Simple Summary
Patients with previous cancer are more likely to suffer from a heart attack than those without cancer. Although risk stratification scores are commonly used both to guide management and estimate mortality after a heart attack, it is unclear if such scores can be applied to patients with previous cancer. As such, this study aimed to determine the performance of the well-established GRACE (Global Registry of Acute Coronary Events) score by combining large national cancer and heart attack databases. This study showed that although the GRACE score was more accurate in patients without cancer, it greatly underestimated the risk of death in patients with previous cancer. Specific risk stratification scores are required for patients with previous cancer who have a heart attack.
Abstract
Background Patients with prior cancer are at increased risk of acute coronary syndrome (ACS) with poorer post-ACS outcomes. We aimed to ascertain if the Global Registry of Acute Coronary Events (GRACE) score accurately predicts mortality risk among patients with ACS and prior cancer. Methods We linked nationwide ACS and cancer registries from 2007 to 2018 in Singapore. A total of 24,529 eligible patients had in-hospital and 1-year all-cause mortality risk calculated using the GRACE score (2471 prior cancer; 22,058 no cancer). Results Patients with prior cancer had two-fold higher all-cause mortality compared to patients without cancer (in-hospital: 22.8% versus 10.3%, p < 0.001; 1-year: 49.0% vs. 18.7%, p < 0.001). Cardiovascular mortality did not differ between groups (in-hospital: 5.2% vs. 4.8%, p = 0.346; 1-year: 6.9% vs. 6.1%, p = 0.12). The area under the receiver operating characteristic curve of the GRACE score for prediction of all-cause mortality was less for prior cancer (in-hospital: 0.64 vs. 0.80, p < 0.001; 1-year: 0.66 vs. 0.83, p < 0.001). Among patients with prior cancer and a high-risk GRACE score > 140, in-hospital revascularization was not associated with lower cardiovascular mortality than without in-hospital revascularization (6.7% vs. 7.6%, p = 0.50). Conclusions The GRACE score performs poorly in risk stratification of patients with prior cancer and ACS.
1. Introduction
Due to improvements in cancer detection and treatment, more patients with cancer are living longer [1]. Although cancer-related outcomes continue to improve, cancer survivors are increasingly recognized to be at higher risk for cardiovascular disease, including acute coronary syndrome (ACS) [2,3,4]. However, due to a high burden of comorbidities and uncertain prognosis, best treatment practices for ACS in patients with cancer remain unclear. Percutaneous coronary intervention (PCI), which is routinely performed for ACS within the general population, has been associated with an increased risk of mortality and bleeding post-PCI in patients with cancer [5,6].
Although current guidelines advocate an invasive strategy in patients with cancer presenting with high-risk ACS, there is no clear recommendation on how best to risk stratify [7]. Guidelines advocate the use of risk stratification scores in guiding management of patients with ACS [8,9]. The Global Registry of Acute Coronary Events (GRACE) score is a well-established risk stratification score used to calculate mortality in patients presenting with ACS within the general population [10]. The European Society of Cardiology recommends the use of the GRACE score to estimate prognosis in patients with ACS and to identify suitable patients to undergo revascularization via PCI within 24 h as an early invasive strategy [9]. This is due to the GRACE score offering the best discriminative performance across other clinical risk scores [9,11]. Presently, expert consensus statements recommend using the same algorithms for diagnosis and monitoring of ACS in non-cancer patients to patients with cancer presenting with ACS [12]. Given the increased complications and poorer outcomes post-PCI observed in cancer patients, it is unclear if similar risk stratification with the GRACE score would be applicable to patients with cancer. Due to unaccounted risk variables, we hypothesized that the GRACE score would underestimate mortality in patients with cancer presenting with ACS compared to patients without cancer.
We linked data from nationwide registries to derive GRACE scores in patients presenting with ACS and compared scores in those with prior cancer to those without. Our objectives were, first, to determine if prior cancer was associated with a higher predicted risk of mortality via the GRACE score than those without cancer, and second, to compare the performance of the GRACE score in predicting mortality between the two groups of patients.
2. Materials and Methods
2.1. Study Design and Participants
Data from two national registries (Singapore Myocardial Infarction Registry and Cancer Registry) under the National Registry of Diseases Office of Singapore were combined in this population-based cohort study. [13]. In Singapore, notification of selected diseases, including cancer and ACS, is mandatory under the National Registry of Diseases Act. The Singapore Myocardial Infarction Registry is comprised of retrospectively collected records across all hospitals in Singapore of patients who presented with ACS beginning in 2007. Trained coordinators collect information, including demographics, clinical presentation, inpatient laboratory and echocardiographic parameters, and use of medications, prior to and at discharge [14]. As individual-level blood pressure and heart rate values were only available within the registry from 2017, data on blood pressure and heart rate were extracted from hospital-level databases from two centers and merged with the national registry for the years 2007 to 2016. The Cancer Registry is comprised of retrospectively collected records across all hospitals of all patients diagnosed with cancer since 1968. This database captures demographics and details on diagnosis and treatment within the first six months after diagnosis [15]. This study was approved by the local institutional review board (Domain Specific Review Board-C, National Healthcare Group, 2021/00141). Waiver of consent was granted for studies of public health importance using de-identified registry data.
Data linkage was performed between the Singapore Myocardial Infarction Registry and the Cancer Registry by using the unique National Registration Identity Card number for each patient. This determined if there was a prior diagnosis of cancer for patients presenting with ACS over 12 years from 1 January 2007 to 31 December 2018. The data were then subsequently matched with data from the Registry of Births and Deaths. As the reporting of deaths is mandatory by law in Singapore, this allowed us to obtain the survival status of all patients. All eligible patients presenting with ACS were classified into two groups for analysis: one with a prior diagnosis of cancer (prior cancer) and the other without (no cancer) forming the control group. Each patient had their individual GRACE score disease calculated at incident ACS. We calculated risk for each patient of in-hospital all-cause mortality through the GRACE score and 1-year all-cause mortality through the GRACE 2.0 score [16,17]. The GRACE score comprises eight variables: age, heart rate, systolic blood pressure, initial serum creatinine, Killip class, cardiac arrest, initial cardiac enzyme, and ST segment deviation [15]. The GRACE 2.0 score incorporates values from regression models based on these variables, which are then used to derive a sum estimate of the probability of adverse outcomes instead of a point system per the original GRACE score [17]. We excluded patients who developed cancer after their first ACS between 1 January 2007 and 31 December 2018 and patients with incomplete data resulting in incomplete calculation of an individual GRACE score. To maintain data in its original form, we excluded missing data from the analyses through case deletion without imputation. The outcomes of interest were in-hospital all-cause mortality and 1-year all-cause mortality post-ACS. STATA SE version 13 was used to perform all analyses.
2.2. Statistical Analysis
We compared demographics, comorbidities, and clinical characteristics for incident ACS in patients with prior cancer between patients without prior cancer using the chi-square test for categorical variables and the Wilcoxon rank-sum test for numeric variables. Among those with prior cancer, the predicted mortality in terms of GRACE score was compared across their cancer characteristics using the Kruskal–Wallis rank test and Wilcoxon rank-sum test, while the actual observed mortality was compared using the chi-square test. Aside from plotting the distribution of patients with prior cancer and those without cancer by their GRACE risk score, the Kaplan–Meier survival curves for the two groups of patients were also plotted. Area under the receiver operating characteristic curve (AUC) and Hosmer–Lemeshow goodness-of-fit test (GOF) were used to evaluate the diagnostic accuracy of the GRACE score for predicting mortality in the two groups of patients. p-Values < 0.05 were considered statistically significant.
3. Results
3.1. Baseline Demographics
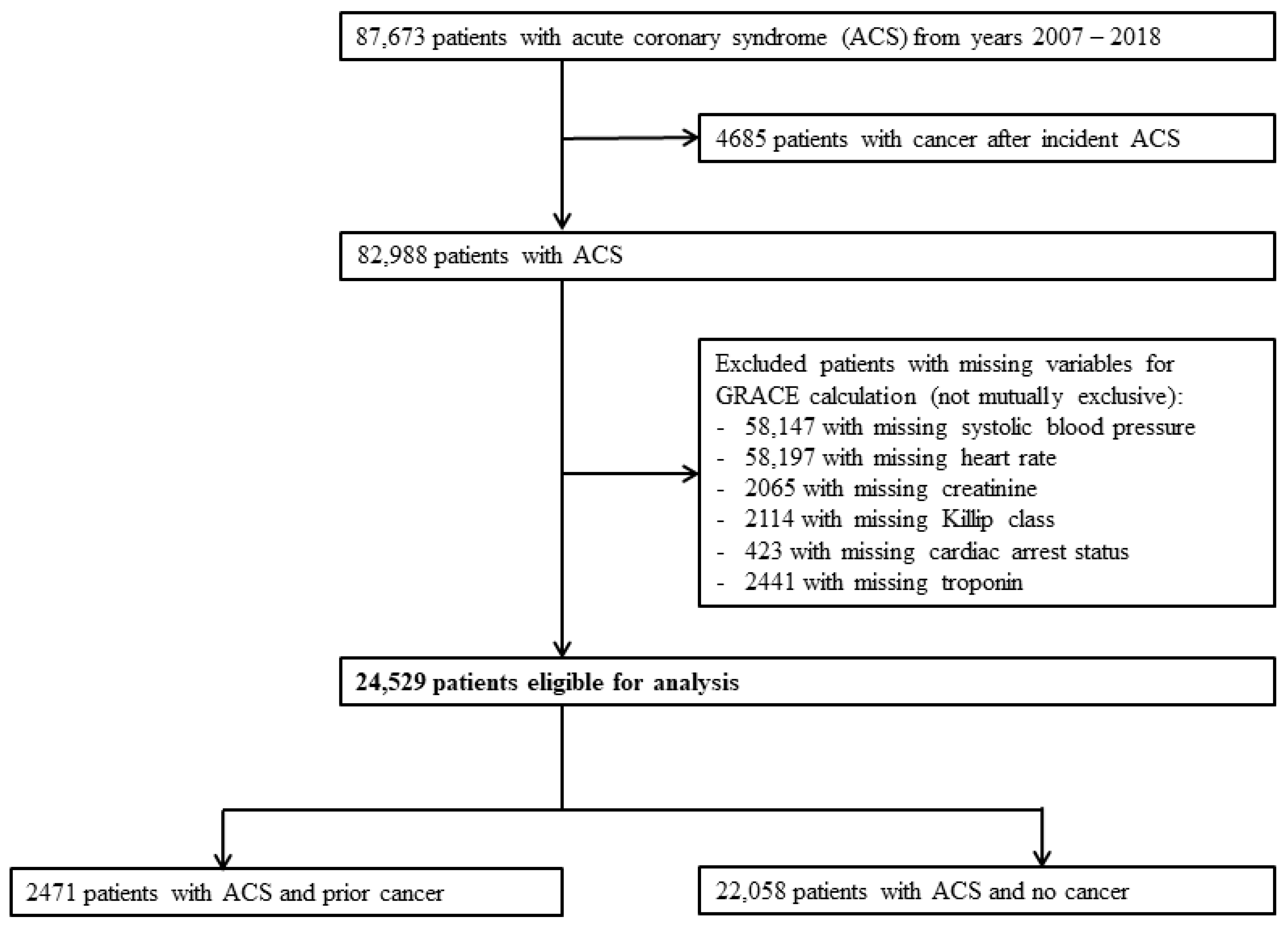
We identified 87,673 patients with ACS between 1 January 2007 and 31 December 2018. After excluding patients who developed cancer after incident ACS, 82,988 patients were available for analysis. We then further excluded patients with incomplete data for the calculation of GRACE score. A total of 24,529 patients were available for final analysis, of which 2471 patients had prior cancer (prior-cancer group) and 22,058 patients did not have cancer (no-cancer group). (Figure 1) The median duration from cancer diagnosis to ACS was 1911 days (5 years and 85 days) among those with prior cancer.
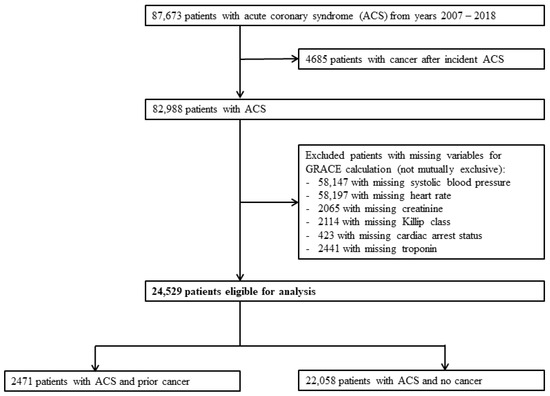
Figure 1.
Flowchart of study patients. Study flowchart demonstrating eventual number of eligible subjects for analysis after exclusion. ACS: acute coronary syndrome.
Baseline characteristics of the two groups with incident ACS are presented in Table 1. The prior-cancer group was older, with a lower proportion of men, higher proportion of Chinese ethnicity, and a lower median body mass index. The proportion of current smokers was lower in the prior-cancer group. The prevalence of hypertension was higher in the prior-cancer group, but the median systolic and diastolic blood pressures were lower for incident ACS. The prevalence of hyperlipidemia was lower in the prior-cancer group, where the median total cholesterol and low-density lipoprotein cholesterol concentrations were also lower for incident ACS. There was no significant difference in the prevalence of diabetes mellitus or history of myocardial infarction and revascularization between the two groups.

Table 1.
Demographic and clinical characteristics of patients with incident ACS.
The proportions of ST-segment elevation myocardial infarctions and cardiac arrests were lower in the prior-cancer group, and they were less likely to undergo revascularization (Table 1). Although the prior-cancer group had a lower proportion of heart failure (Killip class II, III or IV), there was no significant difference in the proportion of cardiogenic shock or median left ventricular ejection fraction. At the point of discharge after incident ACS, the prior-cancer group was less likely to be prescribed with guideline directed medical therapy.
The predicted risk from GRACE score for both in-hospital and 1-year all-cause mortality were significantly higher in the prior-cancer group compared to the no-cancer group (Table 1). Similarly, the observed in-hospital and 1-year all-cause mortality was also significantly higher in the prior-cancer group. However, there was no significant difference in in-hospital and 1-year cardiovascular mortality.
3.2. Predicted and Observed All-Cause Mortality by Cancer Status
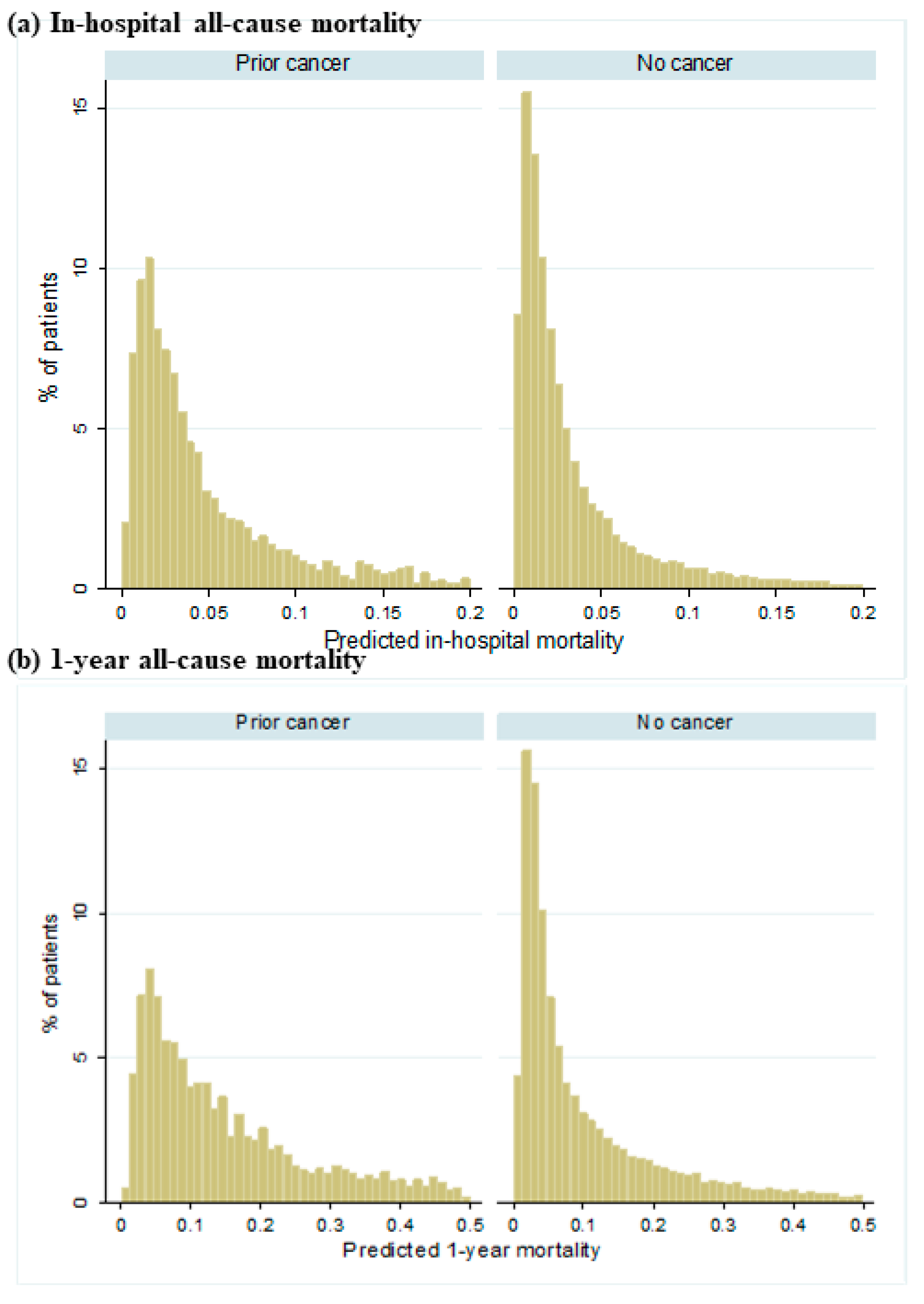
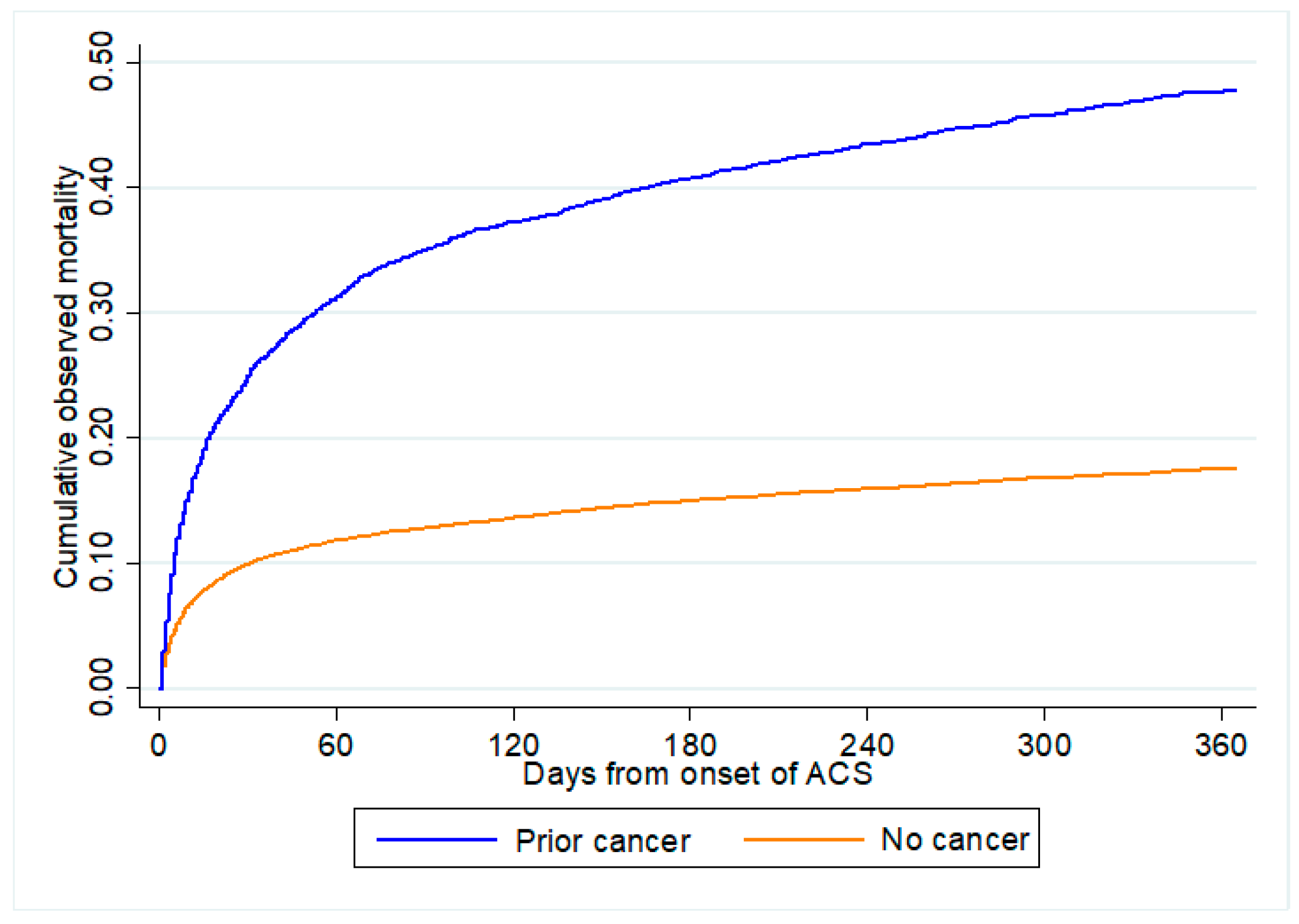
Figure 2 shows the distribution of predicted all-cause mortality by cancer status. A greater proportion of patients with prior cancer were predicted to have higher risk for both in-hospital mortality and 1-year all-cause mortality than those without cancer. Figure 3 shows the cumulative observed all-cause mortality by cancer status. Observed all-cause mortality was higher in those with prior cancer than no cancer regardless of the post-ACS period.
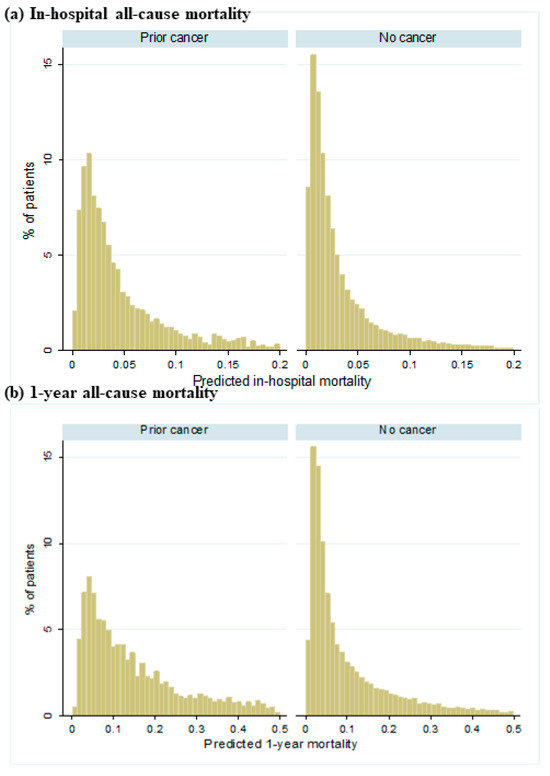
Figure 2.
Distribution of predicted all-cause mortality by cancer status. (a) demonstrates the predicted in-hospital all-cause mortality between patients with prior cancer and no cancer. (b) demonstrates the predicted 1-year all-cause mortality between patients with prior cancer and no cancer.
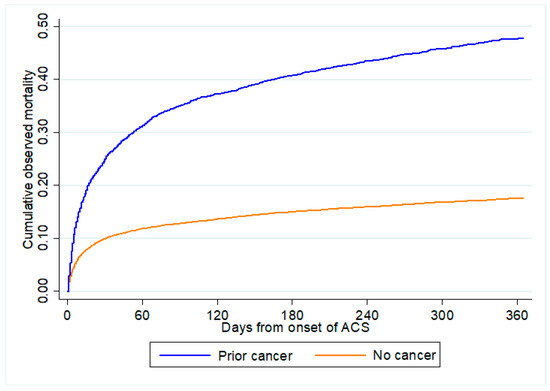
Figure 3.
Observed all-cause mortality by cancer status. All-cause mortality comparing between patients with prior cancer and no cancer from onset of ACS. ACS: acute coronary syndrome.
3.3. Performance of GRACE Score by Cancer Status
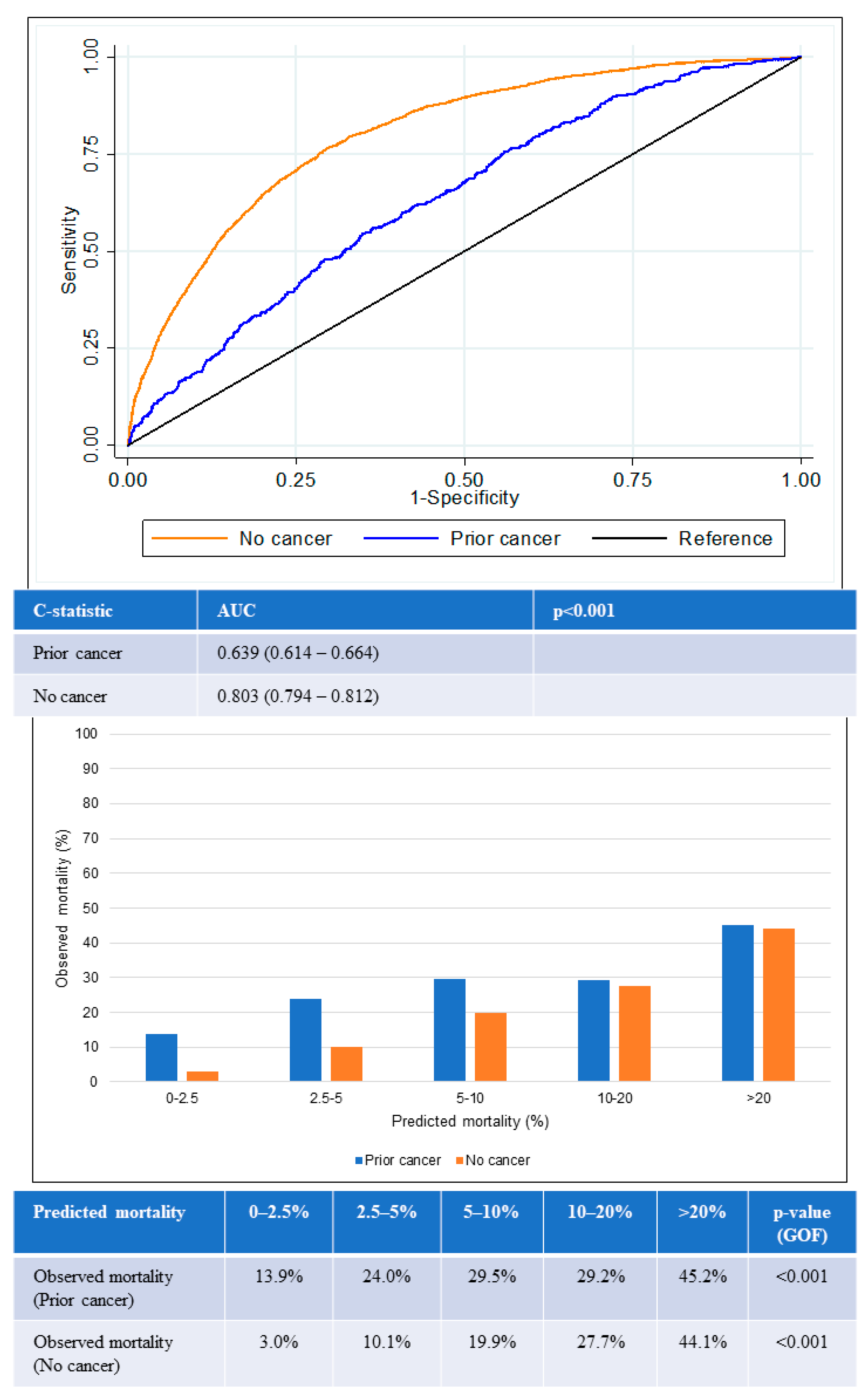
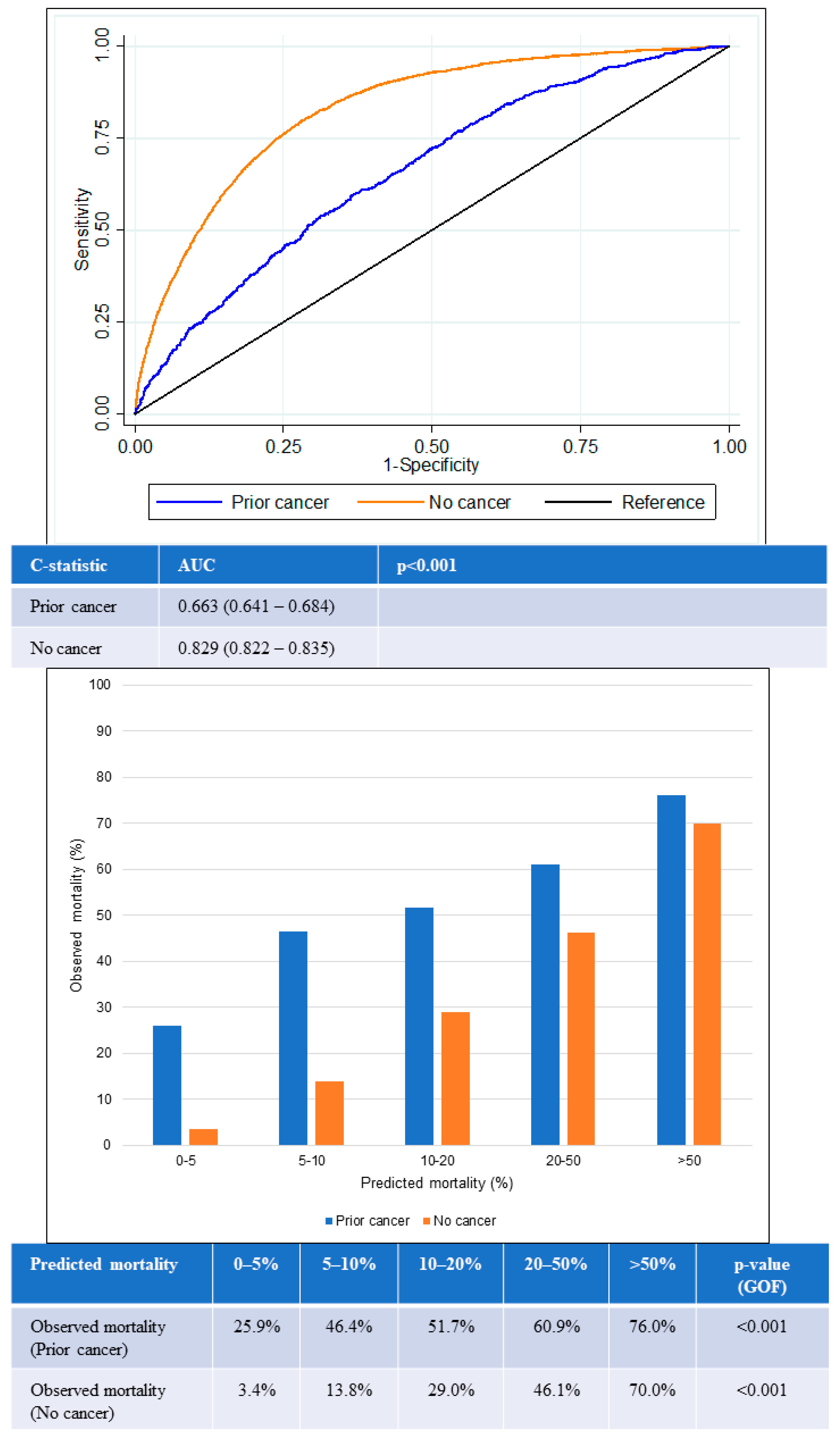
The AUC for in-hospital all-cause mortality among patients with prior cancer was 0.64 (95% confidence interval [CI] 0.61–0.66), which was significantly lower than the AUC among those with no cancer (0.80, 95% CI 0.79–0.81) (p < 0.001) (Figure 4). The lower AUC for the prior-cancer group indicated that the GRACE score was poorer in differentiating the mortality status of patients with prior cancer. The GOF test for in-hospital all-cause mortality was statistically significant in both groups of patients, suggesting that predicted mortality risk from GRACE score was not close to the observed mortality regardless if the patients had prior cancer or no cancer.
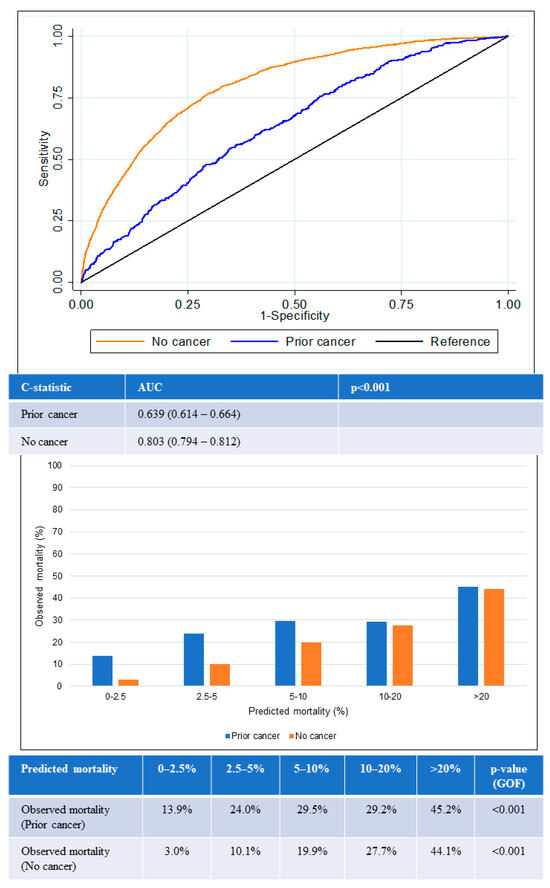
Figure 4.
Performance of GRACE score for in-hospital mortality according to cancer status. Receiver operating curves (upper) showing AUC for the GRACE score according to cancer status. Chart (lower) comparing predicted mortality against observed mortality according to cancer status. AUC: area under the receiver operating curve; GOF: goodness of fit.
Likewise, similar trends were observed for 1-year all-cause mortality (Figure 5). The AUC for the prior-cancer group was 0.66 (95% CI 0.64–0.68), while the AUC for the no-cancer group was 0.83 (95% CI 0.82–0.84). This was again significantly lower in the prior-cancer group (p < 0.001), indicating poorer discrimination by the GRACE score among patients in this group. The GOF test for 1-year all-cause mortality was again statistically significant in both groups of patients.
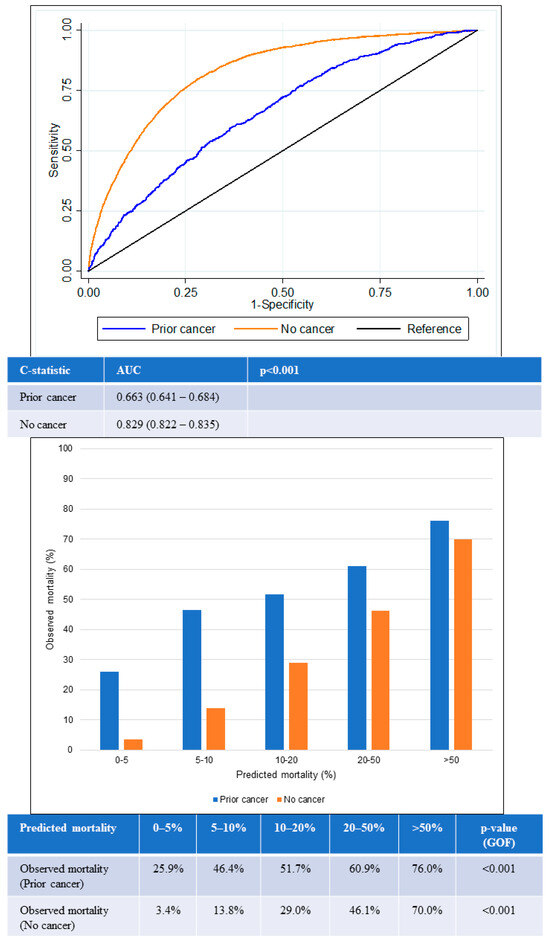
Figure 5.
Performance of GRACE score for 1-year mortality according to cancer status. Receiver operating curves (upper) showing AUC for the GRACE score according to cancer status. Chart (lower) comparing predicted mortality against observed mortality according to cancer status. AUC: area under the receiver operating curve; GOF: goodness of fit.
The GRACE score showed suboptimal calibration among both prior cancer and no-cancer groups. Underestimation of mortality in the prior-cancer group was worse than for the no-cancer group across all quintiles of risk. The greatest underestimation of mortality was observed in the lowest two quintiles of risk in the prior-cancer group in which observed mortality exceeded predicted mortality by two- to six-fold in the lower two quintiles of risk.
3.4. Mortality by Revascularization Status for Low and High GRACE Score
In-hospital revascularization was associated with lower in-hospital and 1-year all-cause mortality regardless of cancer status and GRACE score (Table 2). In-hospital revascularization was not associated with lower cardiovascular mortality in the prior-cancer group but was instead associated with lower cardiovascular mortality in the no-cancer group.

Table 2.
Mortality by in-hospital revascularization status in patients with prior cancer. (A) Among Those with Prior Cancer; (B) Among Those with No Cancer.
When classified according to the GRACE score, in-hospital revascularization in the prior-cancer group with a high-risk GRACE score (>140) was not associated with lower cardiovascular mortality but was associated with lower 1-year cardiovascular mortality in the no-cancer group with a high-risk GRACE score (Table 2).
3.5. Predicted and Observed Mortality by Cancer Characteristics among Those with Prior Cancer
Table 3 shows the predicted and observed in-hospital all-cause mortality by cancer characteristics among those with prior cancer. While there was no significant difference in predicted in-hospital all-cause mortality across cancer stages, observed mortality increased significantly with cancer stage. Predicted in-hospital all-cause mortality was lowest in those most recently diagnosed with cancer, but observed in-hospital all-cause mortality was highest in this group. Head and neck cancer patients were predicted to have the lowest in-hospital all-cause mortality, while skin cancer patients were predicted to have the highest in-hospital all-cause mortality. Observed in-hospital all-cause mortality was lowest in thyroid cancer patients and highest in lung cancer patients. Predicted in-hospital all-cause mortality was lower for most patients who received treatment for cancer. However, aside from lower observed in-hospital all-cause mortality in patients who underwent surgery than those who did not, there was no other significant difference in observed in-hospital all-cause mortality by cancer treatment.

Table 3.
In-hospital all-cause mortality by cancer characteristics in patients with prior cancer.
Table 4 shows the predicted and observed 1-year all-cause mortality by cancer characteristics among those with prior cancer. Predicted 1-year all-cause mortality was highest in early stage cancer, but observed 1-year all-cause mortality increased with cancer stage. Predicted 1-year all-cause mortality was lowest in those most recently diagnosed with cancer, but observed 1-year all-cause mortality was again highest in this group. When classified according to cancer subtype or cancer treatment, the 1-year all-cause mortality trends were similar to that of in-hospital all-cause mortality.

Table 4.
One-year all-cause mortality by cancer characteristics in patients with prior cancer.
Specific to cardiovascular mortality, both in-hospital and 1-year cardiovascular mortality was significantly lower in patients recently diagnosed with cancer (within the first quartile of 425 days) compared to patients with a longer interval from cancer diagnosis (across the other three quartiles) (Table 5). When classified according to cancer stage or cancer therapy received, there was no significant difference in cardiovascular mortality.

Table 5.
Cardiovascular mortality by cancer characteristics in patients with prior cancer.
4. Discussion
This study compared the performance of the GRACE score in prediction of all-cause mortality after ACS among patients with and without prior cancer. The AUC of the GRACE score was substantially lower in the prior-cancer group compared with the no-cancer group for both in-hospital all-cause mortality (0.64 vs. 0.80, p < 0.001) and 1-year all-cause mortality (0.66 vs. 0.83, p < 0.001). The GRACE score performed worse among the lowest two quintiles of risk, in which observed mortality was two- to six-fold higher than predicted mortality. In patients with high GRACE scores, in-hospital revascularization was not associated with lower cardiovascular mortality in the prior-cancer group but was associated with lower cardiovascular mortality in the no-cancer group. Our findings highlight that patients with cancer who develop ACS have a very high risk of mortality that is not accurately predicted by a conventional ACS risk score.
Several reasons can account for the poorer performance of the GRACE score in patients with prior cancer. Firstly, the GRACE score does not account for the cardiotoxic effects of cancer therapies. Cancer therapies are independently associated with increased risk of cardiovascular toxicities [18,19]. These include common associations such as hypertension from tyrosine kinase inhibitors, heart failure from anthracyclines and trastuzumab, vasospasm from 5-fluorouracil and its derivatives, ischemia from vascular endothelial growth factor inhibition, and even myocarditis and accelerated atherosclerosis from newer agents such as immune-checkpoint inhibitors [20,21,22]. Radiotherapy can also lead to atherosclerosis as well as valvular, myocardial, and pericardial dysfunction [23].
Secondly, the GRACE score does not include cancer characteristics when predicting all-cause mortality. This was most evident through our findings where patients with advanced stage cancer were conversely of a lower predicted risk of mortality than those with early stage cancer when the GRACE score was applied. This was despite the markedly elevated observed mortality in those with advanced stage cancer. Other cancer characteristics such as cancer subtypes may be of similar relevance although more research will be required. For example, we identified patients with thyroid cancer to have the lowest in-patient all-cause mortality and those with head and neck cancer to have the lowest 1-year all-cause mortality. Patients with lung cancer had the highest in-patient and 1-year all-cause mortality. This is consistent with other studies also demonstrating highest mortality in patients with lung cancer post-myocardial infarction or post-PCI [24,25]. We also identified that patients with a recent diagnosis of cancer, within 425 days (first quartile within the study population), had the highest all-cause mortality but lowest cardiovascular mortality when compared to those with a cancer diagnosis beyond this period. This may suggest that in patients with an early diagnosis of cancer within 1 year, especially lung cancer, the benefit of an early invasive revascularization strategy may be less pronounced. The GRACE score could therefore be further optimized by incorporating cancer characteristics, such as cancer type, staging, and duration, from cancer diagnosis to better guide revascularization strategies in patients with cancer.
Thirdly, there are significant treatment biases which exist when treating prior cancer patients with ACS. Similar to other studies, we found that patients with prior cancer were less likely to undergo revascularization or receive guideline-directed medical therapy compared to patients without a history of cancer [25,26,27]. These treatment biases would inadvertently lead to the increased mortality in patients with prior cancer unaccounted for by the GRACE score. Increased education and awareness in both patients and medical practitioners are required to address this gap in guideline directed care within this challenging and often neglected patient population.
Therefore, risk stratification and management of the patient with cancer with ACS currently lacks a strong scientific evidence base. Guidelines recommend pursuing an early invasive strategy for revascularization guided by use of the GRACE score [9]. However, our findings demonstrate that this grossly underestimated mortality risk in patients with cancer. Although revascularization was associated with lower all-cause mortality in prior cancer patients, this occurred regardless of GRACE score. This observed lower all-cause mortality is likely due to selection bias similar to other studies, where those with a better prognosis were more likely to undergo revascularization [25]. However, among high-risk patients with prior cancer identified via the GRACE score, in-hospital revascularization did not reduce cardiovascular mortality compared to those who did not undergo revascularization. Hence, the benefit of pursuing an aggressive revascularization strategy in patients with prior cancer guided by the GRACE score may not be unequivocally beneficial. The expected benefit of revascularization in cancer patients could be reduced by an overall poor prognosis from cancer or possible increased thrombotic or bleeding complications after revascularization in prior cancer patients [5,6,28,29]. We suggest adopting a more individualized approach to revascularization in prior cancer patients, similar to how cancer patients are risk assessed prior to commencement of cancer treatment [30]. In the absence of a well validated risk stratification tool specific to prior cancer patients, we propose a multi-disciplinary approach involving the cancer physician, cardio-oncologist, and interventional cardiologist to guide revascularization decisions.
To the best our knowledge, this is the first study demonstrating the performance of the GRACE score in patients with both ACS and cancer. However, there are several limitations to our study. First, data were not available on the etiology of ACS as we were not able to retrieve details on whether the ACS was secondary to atherosclerosis or complications from cancer therapy, such as vasospasm. Second, data on PCI characteristics, such as the use of balloon angioplasty or choice of stents deployed, the use of intravascular imaging to optimize PCI outcomes in cancer patients which have been recommended by guidelines, and the duration of dual anti-platelet therapy, were all not available [31]. Third, the GRACE score was developed in a predominantly European population and not a multiethnic Asian population. However, our results remained similar despite recalibration of the GRACE score for use within the local population [32].
5. Conclusions
In conclusion, the GRACE score performed much worse among patients with ACS and prior cancer than patients without cancer. Only a minority of patients with cancer underwent in-hospital revascularization. Among patients identified as high-risk through the GRACE score, in-hospital revascularization was not associated with lower cardiovascular mortality in patients with prior cancer but was associated with lower 1-year cardiovascular mortality in patients with no cancer. Further research is needed to identify other risk variables unique to this special population to better risk stratify and guide treatment strategies for patients with prior cancer and ACS.
Author Contributions
Conceptualization, C.-Y.K. and M.Y.C.; methodology and formal analysis, C.-Y.K., H.Z. and M.Y.C.; writing—original draft preparation, C.-Y.K.; writing—review and editing, H.Z., L.-L.T., L.-L.F., E.S., D.J.H., R.A.S., A.S.W., A.M.R., C.-H.L. and M.Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the local Institutional Review Board (Domain Specific Review Board-C, National Healthcare Group, 2021/00141).
Informed Consent Statement
Patient consent was waived for studies with public health importance using de-identified registry data.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The patient-identifiable data are not publicly available as they are under the jurisdiction of the Ministry of Health Singapore.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Armenian, S.H.; Xu, L.; Ky, B.; Sun, C.; Farol, L.T.; Pal, S.K.; Douglas, P.S.; Bhatia, S.; Chao, C. Cardiovascular Disease among Survivors of Adult-Onset Cancer: A Community-Based Retrospective Cohort Study. J. Clin. Oncol. 2016, 34, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Strongman, H.; Gadd, S.; Matthews, A.; Mansfield, K.E.; Stanway, S.; Lyon, A.R.; Dos-Santos-Silva, I.; Smeeth, L.; Bhaskaran, K. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: A population-based cohort study using multiple linked UK electronic health records databases. Lancet 2019, 394, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Koo, C.Y.; Zheng, H.; Tan, L.L.; Foo, L.-L.; Seet, R.; Chong, J.-H.; Hausenloy, D.J.; Chng, W.-J.; Richards, A.M.; Lee, C.-H.; et al. Lipid profiles and outcomes of patients with prior cancer and subsequent myocardial infarction or stroke. Sci. Rep. 2021, 11, 21167. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, M.; D’ascenzo, F.; Vadalà, P.; Wilton, S.B.; Noussan, P.; Colombo, F.; Roubín, S.R.; Abu Assi, E.; González-Juanatey, J.R.; Henriques, J.P.S.; et al. Prevalence and outcome of patients with cancer and acute coronary syndrome undergoing percutaneous coronary intervention: A BleeMACS substudy. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Wong, C.W.; Kontopantelis, E.; Barac, A.; Brown, S.-A.; Velagapudi, P.; Hilliard, A.A.; Bharadwaj, A.S.; Alraies, M.C.; Mohamed, M.; et al. Percutaneous coronary intervention in patients with cancer and readmissions within 90 days for acute myocardial infarction and bleeding in the USA. Eur. Heart J. 2021, 42, 1019–1034. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 78, e187–e285. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Fox, K.A.A.; Dabbous, O.H.; Goldberg, R.J.; Pieper, K.S.; Eagle, K.A.; Van de Werf, F.; Avezum, Á.; Goodman, S.G.; Flather, M.D.; Anderson, F.A.; et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: Prospective multinational observational study (GRACE). BMJ 2006, 333, 1091. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; Biondi-Zoccai, G.; Moretti, C.; Bollati, M.; Omedè, P.; Sciuto, F.; Presutti, D.G.; Modena, M.G.; Gasparini, M.; Reed, M.J.; et al. TIMI, GRACE and alternative risk scores in Acute Coronary Syndromes: A meta-analysis of 40 derivation studies on 216,552 patients and of 42 validation studies on 31,625 patients. Contemp. Clin. Trials 2012, 33, 507–514. [Google Scholar] [CrossRef]
- Gevaert, S.A.; Halvorsen, S.; Sinnaeve, P.R.; Sambola, A.; Gulati, G.; Lancellotti, P.; Van Der Meer, P.; Lyon, A.R.; Farmakis, D.; Lee, G.; et al. Evaluation and management of cancer patients presenting with acute cardiovascular disease: A Consensus Document of the Acute CardioVascular Care (ACVC) association and the ESC council of Cardio-Oncology—Part 1: Acute coronary syndromes and acute pericardial diseases. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 947–959. [Google Scholar] [CrossRef]
- National Registry of Diseases Office. Available online: https://www.nrdo.gov.sg (accessed on 30 January 2022).
- Sim, H.W.; Zheng, H.; Richards, A.M.; Chen, R.W.; Sahlen, A.; Yeo, K.-K.; Tan, J.W.; Chua, T.; Tan, H.C.; Yeo, T.C.; et al. Beta-blockers and renin-angiotensin system inhibitors in acute myocardial infarction managed with inhospital coronary revascularization. Sci. Rep. 2020, 10, 15184. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P. Monitoring cancer incidence and risk factors in Singapore. Ann. Acad. Med. Singap. 1990, 19, 133–138. [Google Scholar] [PubMed]
- Granger, C.B.; Goldberg, R.J.; Dabbous, O.; Pieper, K.S.; Eagle, K.A.; Cannon, C.P.; Van de Werf, F.; Avezum, A.; Goodman, S.G.; Flather, M.D.; et al. Predictors of Hospital Mortality in the Global Registry of Acute Coronary Events. Arch. Intern. Med. 2003, 163, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.A.A.; FitzGerald, G.; Puymirat, E.; Huang, W.; Carruthers, K.; Simon, T.; Coste, P.; Monsegu, J.; Steg, P.G.; Danchin, N.; et al. Should patients with acute coronary disease be stratified for management according to their risk? Derivation, external validation and outcomes using the updated GRACE risk score. BMJ Open 2014, 4, e004425. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, J.; Cautela, J.; Ederhy, S.; Damaj, G.L.; Salem, J.; Barlesi, F.; Farnault, L.; Charbonnier, A.; Mirabel, M.; Champiat, S.; et al. Cardiovascular Toxicity Related to Cancer Treatment: A Pragmatic Approach to the American and European Cardio-Oncology Guidelines. J. Am. Heart Assoc. 2020, 9, e018403. [Google Scholar] [CrossRef]
- Rao, V.U.; Reeves, D.J.; Chugh, A.R.; O’quinn, R.; Fradley, M.G.; Raghavendra, M.; Dent, S.; Barac, A.; Lenihan, D. Clinical Approach to Cardiovascular Toxicity of Oral Antineoplastic Agents: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 2693–2716. [Google Scholar] [CrossRef]
- López-Sendón, J.; Álvarez-Ortega, C.; Zamora Auñon, P.; Buño Soto, A.; Lyon, A.R.; Farmakis, D.; Cardinale, D.; Canales Albendea, M.; Feliu Batlle, J.; Rodríguez Rodríguez, I.; et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: The CARDIOTOX registry. Eur. Heart J. 2020, 41, 1720–1729. [Google Scholar] [CrossRef]
- Drobni, Z.D.; Alvi, R.M.; Taron, J.; Zafar, A.; Murphy, S.P.; Rambarat, P.K.; Mosarla, R.C.; Lee, C.; Zlotoff, D.A.; Raghu, V.K.; et al. Association between Immune Checkpoint Inhibitors with Cardiovascular Events and Atherosclerotic Plaque. Circulation 2020, 142, 2299–2311. [Google Scholar] [CrossRef]
- Mihalcea, D.; Memis, H.; Mihaila, S.; Vinereanu, D. Cardiovascular Toxicity Induced by Vascular Endothelial Growth Factor Inhibitors. Life 2023, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.D.; Cehic, D.A.; Morgia, M.; Bergrom, C.; Toohey, J.; Guerrero, P.A.; Ferencik, M.; Kikuchi, R.; Carver, J.R.; Zaha, V.G.; et al. Cardiovascular Manifestations from Therapeutic Radiation: A Multidisciplinary Expert Consensus Statement from the International Cardio-Oncology Society. JACC CardioOncol. 2021, 3, 360–380. [Google Scholar] [CrossRef]
- Potts, J.E.; Iliescu, C.A.; Mattei, J.C.L.; Martinez, S.C.; Holmvang, L.; Ludman, P.; De Belder, M.A.; Kwok, C.S.; Rashid, M.; Fischman, D.L.; et al. Percutaneous coronary intervention in cancer patients: A report of the prevalence and outcomes in the United States. Eur. Heart J. 2019, 40, 1790–1800. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, A.; Potts, J.; O Mohamed, M.; Parwani, P.; Swamy, P.; Lopez-Mattei, J.C.; Rashid, M.; Kwok, C.S.; Fischman, D.L.; Vassiliou, V.S.; et al. Acute myocardial infarction treatments and outcomes in 6.5 million patients with a current or historical diagnosis of cancer in the USA. Eur. Heart J. 2020, 41, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.O.; Van Spall, H.G.C.; Kontopantelis, E.; Alkhouli, M.; Barac, A.; Elgendy, I.Y.; Khan, S.U.; Kwok, C.S.; Shoaib, A.; Bhatt, D.L.; et al. Effect of primary percutaneous coronary intervention on in-hospital outcomes among active cancer patients presenting with ST-elevation myocardial infarction: A propensity score matching analysis. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Witassek, F.; Erne, P.; Rickli, H.; Radovanovic, D. Treatment of patients with myocardial infarction depends on history of cancer. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 639–645. [Google Scholar] [CrossRef]
- Ueki, Y.; Vögeli, B.; Karagiannis, A.; Zanchin, T.; Zanchin, C.; Rhyner, D.; Otsuka, T.; Praz, F.; Siontis, G.C.; Moro, C.; et al. Ischemia and Bleeding in Cancer Patients Undergoing Percutaneous Coronary Intervention. JACC CardioOncol. 2019, 1, 145–155. [Google Scholar] [CrossRef]
- Guo, W.; Fan, X.; Lewis, B.R.; Johnson, M.P.; Rihal, C.S.; Lerman, A.; Herrmann, J. Cancer Patients Have a Higher Risk of Thrombotic and Ischemic Events after Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2021, 14, 1094–1105. [Google Scholar] [CrossRef]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar] [CrossRef]
- Iliescu, C.A.; Grines, C.L.; Herrmann, J.; Yang, E.H.; Cilingiroglu, M.; Charitakis, K.; Hakeem, A.; Toutouzas, K.P.; Leesar, M.A.; Marmagkiolis, K. SCAI Expert consensus statement: Evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the cardiological society of india, and sociedad Latino Americana de Cardiologıa intervencionista). Catheter. Cardiovasc. Interv. 2016, 87, E202–E223. [Google Scholar] [CrossRef]
- Chan, M.Y.; Shah, B.R.; Gao, F.; Sim, L.L.; Chua, T.; Tan, H.C.; Yeo, T.C.; Ong, H.Y.; Foo, D.; Goh, P.P.; et al. Recalibration of the Global Registry of Acute Coronary Events risk score in a multiethnic Asian population. Am. Heart J. 2011, 162, 291–299. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).