Simple Summary
Immunotherapy is emerging as an improved systemic treatment for select patients with advanced unresectable hepatocellular carcinoma. An objective response is reported in 30% of patients, yet a complete response allowing for curative-intent surgery is rare. Locoregional therapies seem to show synergistic effects with immunotherapy, though this effect has not been scientifically reported. We report a cohort of patients showing a complete response to this combination immunotherapy + LRT and aim to present this as a proposed treatment approach for locally unresectable disease. We also report how liquid biopsy using ctDNA was cleared using this approach and discuss how this testing modality may assist patients with this type of disease.
Abstract
Background: Immunotherapy has emerged as an improved systemic treatment for select patients with advanced unresectable HCC. Objective response is reported in 30% of patients, yet complete response (pCR) allowing for curative-intent resection is rare. Locoregional therapies (LRTs) seem to show synergistic effects with immunotherapy, though this effect has not been scientifically reported. We report a cohort of patients showing pCR to immunotherapy + LRT as a proof of concept for the proposed treatment approach for locally unresectable HCC. Methods: Patients with unresectable HCC treated with immunotherapy as an intended destination therapy from 2016 to 2023 were included. The electronic health record was queried for oncologic information, locoregional therapies, surgical interventions, and long-term outcomes. Circulating tumor DNA (ctDNA) testing was obtained using Guardant360, and tumor mutational burden (TMB) was defined as the number of somatic mutations per megabase. Results: Ninety-six patients with advanced HCC received immunotherapy + LRT as a destination therapy. In total, 11 of 96 patients showed a complete response according to mRECIST criteria. Four of these (36.4%) ultimately underwent curative-intent resection. The median follow-up was 24.9 (IQR 15.6–38.3) months. Overall survival rates in those with complete response at 1, 3, and 5 years were 100%, 91%, and 81.8%, respectively, which were significantly improved compared to those of the cohort not achieving pCR (p < 0.001). All four patients undergoing immunotherapy + LRT followed by curative-intent hepatectomy have no evidence of disease (NED). Of those undergoing surgery, ctDNA was cleared in 75% (n = 3), providing an additional objective measurement of complete response. All four patients were TMB+ before beginning this treatment course, with three being TMB-, indicating stable and complete disease response. Conclusions: Immunotherapy + locoregional therapy can help downstage a significant proportion of patients with initially unresectable HCC, allowing for curative-intent surgery. The survival benefit associated with complete response seems durable up to 3 years after achieving this response. ctDNA measurement was converted from positive to negative in this cohort, providing additional indication of response.
1. Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver tumor and one of the leading causes of cancer-related death worldwide [1]. Approximately 60% of HCC cases are diagnosed at an advanced stage and cannot be treated with surgery or locoregional therapies [2]. These patients received systemic therapy [3]. Immunotherapy has emerged as an improved systemic treatment for select patients with advanced HCC [4]. The current standard first-line therapy is a combination of the immune checkpoint inhibitor (ICI) atezolizumab (PDL1 inhibitor) and the vascular endothelial growth factor inhibitor (VEGF) bevacizumab [4,5,6]. This treatment offers an objective response in only about one-third of patients, and survival remains limited; however, a complete response is occasionally observed, allowing liver resection [4,5,6]. Additionally, some studies suggest that locoregional therapies show synergistic effects with immunotherapy in some cases and improve response rates and outcomes [7].
Circulating tumor DNA (ctDNA) is an emerging tool for HCC. In other cancers, ctDNA has been shown to predict response to systemic therapy and to predict recurrence after resection [8,9,10,11]. One key component of ctDNA is tumor mutational burden (TMB). TMB can be obtained from both tissue and blood; the tissue-based results have been associated with worse prognosis in HCC, and with predicting response to immunotherapy [10,12,13,14,15,16]. The liquid biopsy (LB)-based approach has not been validated in this cohort, and results of ctDNA have not yet been reported in conjunction with immunotherapy results.
Herein, we present the largest cohort of patients yet reported that showed exceptional response to systemic immunotherapy with or without concurrent locoregional interventions, demonstrating prolonged survival despite presenting with advanced-stage HCC.
2. Methods
The prospectively collected comprehensive liver database was queried for patients with advanced HCC who received immunotherapy after obtaining approval from our Institutional Review Board (IRB). Data collected from the electronic medical records included cross-sectional images, laboratory values, serum biomarkers, pathology reports, and follow-up and recurrence information. The aim of this study was to describe the outcomes in patients who responded to systemic treatment with immunotherapy. Patient response to immunotherapy was evaluated using the modified Response Evaluation Criteria in Solid Tumors (mRECIST) scoring system. Atezolizumab/bevacizumab is preferred as a first-line immunotherapy at our institution, unless it is unavailable due to cost or manufacturing issues. Nivolumab is also sometimes used in refractory cases that have already received atezolizumab/bevacizumab, or in cases where there is an issue with tolerance of the atezo/bev regimen. Changes in alpha-fetoprotein (AFP) levels were also considered. Patient survival was also recorded. IRB approval was obtained; consent was waived due to the retrospective nature of the review.
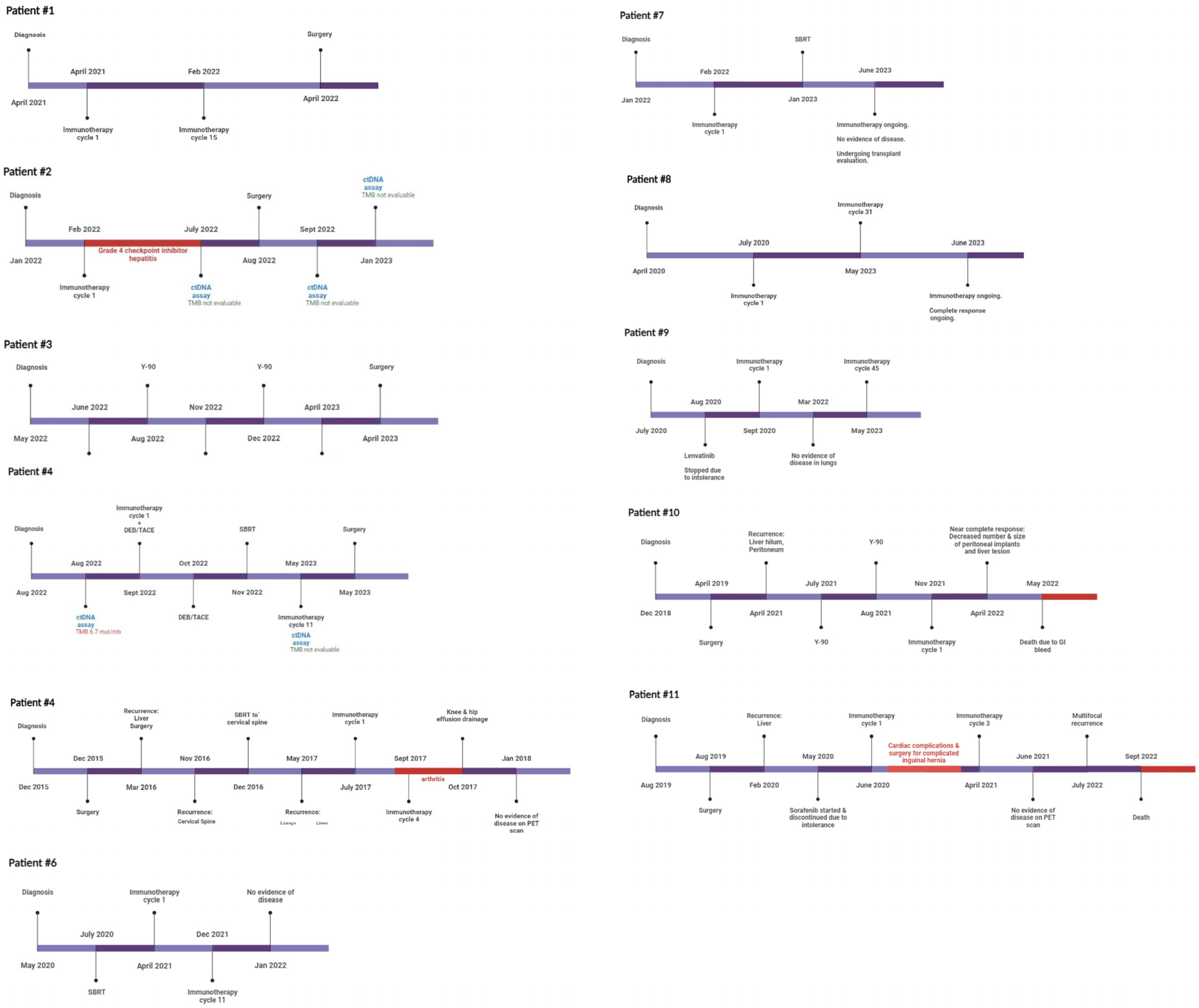
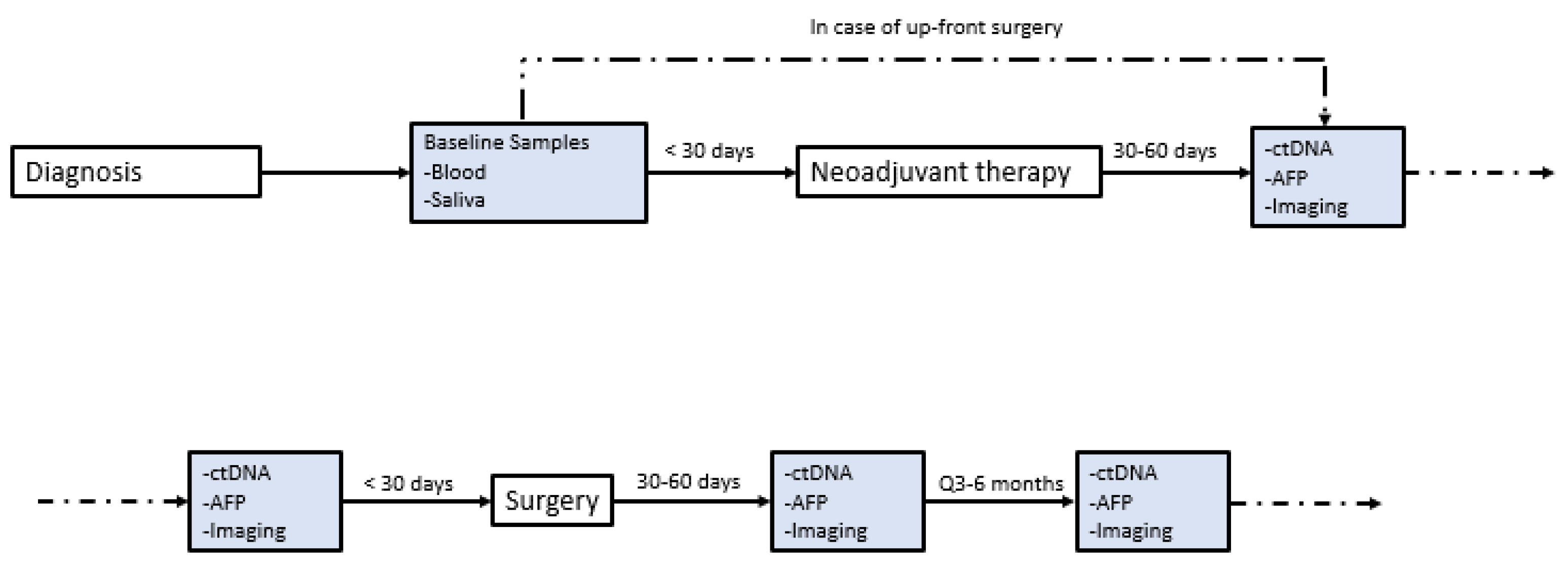
At the initiation of this study, ctDNA was assessed at the discretion of the treating physician without a clear institutional protocol. Because of this variable timing, contains a timeline annotation of the timing of ctDNA draws for each included patient with exceptional response. Our current institutional protocol for patients with HCC undergoing curative-intent surgery was developed and implemented in January 2021. Patients now receive standard-of-care ctDNA draws within 30 days preoperatively, 30–60 days postoperatively, and q3–6 months thereafter (Figure 1). ctDNA was obtained using the Guardant360 platform (Guardant Health, Redwood, CA, USA). The panel reports specific mutations for a pan-cancer 83 gene panel, and assesses mutations over 500 genes to establish a genomic footprint for the calculation of tumor mutational burden (TMB). Blood draws were performed in our facilities’ outpatient laboratory or coordinated by using mobile phlebotomy at patients’ homes based on patient preference. TMB in the included assay is defined as the number of somatic mutations per megabase. No detectable mutations are considered “TMB-ND” and >0 mutations are considered “TMB-detectable” Note that tissues of primary tumors were not sequenced in this study, though this has since become our standard practice.
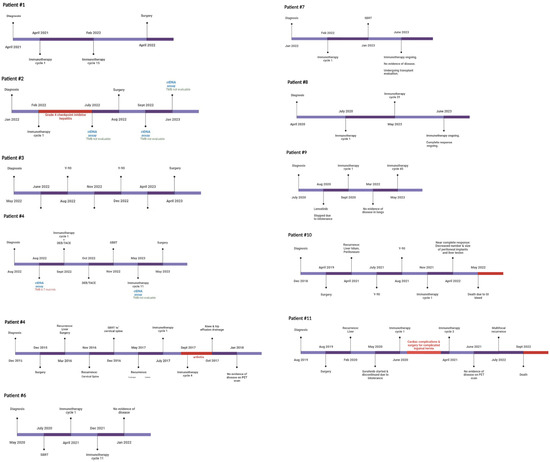
Figure 1.
Timeline of treatment and disease response. Treatment and testing timeline for each patient with complete response to neoadjuvant immunotherapy regimens.
Descriptive statistics were used to summarize patient demographics, clinical characteristics, and treatment details. Continuous variables were presented as means with standard deviations or medians with interquartile ranges, depending on their distribution. Categorical variables were presented as frequencies and percentages. Kaplan–Meier survival curves were constructed to estimate OS in patients with exceptional responses to immunotherapy. All statistical analyses were conducted using SPSS Version 29.0 with statistical significance defined as p < 0.05.
3. Results
Between 2016 and 2023, 96 patients with advanced HCC underwent systemic treatment with immunotherapeutic agents. In total, 11 of these 96 patients showed complete response according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST). Nine (81.8%) of the eleven complete responders were male. The mean age at diagnosis was 66.7 ± 9.7 years (Table 1). Five patients (45.4%) had cirrhosis, most commonly due to HCV infection (n = 3, 27%). Three (27%) patients had metastatic disease (stage IVB), one (9%) had nodal disease (stage IVA), and four (36%) had major vascular invasion (stage IIIB) at the time of starting immunotherapy. The mean pre-treatment AFP was 28,035.25 ± 58,034.21 ng/mL, with the highest noted AFP being 200,000 ng/mL. Three patients had a history of surgical resection of their cancer and were treated with immunotherapy for recurrent disease. Four patients (36%) did not receive any locoregional or systemic therapy prior to or concurrent with immunotherapy (Table 2). The timeline of treatment, ctDNA testing, and disease response in the 11 patients is provided (Figure 1). A summary of previously presented case reports on this topic is also provided (Table 3).

Table 1.
Patient demographics.

Table 2.
Treatment details and outcomes. M = male, F = female, OS = overall survival, NED = no evidence of disease, and pCR = pathologic complete response.

Table 3.
Summary of published case reports describing response to immunotherapy in advanced HCC.
The mean number of cycles received was 15 ± 12 (min = 1, max = 45). Three patients experienced immunotherapy-related adverse events, and one patient discontinued treatment. The mean post-treatment AFP was 32.42 ± 49.39 ng/mL, with the highest value being 158.6 ng/mL.
The median follow-up was 24.9 (IQR 15.6–38.3) months. In total, 9 of 11 patients were alive at the time of this report. One patient died due to bevacizumab-related complications, while the second patient experienced recurrence 15 months after stopping immunotherapy and died of metastatic disease. Four patients underwent resection after successful downstaging, while five patients remained unsuitable for surgery due to their comorbidities. One patient had a complete pathologic response (pCR) while three had <50% of viable tumors, corresponding to tumor response grade (TRG) 1. Overall survival (OS) rates at 1, 3, and 5 years were 100%, 91%, and 81.8%, respectively.
Four patients underwent surgical resection of the remaining disease with available pre- and post-treatment ctDNA levels. All four patients had identifiable TMB on their pre-treatment ctDNA. However, three of four patients (75%) were rendered TMB-ND after their resection (Table 4). All three of these patients are currently alive with NED, with a mean follow-up of 43.2 months.

Table 4.
ctDNA testing pre- and post-treatment course for patients (n = 4) with available testing.
4. Discussion
To our knowledge, this represents the largest series of patients describing a significant to complete response to neoadjuvant immunotherapy for advanced-stage HCC. This proof-of-concept manuscript demonstrates a high rate (>10%) of patients who presented with unresectable HCC and were able to achieve prolonged survival after multimodal treatment, including systemic immunotherapy, ablation, transarterial chemoembolization (TACE), transarterial radioembolization (TARE) with Y-90, and stereotactic body radiation therapy (SBRT), and underwent surgery after successful downstaging. We also show that systemic immunotherapy + LRT downstaging can allow for eventual curative-intent resection even in very advanced cases. Finally, we show that this sequence leading to curative-intent resection can lead to clearance of previously identifiable blood-based TMB, further indicating successful systemic treatment.
Although surgery offers the best outcomes in patients with HCC, only 30–40% present with resectable disease at the time of diagnosis [36,37]. Furthermore, some patients cannot undergo surgery because of comorbidities and compromised liver function [38]. Immunotherapy has ushered in a new era in the treatment of advanced HCC in such patients, with an objective response achieved in approximately 30% of patients and a smaller subset of just 1–5% showing complete response [39,40]. In patients with good functional status and preserved liver function, resection of the residual tumor can be achieved. In patients who are not candidates for surgery because of compromised liver function and other comorbidities, systemic immunotherapy with or without the aid of LRTs can offer prolonged overall or progression-free survival [4,5,6]. Our study further demonstrates this utility of immunotherapy, with a salvage therapy rate of over 10%. Preliminary work has focused on clinical predictors of response to immunotherapy, but additional work should focus on identifying those that will experience the described robust response [41].
Some studies suggest that locoregional intra-arterial and ablative treatments (LRTs) provide a synergistic action with systemic immunotherapy and improve tumor response rates [42,43,44,45,46]. For example, Singh et al. have shown that LRT alone has immunomodulating effects that may sensitize the tumor to the effects of systemic immunotherapy [45]. This synergy was reflected in the results of our study, given the high rate of systemic response to immunotherapy and LRT. In our cohort, bevacizumab was held four weeks before LRT as per safety recommendations, and atezolizumab or nivolumab was held only if the scheduled LRT coincided with the day of the ICI cycle. Thus, these results also confirm that concurrent treatment with LRTs in patients receiving immunotherapy is feasible and can be administered without long breaks between doses. Sangro et al. report in a review and meta-analysis a complete response rate of 1–5% with immunotherapy for HCC [40]. There is also a known rate of recurrence after treatment with LRT alone, as demonstrated by Facciorusso et al. and others [47,48]. The addition of immunotherapy could theoretically help lessen the likelihood of post-LRT recurrence, as evidenced by the subset of patients who had LRT + immunotherapy alone and are currently NED, though this cannot be directly proven in this study. Given the small sample size, this study is intended to serve as a proof of concept. Thus, though our findings are not definitive, the >10% complete response rate in our study may provide additional evidence that combined LRT and immunotherapy has significant potential for downstaging to potential curative-intent resection in advanced HCC.
Immune checkpoint inhibitors also have a milder side effect profile than that of known tyrosine kinase inhibitors previously used in the management of advanced HCC [4,6]. Only one patient in our study discontinued treatment due to immunotherapy-related grade 4 hepatitis. However, it is interesting to note that this patient received only one cycle of atezolizumab and achieved complete response. One patient developed immune-related hepatitis, and one patient developed immune-related arthritis. These patients were managed by holding immunotherapy and prescribing steroids, respectively, and resumed treatment after the successful control of adverse effects. One patient receiving a combination of atezolizumab and bevacizumab died due to gastrointestinal hemorrhage. Bevacizumab is reported to increase the risk of gastrointestinal hemorrhage or perforation fourfold in patients with advanced disease [5,49]. Immunotherapy was generally well tolerated in this study, but these side effects are of important clinical significance for providers.
ctDNA-based liquid biopsy is a potentially useful tool for judging the response to treatment as well as identifying potential biomarkers in addition to TMB, which can be associated with response or resistance to immunotherapy [8,9,10,11]. New research has also investigated the utility of ctDNA as a method of detecting minimal residual disease (MRD), a testing approach that identifies the smallest number of remaining tumor cells or tumor DNA needed to create a disease recurrence. This concept has been discussed and preliminarily reported in HCC, but there no globally accepted approach for this at this time [50,51,52]. TMB is not specifically an MRD marker, but low or nondetectable TMB is associated with reduced progression [53,54], and thus, the conversion of TMB-detectable to nondetectable in our patients may provider further support for this approach.
This study has limitations. While this is the largest published series, we report a very small sample size of patients with complete radiologic response overall. This may limit the broader applicability of these findings, and we cannot make any definitive claims about how other patients might respond to the aforementioned treatment regimens. There was a range of LRTs used in the study which may have influenced patient outcomes, and patients may have responded differently to each combination of LRT + immunotherapy. The sample size precluded a statistical analysis of the factors that might have influenced either the response to this approach or recurrence-free survival after resection in this cohort. Not all patients received resection, so the degree of pathologic response could not be determined. We also recognize that ctDNA was not obtained in a consistent fashion for the included patients, and that only having data on four patients limits any conclusion of ctDNA and/or TMB as a biomarker. As shown in Figure 2, we have now established standardized protocols such that we can study ctDNA and TMB as a biomarker in more robust fashion.
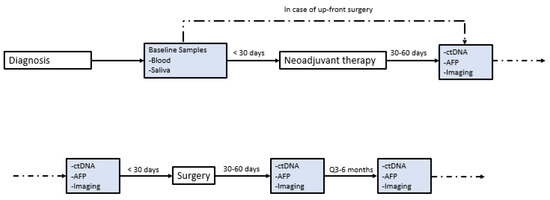
Figure 2.
Institutional protocol for ctDNA. Institutional protocol for circulating tumor DNA (ctDNA) screening in patients with hepatocellular carcinoma (HCC) seen in our multidisciplinary liver tumor clinic. This protocol was implemented in January 2021.
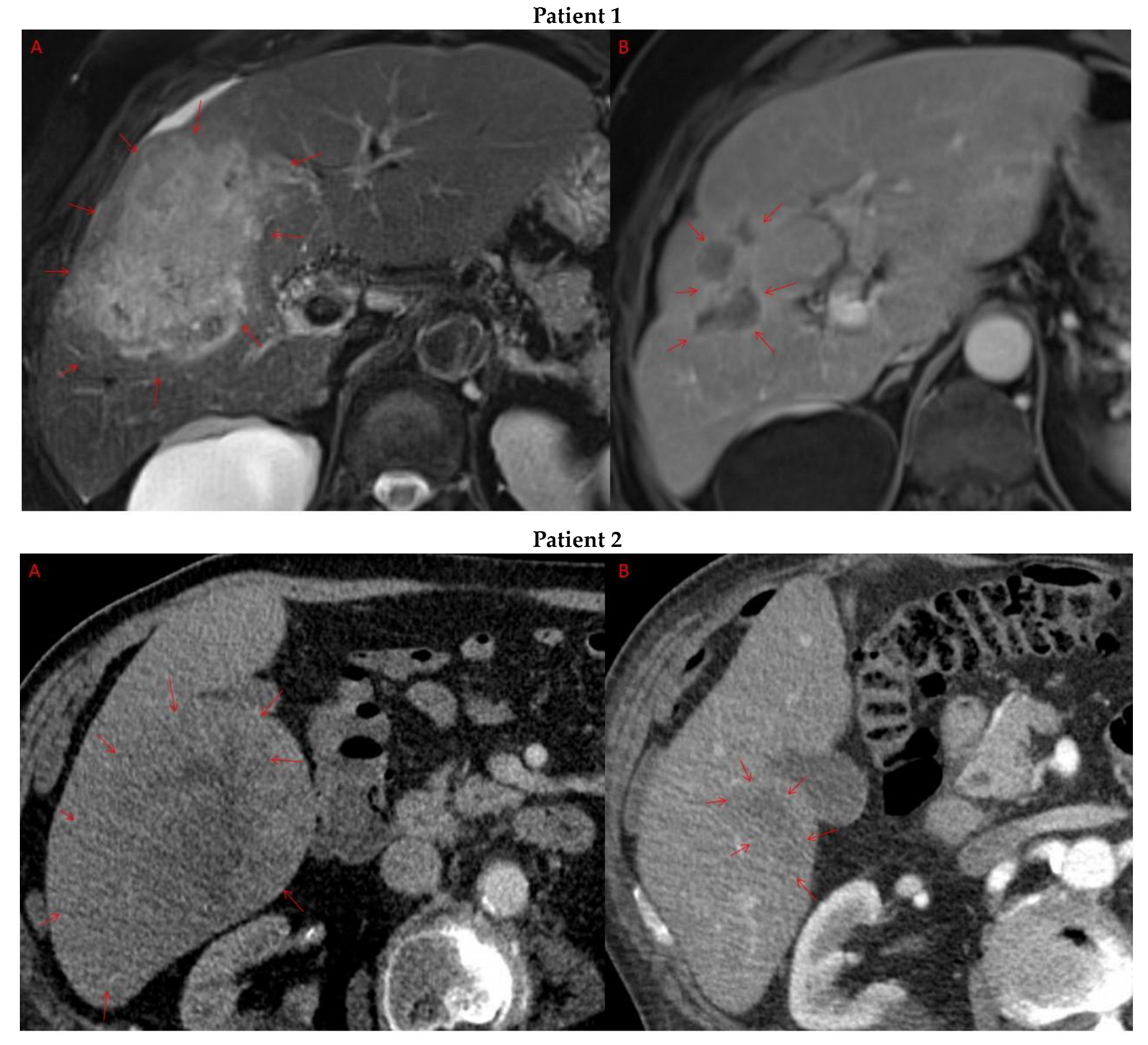
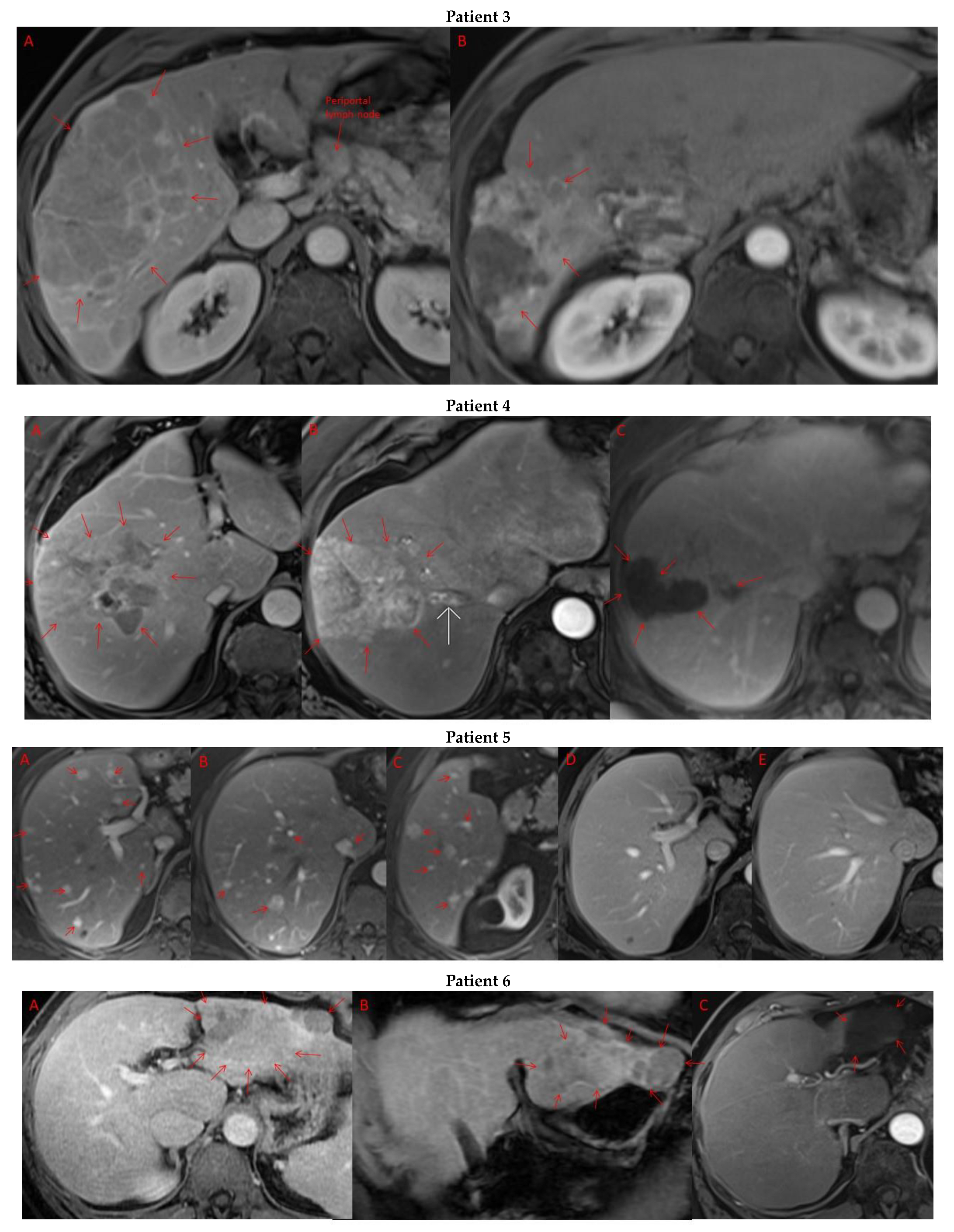
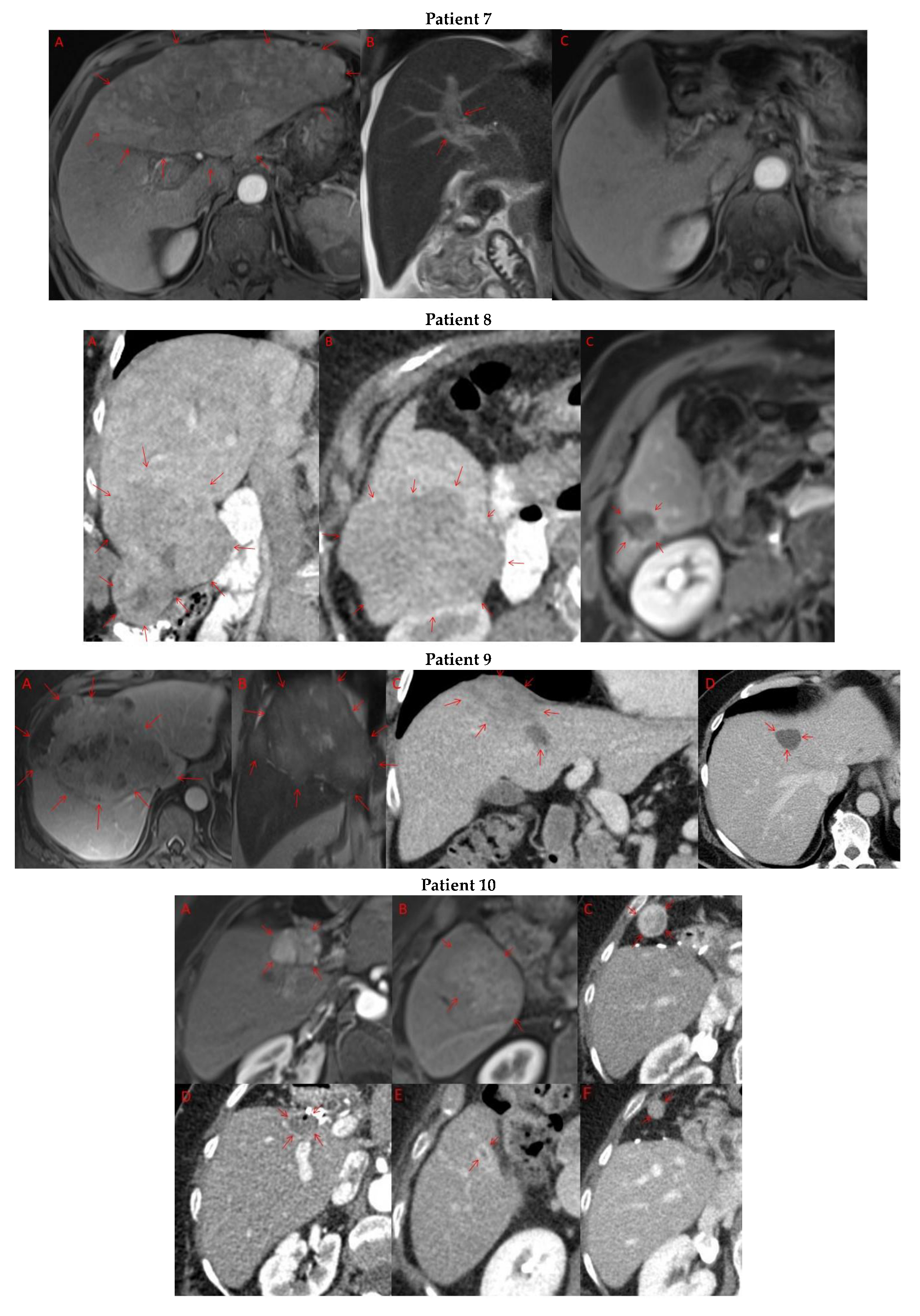
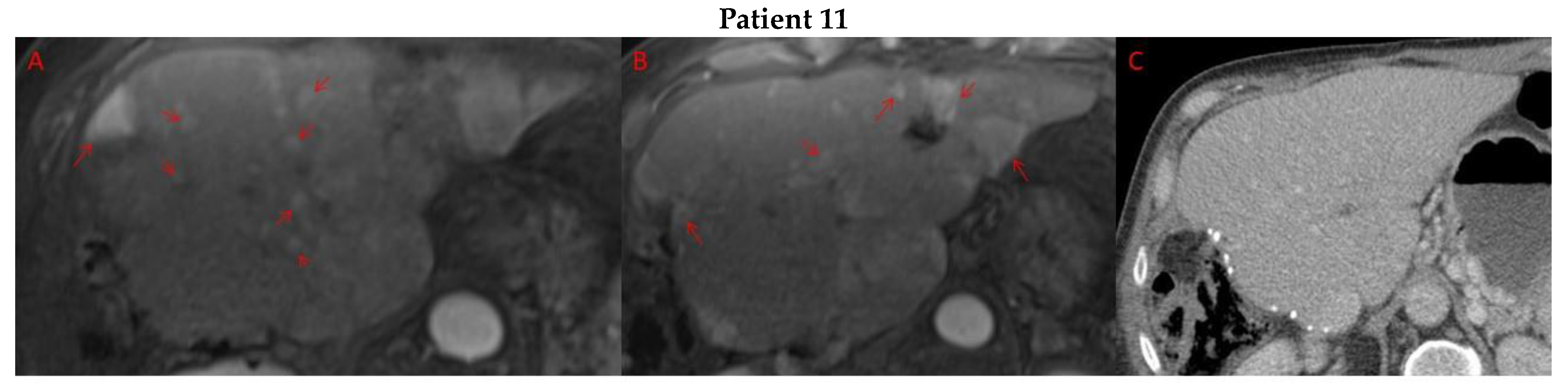
Pre- and post-treatment imaging for all exceptional responders is provided (Figure 3).
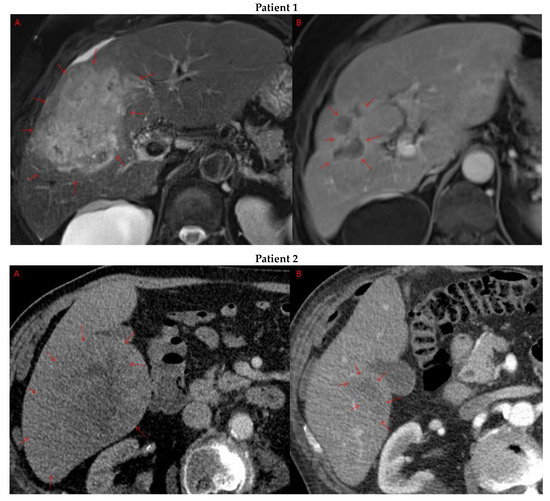
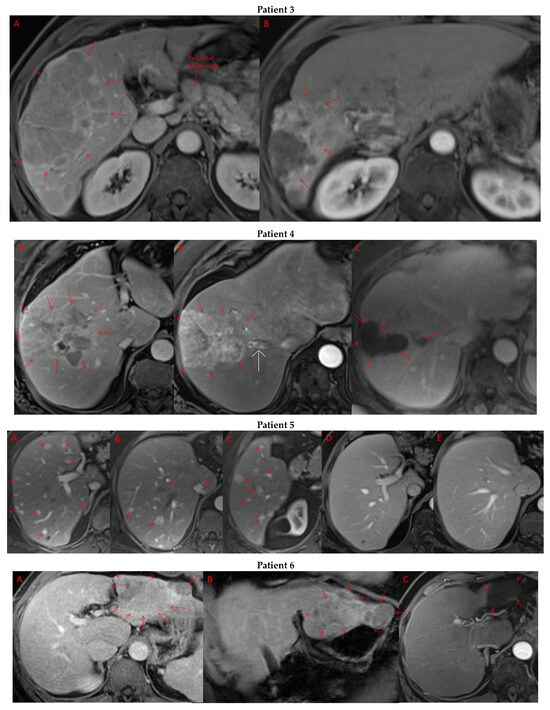
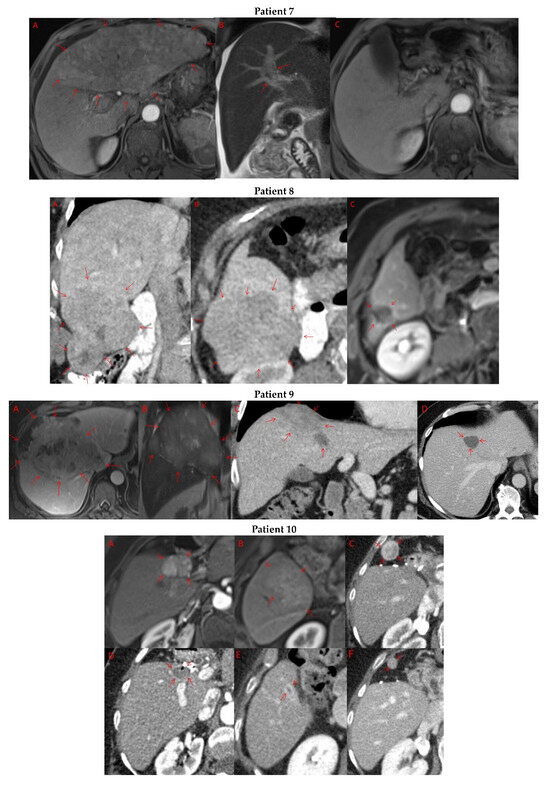
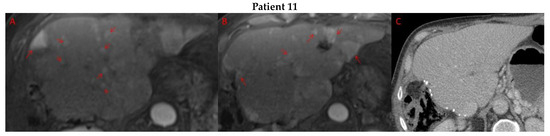
Figure 3.
Imaging findings in patients (n = 11) with complete response to neoadjuvant immunotherapy. Cross-sectional imaging for each patient with complete response to immunotherapy. Images represent the scan most recently taken prior to beginning the course of neoadjuvant treatment and the scan taken most recently after completion. (Patient 1) MRI showing large pre-treatment infiltrative tumor (A) followed by decrease in size from 10 cm to 5.6 cm status post 15 cycles of immunotherapy (B). (Patient 2) Large ill-defined 11cm tumor at diagnosis (A) followed by decrease in size to 3.6cm after 1 cycle Atezolizumab (B). (Patient 3) 13.3 cm mass with periportal lymphadenopathy (A) and subsequent decrease in size to 6.9 cm after 5 cycles of immunotherapy+1 round Y90 (B). (Patient 4) 8.1 cm mass in segment VIII with extension to right hepatic vein (A,B), and post-treatment tumor shrinkage after 11 cycles immunotherapy and locoregional treatment (C). (Patient 5) Recurrent scattered HCC lesions after previous resection (A–C). Near complete resolution after 4 cycles of immunotherapy and 1 Y90 (D,E). (Patient 6) 8 cm mass in segment II/III with portal vein invasion (A,B) and complete resolution after 22 cycles immunotherapy, Y90 and SBRT (C). (Patient 7) Infiltrative 20.3 cm mass in the left lobe with extensive tumor-in-vein in portal venous system (A,B) followed by complete resolution on 20 cycles immunotherapy, locoregional treatment and SBRT (C). (Patient 8). 7.1 cm mass in the right lobe (A,B) with reduction to 1.7 cm after 31 cycles immunotherapy (C). (Patient 9) 14.7 cm mass in the right hepatic lobe (A,B) shrinking to 5.8 cm after treatment (C,D). (Patient 10) Patient post-partial resection of segment IV/V for HCC with recurrence demonstrating 3.2 cm segment V/VIII lesion (A), a 4 cm lesion in the Right lower lobe (B) and a peritoneal metastasis (C). After treatment with 9 cycles immunotherapy + multiply Y90 treatments tumor size shrunk significantly (D–F). (Patient 11) Patient post-right hepatectomy for HCC with multiple diffuse recurrent nodules in the liver with largest measuring 2.6 cm (A,B). Post treatment imaging showing no evidence of disease (C).
5. Conclusions
Combined immunotherapy and locoregional therapy can downstage some patients with initially unresectable HCC, allowing for eventual curative-intent surgery. The survival benefit associated with complete response seems durable up to 3 years after achieving this response, and ctDNA measurements support disease-free status in those who ultimately undergo resection. Studies should focus on identifying ideal candidates for this approach using clinical factors and liquid biopsy.
Author Contributions
Conceptualization, R.R., C.J.W. and F.A.; methodology, R.R. and F.A.; software, n/a.; validation, R.R., C.J.W. and F.A.; formal analysis, R.R. and F.A.; resources, D.C.H.K. and F.A.; data curation, D.C.H.K. and F.A.; writing—original draft preparation, R.R., C.J.W. and F.A.; writing—review and editing, R.R., C.J.W., N.A., H.S., W.W.M., S.K. (Smitha Krishnamurthi), B.E., S.K. (Suneel Kamath), D.C.H.K. and F.A.; visualization, R.R., C.J.W. and F.A.; supervision, D.C.H.K. and F.A.; project administration, F.A. funding acquisition, n/a. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Presentation: Partial data from this study was presented at AHPBA 2023. IRB approval was obtained under Cleveland Clinic IRB 09-096.
Informed Consent Statement
Consent was waived due to the retrospective nature of the review.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| HCC | hepatocellular carcinoma |
| VEGF | vascular endothelial growth factor |
| mRECIST | modified Response Evaluation Criteria in Solid Tumors |
| AFP | alpha-fetoprotein |
| ICI | immune checkpoint inhibitor |
| PDL-1 | programmed death ligand 1 |
| ctDNA | circulating tumor DNA |
| TACE | transarterial chemoembolization |
| TARE | transarterial radioembolization |
| Y-90 | yttrium-90 |
| SBRT | stereotactic body radiation therapy |
| TMB | tumor mutational burden |
| LRT | locoregional treatment |
| OS | overall survival |
| NED | no evidence of disease |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Koulouris, A.; Tsagkaris, C.; Spyrou, V.; Pappa, E.; Troullinou, A.; Nikolaou, M. Hepatocellular Carcinoma: An Overview of the Changing Landscape of Treatment Options. J. Hepatocell. Carcinoma 2021, 8, 387–401. [Google Scholar] [CrossRef]
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef]
- Llovet, J.M.; De Baere, T.; Kulik, L.; Haber, P.K.; Greten, T.F.; Meyer, T.; Lencioni, R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 293–313. [Google Scholar] [CrossRef]
- Tie, J.; Cohen, J.D.; Lahouel, K.; Lo, S.N.; Wang, Y.; Kosmider, S.; Wong, R.; Shapiro, J.; Lee, M.; Harris, S.; et al. Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer. N. Engl. J. Med. 2022, 386, 2261–2272. [Google Scholar] [CrossRef]
- Kotani, D.; Oki, E.; Nakamura, Y.; Yukami, H.; Mishima, S.; Bando, H.; Shirasu, H.; Yamazaki, K.; Watanabe, J.; Kotaka, M.; et al. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat. Med. 2023, 29, 127–134. [Google Scholar] [CrossRef]
- Jardim, D.L.; Goodman, A.; de Melo Gagliato, D.; Kurzrock, R. The Challenges of Tumor Mutational Burden as an Immunotherapy Biomarker. Cancer Cell 2021, 39, 154–173. [Google Scholar] [CrossRef]
- Powles, T.; Assaf, Z.J.; Davarpanah, N.; Banchereau, R.; Szabados, B.E.; Yuen, K.C.; Grivas, P.; Hussain, M.; Oudard, S.; Gschwend, J.E.; et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature 2021, 595, 432–437. [Google Scholar] [CrossRef]
- Xie, C.; Wu, H.; Pan, T.; Zheng, X.; Yang, X.; Zhang, G.; Lian, Y.; Lin, J.; Peng, L. A novel panel based on immune infiltration and tumor mutational burden for prognostic prediction in hepatocellular carcinoma. Aging 2021, 13, 8563–8587. [Google Scholar] [CrossRef]
- Yin, L.; Zhou, L.; Xu, R. Identification of Tumor Mutation Burden and Immune Infiltrates in Hepatocellular Carcinoma Based on Multi-Omics Analysis. Front. Mol. Biosci. 2020, 7, 599142. [Google Scholar] [CrossRef]
- Gabbia, D.; De Martin, S. Tumor Mutational Burden for Predicting Prognosis and Therapy Outcome of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 24, 3441. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, D.; Liu, L.; Zhou, X.; Yao, Y.; Li, Z.; Li, J.; Chen, J.; Lei, Q.; Han, X. Development and validation of a robust immune-related risk signature for hepatocellular carcinoma. Medicine 2021, 100, e24683. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Xin, H.Y.; Luo, C.B.; Li, J.; Zhou, Z.J.; Zou, J.X.; Zhou, S.L. Associations among the mutational landscape, immune microenvironment, and prognosis in Chinese patients with hepatocellular carcinoma. Cancer Immunol. Immunother. 2021, 70, 377–389. [Google Scholar] [CrossRef]
- Truong, P.; Rahal, A.; Kallail, K.J. Metastatic Hepatocellular Carcinoma Responsive to Pembrolizumab. Cureus 2016, 8, e631. [Google Scholar] [CrossRef]
- Tighe, S.P.; Iqbal, U.; Fernandes, C.T.; Ahmed, A. Treatment of inoperable hepatocellular carcinoma with immunotherapy. BMJ Case Rep. 2019, 12, e229744. [Google Scholar] [CrossRef]
- Chiang, C.-L.; Chan, A.C.Y.; Chiu, K.W.H.; Kong, F.-M. Combined Stereotactic Body Radiotherapy and Checkpoint Inhibition in Unresectable Hepatocellular Carcinoma: A Potential Synergistic Treatment Strategy. Front. Oncol. 2019, 9, 1157. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; He, X.; Xu, Y.; Wang, X. Complete response to the combination of Lenvatinib and Pembrolizumab in an advanced hepatocellular carcinoma patient: A case report. BMC Cancer 2019, 19, 1062. [Google Scholar] [CrossRef]
- Adcock, C.S.; Puneky, L.V.; Campbell, G.S. Favorable Response of Metastatic Hepatocellular Carcinoma to Treatment with Trans-arterial Radioembolization Followed by Sorafenib and Nivolumab. Cureus 2019, 11, e4083. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Yamauchi, M.; Suehiro, Y.; Yamaoka, K.; Kosaka, Y.; Fuji, Y.; Uchikawa, S.; Kodama, K.; Morio, K.; Fujino, H.; et al. Complete response to pembrolizumab in advanced hepatocellular carcinoma with microsatellite instability. Clin. J. Gastroenterol. 2020, 13, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.G.; Li, H.B.; Yuan, Z.N.; Liu, W.; Yang, Q.; Cheng, Y.; Wang, W.J.; Wang, G.Y.; Li, H. Achievement of complete response to nivolumab in a patient with advanced sarcomatoid hepatocellular carcinoma: A case report. World J. Gastrointest. Oncol. 2020, 12, 1209–1215. [Google Scholar] [CrossRef]
- Bucalau, A.M.; Tancredi, I.; Pezzullo, M.; Covas, A.; Verset, G. Complete response of a hepatocellular carcinoma with complex macrovascular invasion after combined treatment with chemoembolization and immunotherapy: A case report. Acta Gastroenterol. Belg. 2021, 84, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.R.; Yan, Q.; Lin, H.M.; Shi, G.Z.; Cao, Y.; Zeng, H.; Liu, C.; Zhang, R. Anti-programmed cell death ligand 1-based immunotherapy in recurrent hepatocellular carcinoma with inferior vena cava tumor thrombus and metastasis: Three case reports. World J. Clin. Cases 2021, 9, 5988–5998. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Esmail, A.; Umoru, G.; Westhart, K.; Abudayyeh, A.; Saharia, A.; Ghobrial, R.M. Immunotherapy as a Neoadjuvant Therapy for a Patient with Hepatocellular Carcinoma in the Pretransplant Setting: A Case Report. Curr. Oncol. 2022, 29, 4267–4273. [Google Scholar] [CrossRef]
- Goto, Y.; Tajiri, K.; Tanaka, S.; Murayama, A.; Muraishi, N.; Hayashi, Y.; Yasuda, I. A ruptured sarcomatoid hepatocellular carcinoma treated with combined immunotherapy. Clin. J. Gastroenterol. 2023, 16, 244–249. [Google Scholar] [CrossRef]
- Zhong, K.; Xu, Y.; Cheng, Y.; Wen, Y.; Cai, L.; He, G.; Huang, H.; Fu, S.; Zhong, X.; Zheng, Y.; et al. Case report: Primary hepatocellular carcinoma with portal vein tumor thrombus characterized by active tumor immune microenvironment achieving a complete response following treatment of combined immunotherapy. Front. Immunol. 2022, 13, 999763. [Google Scholar] [CrossRef]
- Tsai, K.F.; Tsai, J.C.H.; Li, M.F.; Tan, J.W.H.; Chou, C.K.; Liang, H.L.; Chan, S.H. Complete Response in Metastatic Hepatocellular Carcinoma with Cardiac and Lung Involvement via Multimodality Treatment. Medicina 2021, 57, 849. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.; Gu, X.; Chen, L.; Wu, Q.; Li, H.; Bai, H.; Yang, J.; Qian, J. Case Report: Antiangiogenic Therapy Plus Immune Checkpoint Inhibitors Combined With Intratumoral Cryoablation for Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 740790. [Google Scholar] [CrossRef]
- Swed, B.; Ryan, K.; Gandarilla, O.; Shah, M.A.; Brar, G. Favorable response to second-line atezolizumab and bevacizumab following progression on nivolumab in advanced hepatocellular carcinoma: A case report demonstrating that anti-VEGF therapy overcomes resistance to checkpoint inhibition. Medicine 2021, 100, e26471. [Google Scholar] [CrossRef] [PubMed]
- Krug, S.; Mattheis, L.; Haemmerle, M.; Rosendahl, J.; Kleeff, J.; Michl, P. The impact of atezolizumab and bevacizumab in hepatocellular carcinoma with activated ß-catenin signaling. Cancer Rep. 2022, 5, e1493. [Google Scholar] [CrossRef] [PubMed]
- Nong, X.; Zhang, Y.M.; Liang, J.C.; Xie, J.L.; Zhang, Z.M. Complete response by patients with advanced hepatocellular carcinoma after combination immune/targeted therapy and transarterial chemoembolization: Two case reports and literature review. Transl. Cancer Res. 2022, 11, 2973–2984. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Chen, B.; Peng, D.; He, J.; Zhao, W.; Chen, T.; Xie, Z.; Pang, F. Case Report: Complete response after tislelizumab treatment in a hepatocellular carcinoma patient with abdominal lymph node metastasis. Front. Immunol. 2023, 14, 1163656. [Google Scholar] [CrossRef] [PubMed]
- Shigefuku, R.; Yoshikawa, K.; Tsukimoto, M.; Owa, H.; Tamai, Y.; Tameda, M.; Ogura, S.; Sugimoto, R.; Tanaka, H.; Eguchi, A.; et al. Hepatocellular Carcinoma Pseudoprogression Involving the Main Portal Vein, Right Ventricular Invasion, and Exacerbation of Lung Metastases in a Patient on Atezolizumab Plus Bevacizumab. Intern. Med. 2023, 62, 539–543. [Google Scholar] [CrossRef]
- Galle, P.R.; Foerster, F.; Kudo, M.; Chan, S.L.; Llovet, J.M.; Qin, S.; Schelman, W.R.; Chintharlapalli, S.; Abada, P.B.; Sherman, M.; et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019, 39, 2214–2229. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996, 334, 693–699. [Google Scholar] [CrossRef]
- Foerster, F.; Gairing, S.J.; Ilyas, S.I.; Galle, P.R. Emerging immunotherapy for HCC: A guide for hepatologists. Hepatology 2022, 75, 1604–1626. [Google Scholar] [CrossRef]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef]
- Raj, R.; Aykun, N.; Wehrle, C.J.; Maspero, M.; Krishnamurthi, S.; Estfan, B.; Kamath, S.; Aucejo, F. Immunotherapy for Advanced Hepatocellular Carcinoma-a Large Tertiary Center Experience. J. Gastrointest. Surg. 2023, 27, 2126–2134. [Google Scholar] [CrossRef] [PubMed]
- EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [CrossRef]
- EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [CrossRef]
- Han, J.-W.; Yoon, S.-K. Immune Responses Following Locoregional Treatment for Hepatocellular Carcinoma: Possible Roles of Adjuvant Immunotherapy. Pharmaceutics 2021, 13, 1387. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Toom, S.; Avula, A.; Kumar, V.; Rahma, O.E. The Immune Modulation Effect of Locoregional Therapies and Its Potential Synergy with Immunotherapy in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2020, 7, 11–17. [Google Scholar] [CrossRef]
- Xue, J.; Ni, H.; Wang, F.; Xu, K.; Niu, M. Advances in locoregional therapy for hepatocellular carcinoma combined with immunotherapy and targeted therapy. J. Interv. Med. 2021, 4, 105–113. [Google Scholar] [CrossRef]
- Facciorusso, A.; Del Prete, V.; Antonino, M.; Crucinio, N.; Neve, V.; Di Leo, A.; Carr, B.I.; Barone, M. Post-recurrence survival in hepatocellular carcinoma after percutaneous radiofrequency ablation. Dig. Liver Dis. 2014, 46, 1014–1019. [Google Scholar] [CrossRef]
- Okuwaki, Y.; Nakazawa, T.; Kokubu, S.; Hidaka, H.; Tanaka, Y.; Takada, J.; Watanabe, M.; Shibuya, A.; Minamino, T.; Saigenji, K. Repeat radiofrequency ablation provides survival benefit in patients with intrahepatic distant recurrence of hepatocellular carcinoma. Am. J. Gastroenterol. 2009, 104, 2747–2753. [Google Scholar] [CrossRef]
- Fang, P.; Hu, J.H.; Cheng, Z.G.; Liu, Z.F.; Wang, J.L.; Jiao, S.C. Efficacy and safety of bevacizumab for the treatment of advanced hepatocellular carcinoma: A systematic review of phase II trials. PLoS ONE 2012, 7, e49717. [Google Scholar] [CrossRef]
- Hu, J.; Tang, H.; Xie, F.; Zhang, Y.; Jia, S.; Zhou, J. Abstract 1020: Tissue-informed ctDNA MRD assay detects post-surgery minimal residual disease in HCC patients. Cancer Res. 2023, 83, 1020. [Google Scholar] [CrossRef]
- Pinato, D.J. Circulating-free tumour DNA and the promise of disease phenotyping in hepatocellular carcinoma. Oncogene 2018, 37, 4635–4638. [Google Scholar] [CrossRef]
- Hu, J.; Tang, H.; Xie, F.; Zhang, Y.; Jia, S.; Zhou, J. Ultra-sensitive baseline-informed MRD assay to predict prognosis outcomes in patients with resectable hepatocellular carcinoma. J. Clin. Oncol. 2023, 41, e16228. [Google Scholar] [CrossRef]
- Wong, M.; Kim, J.T.; Cox, B.; Larson, B.K.; Kim, S.; Waters, K.M.; Vail, E.; Guindi, M. Evaluation of tumor mutational burden in small early hepatocellular carcinoma and progressed hepatocellular carcinoma. Hepat. Oncol. 2021, 8, Hep39. [Google Scholar] [CrossRef]
- Yarchoan, M.; Albacker, L.A.; Hopkins, A.C.; Montesion, M.; Murugesan, K.; Vithayathil, T.T.; Zaidi, N.; Azad, N.S.; Laheru, D.A.; Frampton, G.M.; et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. J. Clin. Investig. 2019, 4, e126908. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).