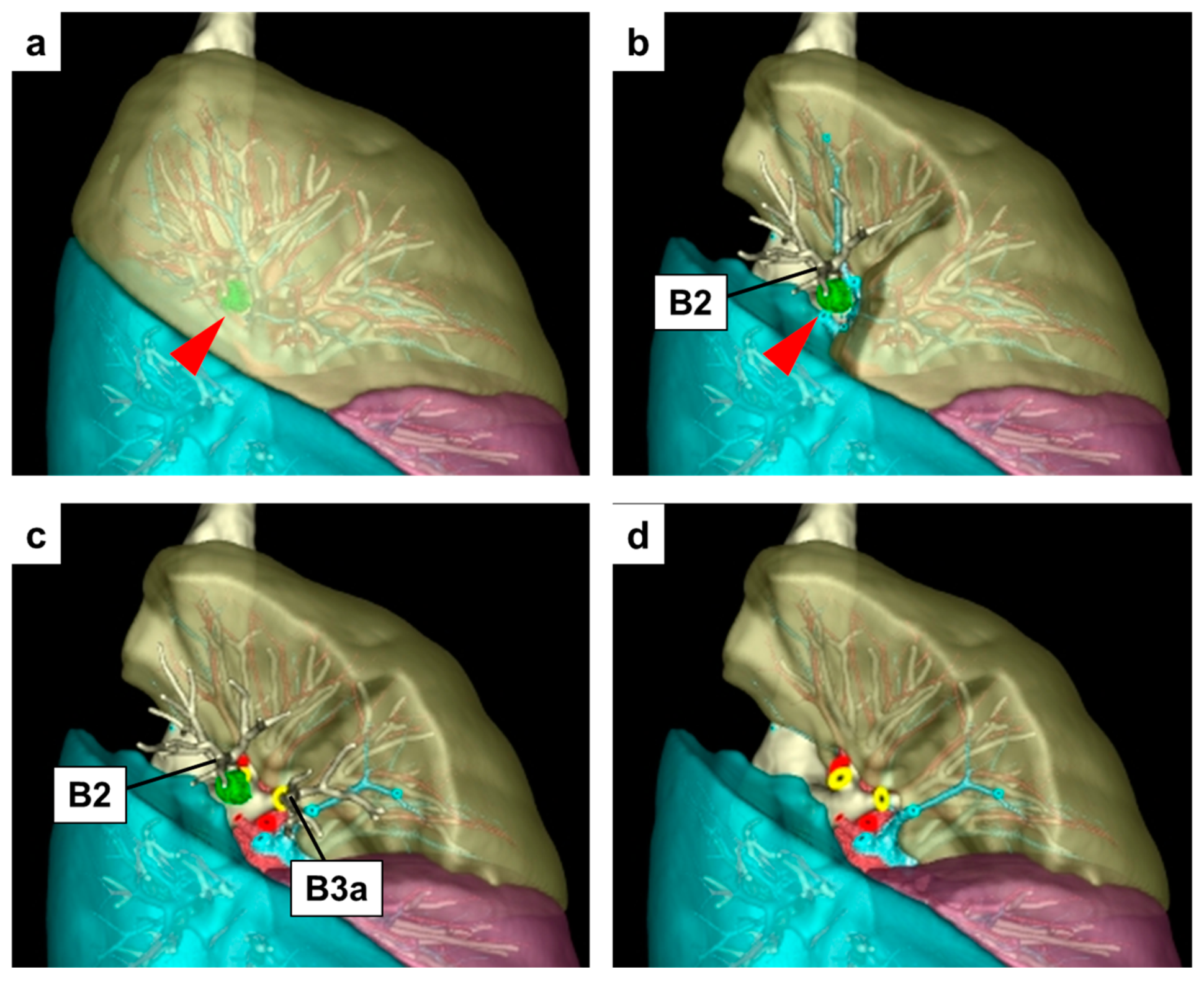
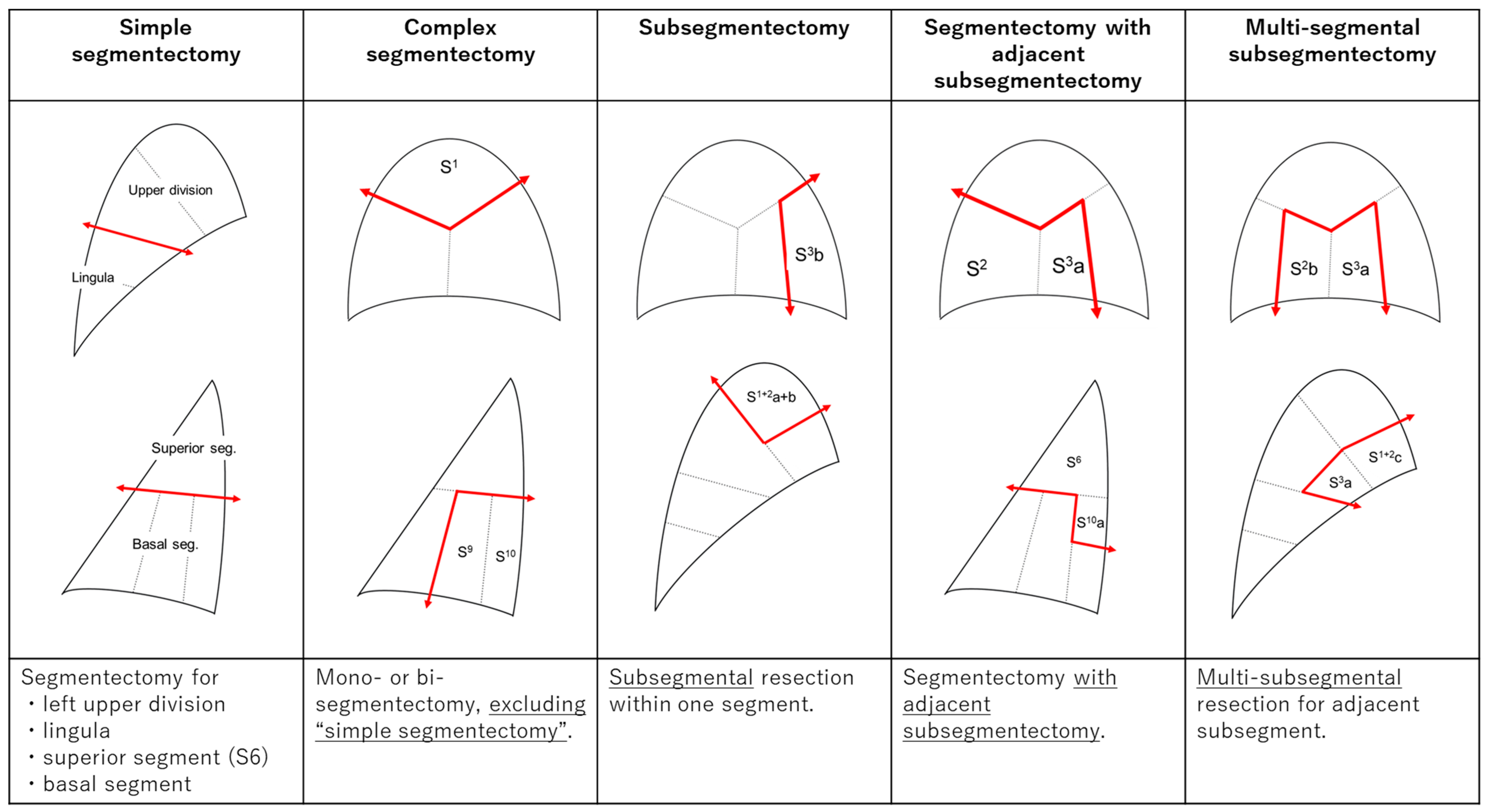
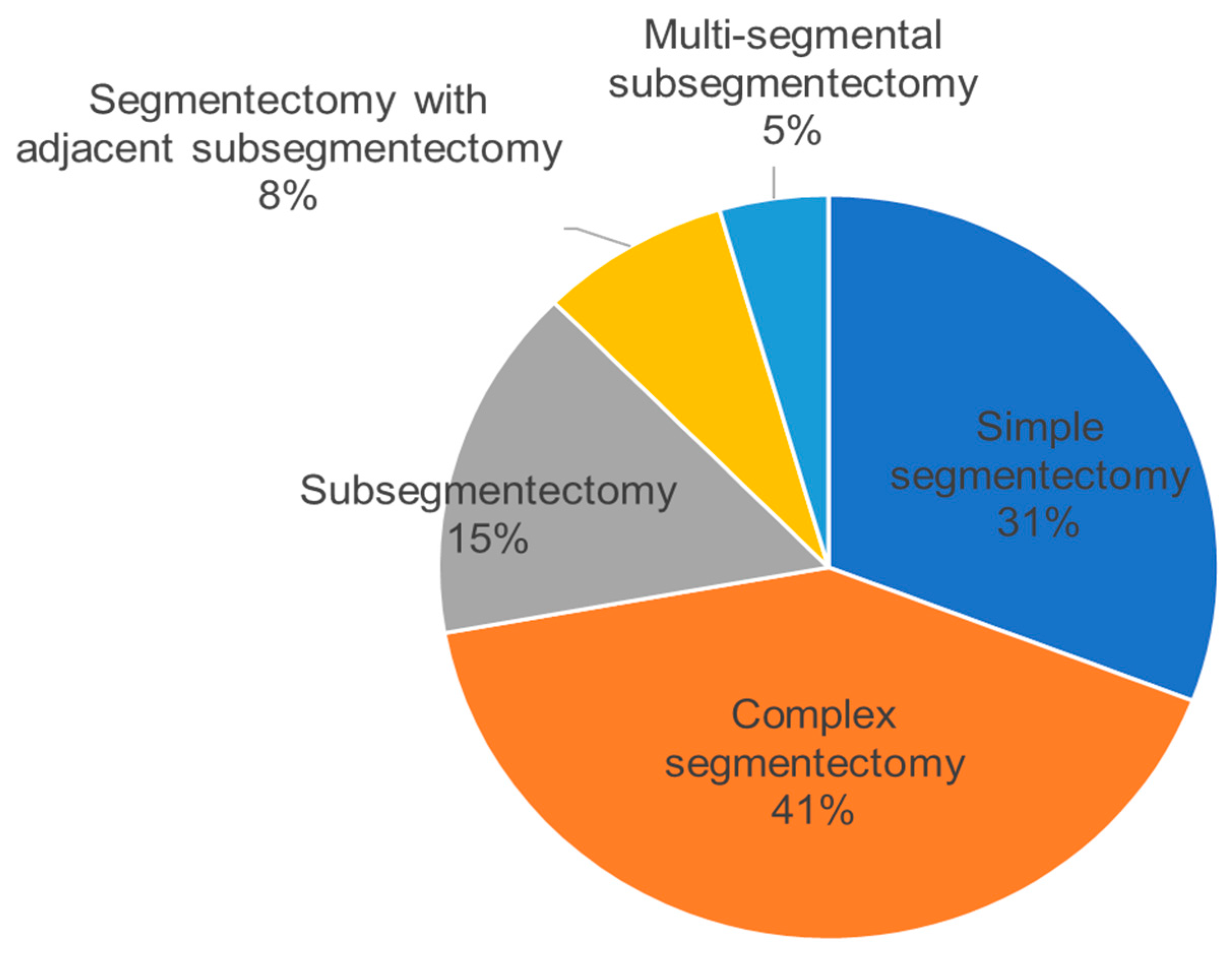
| Simple Segmentectomy | Complex Segmentectomy | Subsegmentectomy | Segmentectomy with Adjacent Subsegmentectomy | Multi-Segmental Subsegmentectomy | |||||
|---|---|---|---|---|---|---|---|---|---|
| left upper devision | 15 | S1 | 12 | S1a | 4 | S1+2 + S3a | 2 | S1a + S2a | 1 |
| Lingula | 15 | S1+ S2 | 2 | S1+2b | 2 | S1+2 + S3a+c | 1 | S2b + S3a | 4 |
| Lingula + S6 | 1 | S1 + S4 | 1 | S1+2c | 2 | S2 + S1a | 1 | S1+2a+b + S3c | 1 |
| Lingula + basal seg. | 1 | S1 + S9-10 | 1 | S1+2a+b | 3 | S2 + S3a | 3 | S1+2c + S3a | 1 |
| S6 | 37 | S1+2 | 11 | S1+2a+c | 1 | S3 + S1+2a | 2 | S1+2c + S3c | 1 |
| Basal seg. | 13 | S1+2 + S6 | 1 | S1+2b+c | 1 | S3 + S4+S5a | 1 | S1+2c + S3a + S4a | 1 |
| S2 | 11 | S2b | 1 | S4 + S3b | 3 | S3a + S4a | 1 | ||
| S3 | 20 | S3b | 10 | S4 + S3a+b | 1 | S8a+S9a | 1 | ||
| S3 + S4+5 | 1 | S3b+c | 1 | S4+S5+S1+2c | 1 | S8a+S9a + S3b+c | 1 | ||
| S4 | 4 | S4a | 2 | S6 + S10a | 2 | ||||
| S5 | 5 | S5a | 1 | S6 + S8a+S9a | 1 | ||||
| S5 + S8 | 1 | S5b | 1 | S8 + S9b | 1 | ||||
| S6 + S10 | 1 | S6a | 1 | S10 + S6c | 1 | ||||
| S7 | 6 | S6b | 1 | S10 + S7b | 1 | ||||
| S7+S8 | 1 | S8b | 5 | ||||||
| S7+S10 | 1 | S9b | 1 | ||||||
| S8 | 5 | S10b+c | 4 | ||||||
| S8+S9 | 6 | ||||||||
| S8+S9+S10 | 3 | ||||||||
| S9 | 5 | ||||||||
| S9+S10 | 7 | ||||||||
| S10 | 5 | ||||||||
| 82 (31%) | 110 (41%) | 41 (15%) | 21 (8%) | 12 (5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamanaka, K.; Miura, K.; Eguchi, T.; Shimizu, K. Harnessing 3D-CT Simulation and Planning for Enhanced Precision Surgery: A Review of Applications and Advancements in Lung Cancer Treatment. Cancers 2023, 15, 5400. https://doi.org/10.3390/cancers15225400
Hamanaka K, Miura K, Eguchi T, Shimizu K. Harnessing 3D-CT Simulation and Planning for Enhanced Precision Surgery: A Review of Applications and Advancements in Lung Cancer Treatment. Cancers. 2023; 15(22):5400. https://doi.org/10.3390/cancers15225400
Chicago/Turabian StyleHamanaka, Kazutoshi, Kentaro Miura, Takashi Eguchi, and Kimihiro Shimizu. 2023. "Harnessing 3D-CT Simulation and Planning for Enhanced Precision Surgery: A Review of Applications and Advancements in Lung Cancer Treatment" Cancers 15, no. 22: 5400. https://doi.org/10.3390/cancers15225400
APA StyleHamanaka, K., Miura, K., Eguchi, T., & Shimizu, K. (2023). Harnessing 3D-CT Simulation and Planning for Enhanced Precision Surgery: A Review of Applications and Advancements in Lung Cancer Treatment. Cancers, 15(22), 5400. https://doi.org/10.3390/cancers15225400







