Endotoxin Tolerance Creates Favourable Conditions for Cancer Development
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Preparation of Lipopolysaccharide (LPS) Solution
2.3. Induction of Endotoxin Tolerance (ET) in RAW 264.7 Macrophage Cells
2.4. Preparation of Conditioned Media from RAW 264.7 Macrophages
2.5. Cell Viability Assay
2.6. Colony Formation Assay
2.7. Scratch Assay
2.8. 3D Spheroidal Assay
2.9. Co-Cultures of Cancer Cells and RAW 264.7 Macrophages
2.10. Analysis of Cytokine Production
2.11. Flow Cytometry Analysis
2.12. Statistical Analysis
3. Results
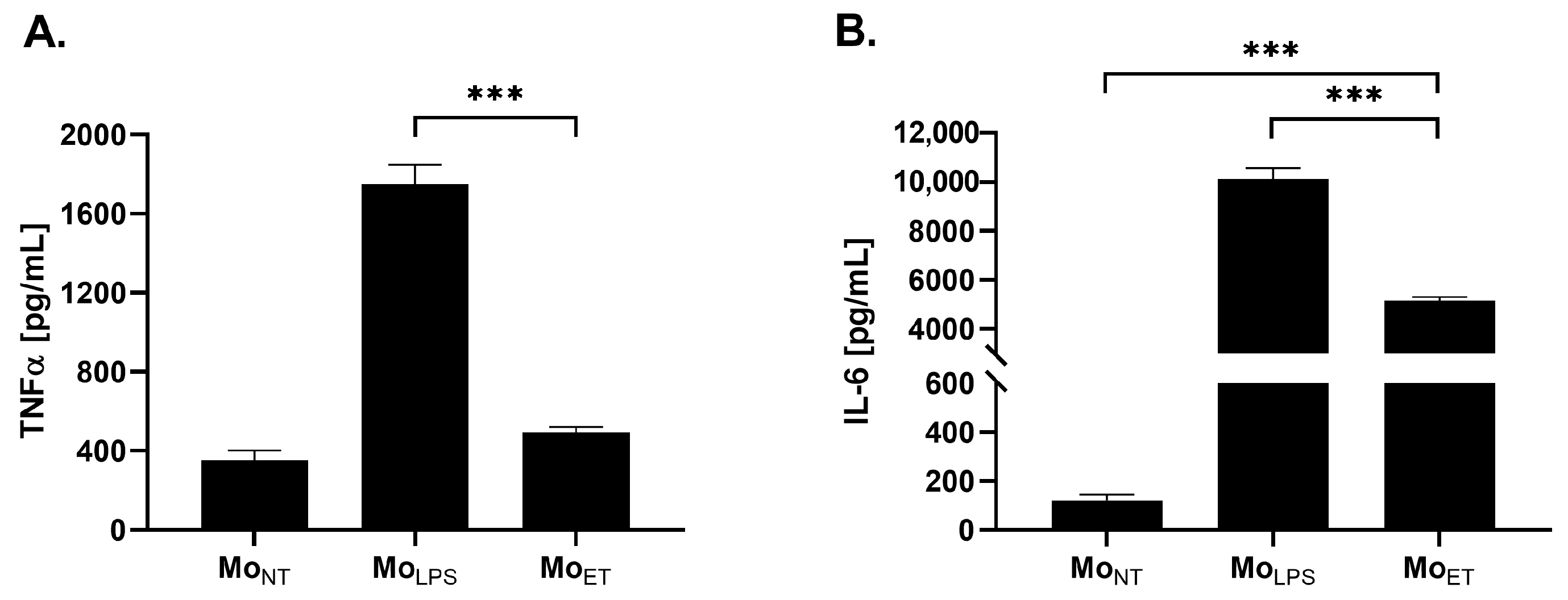
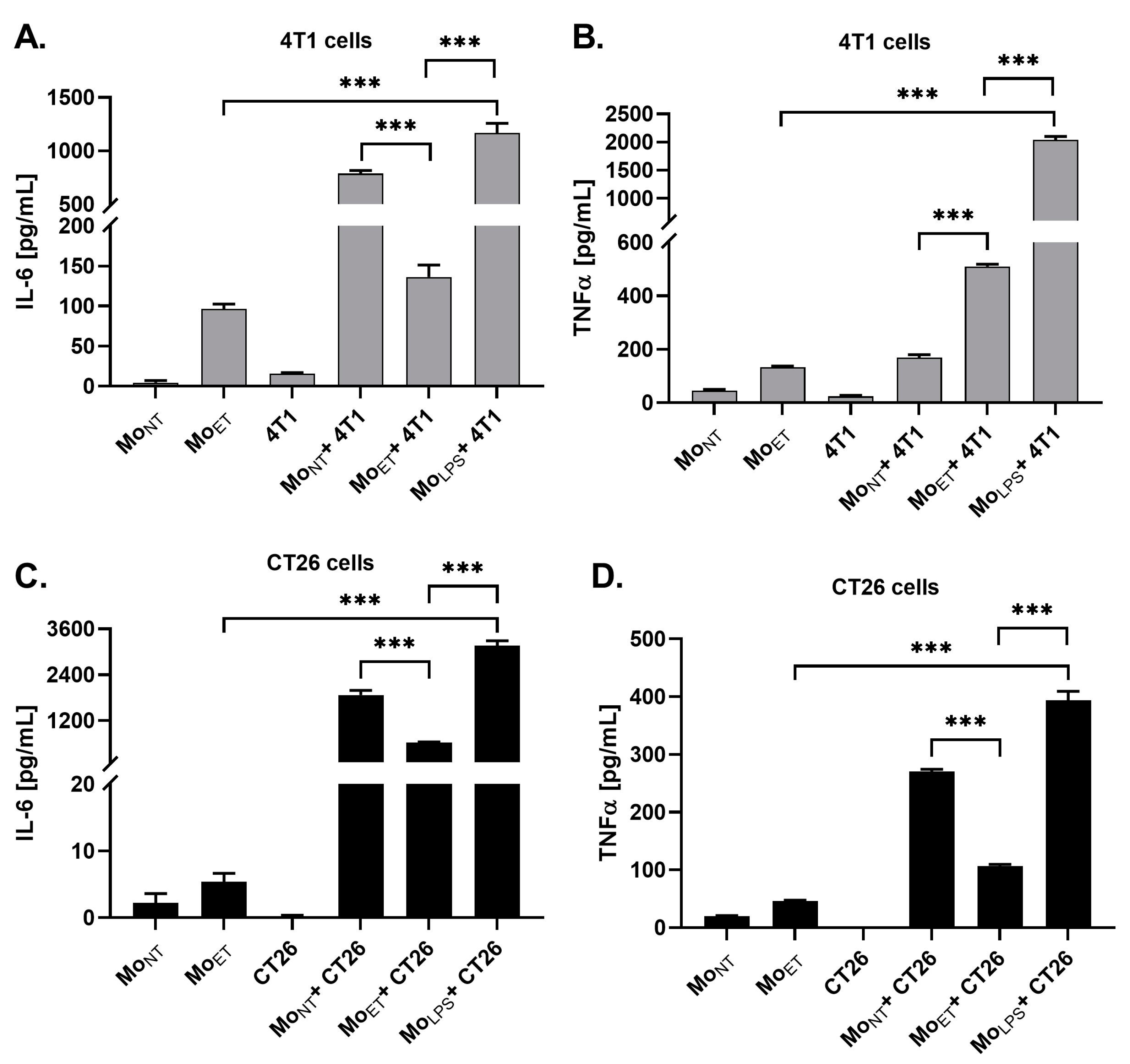
3.1. Endotoxin-Tolerant Macrophages Displayed Decreased Expression of TNFα and IL-6
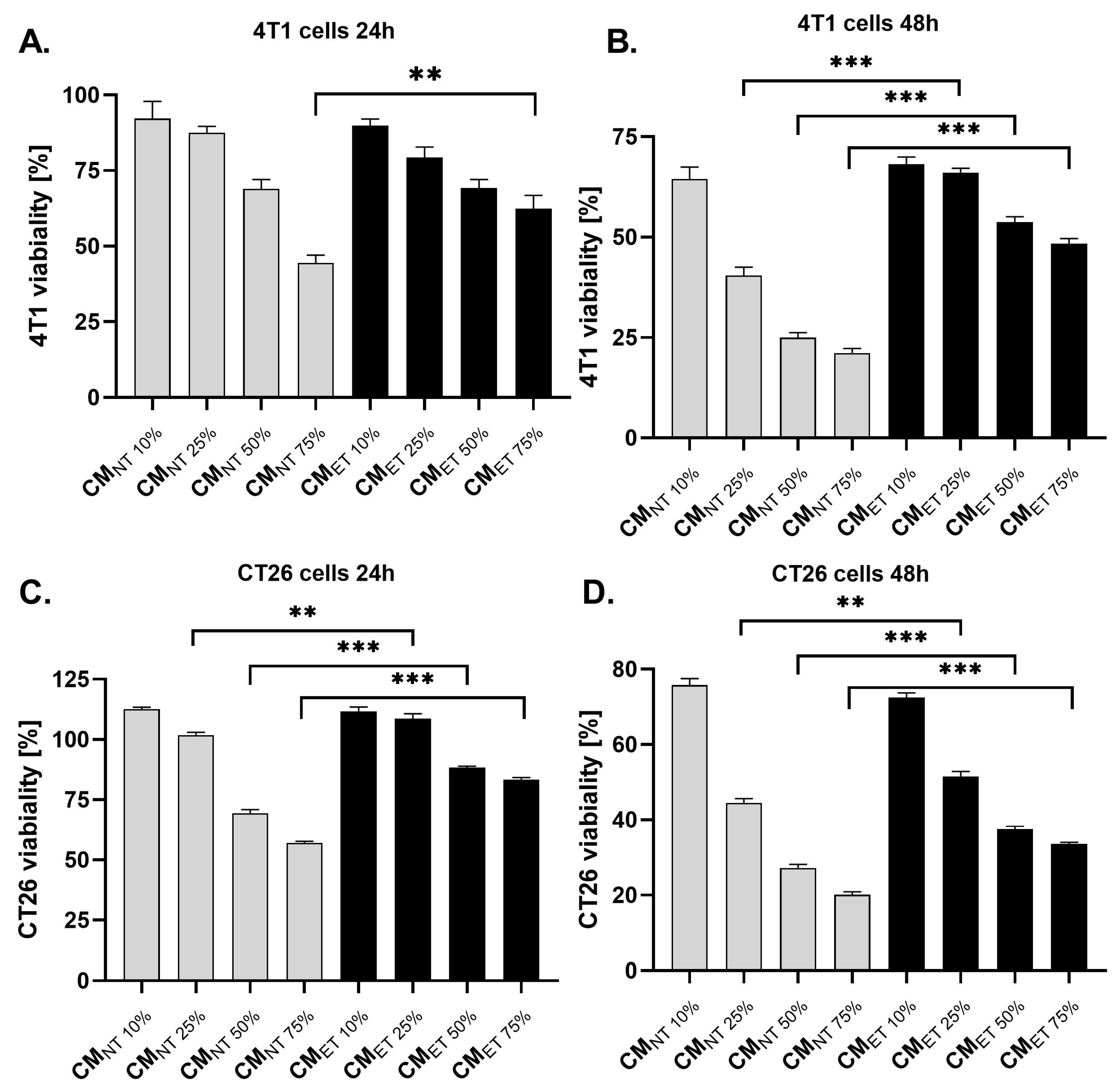
3.2. Conditioned Media Derived from Endotoxin-Tolerant Macrophages Increases the Survival Capacity of Cancer Cells
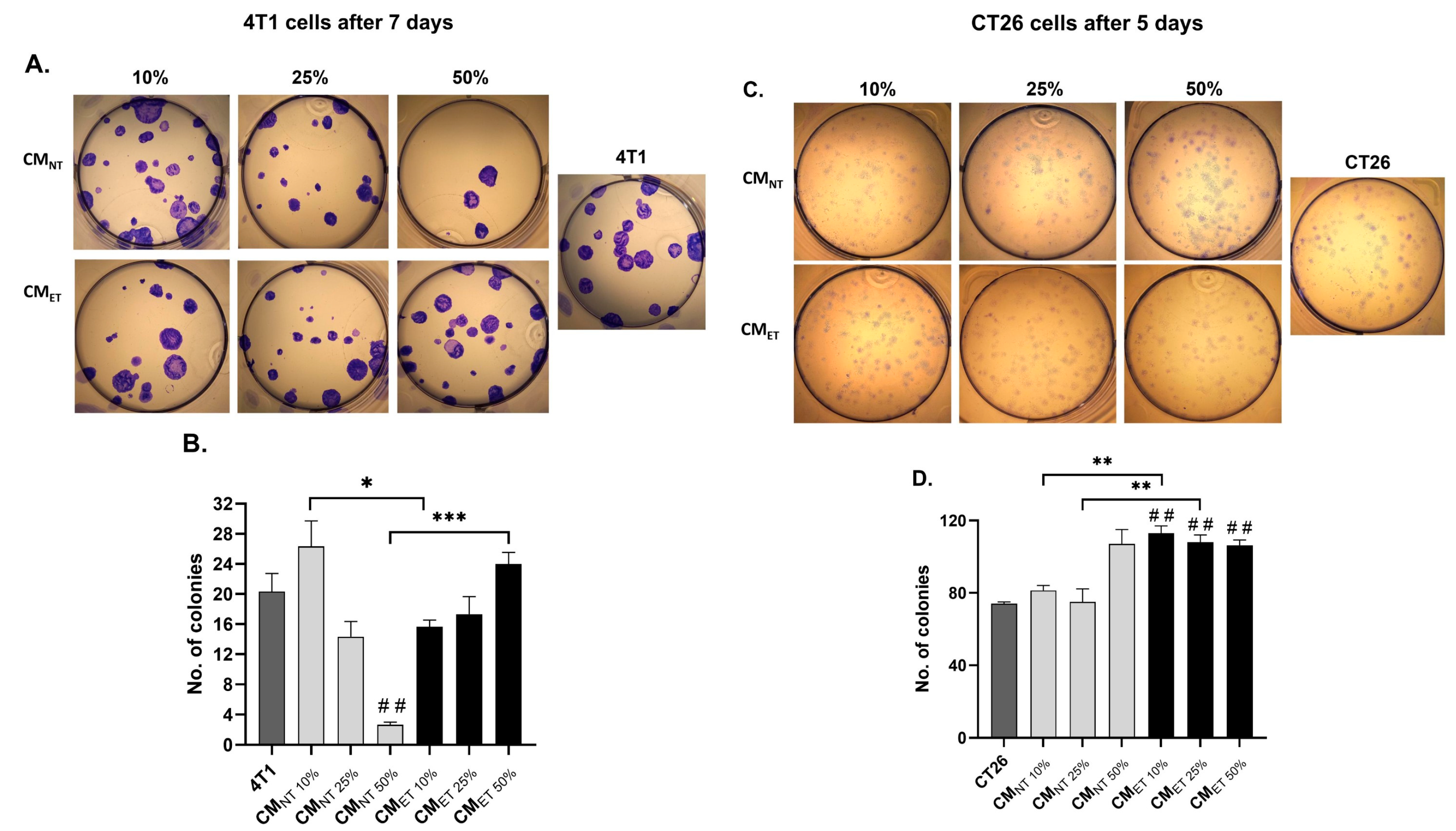
3.3. Exposure of the Cancer Cells to the Conditioned Media Derived from the Tolerant Macrophages Influences Their Clonogenic Potential
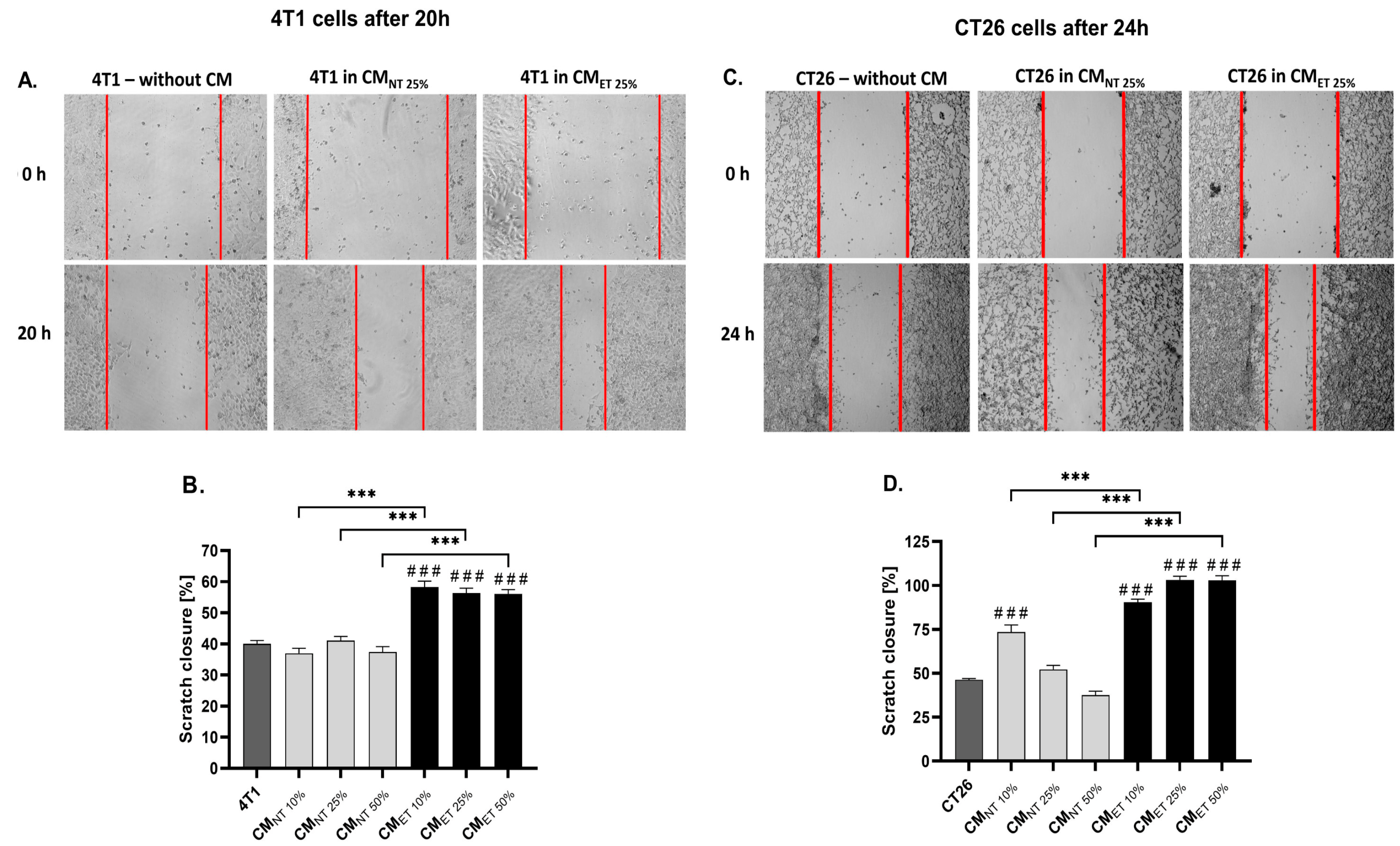
3.4. Conditioned Media Derived from Endotoxin-Tolerant Macrophages Increase Cancer Cell Motility
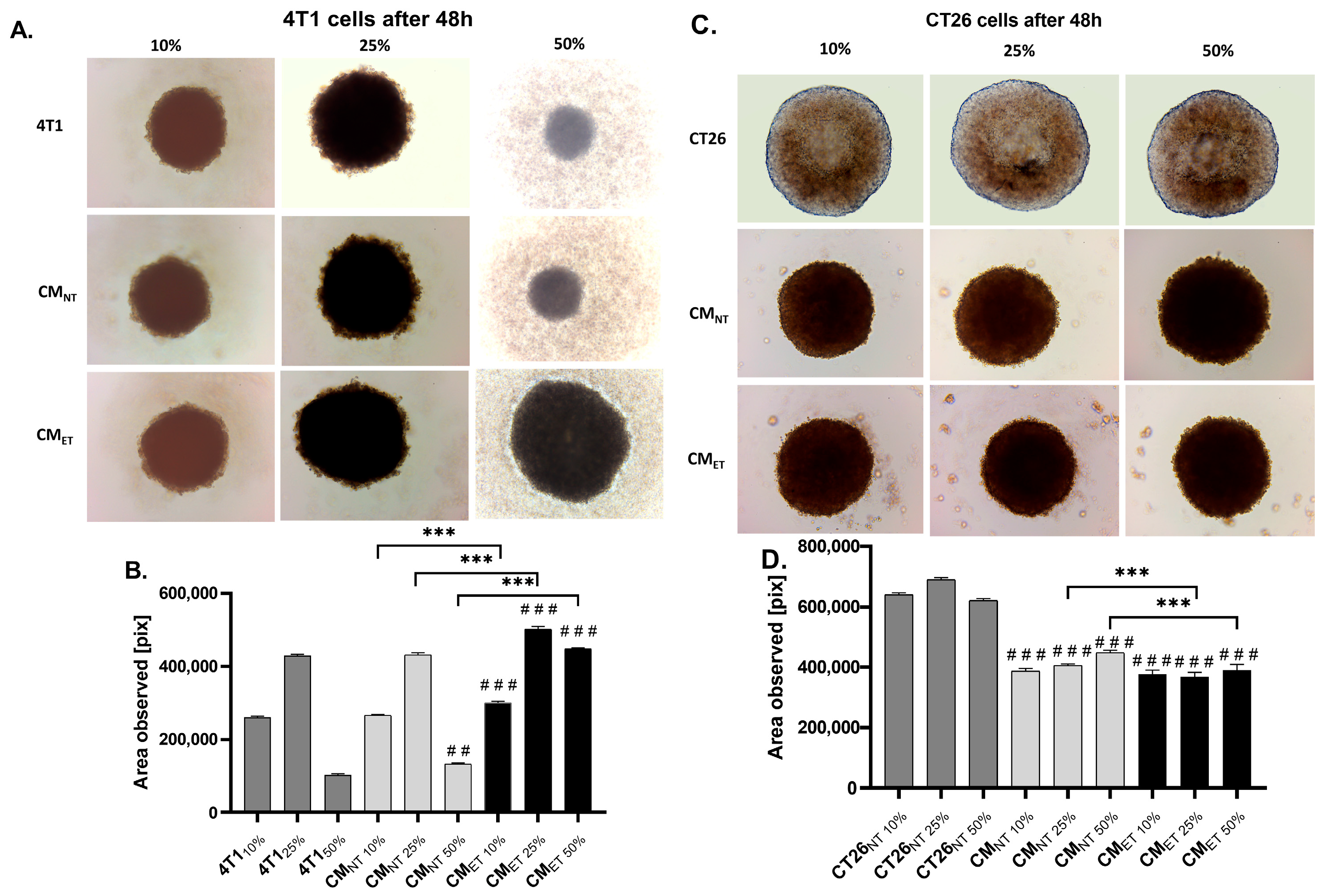
3.5. Conditioned Media from the Tolerant Macrophages Can Affect the Survivability of the Cancer Cells at the 3D Level
3.6. Endotoxin Tolerance Affects Crosstalk between Macrophages and Cancer Cells by Downregulating Expression of IL-6 and TNFα
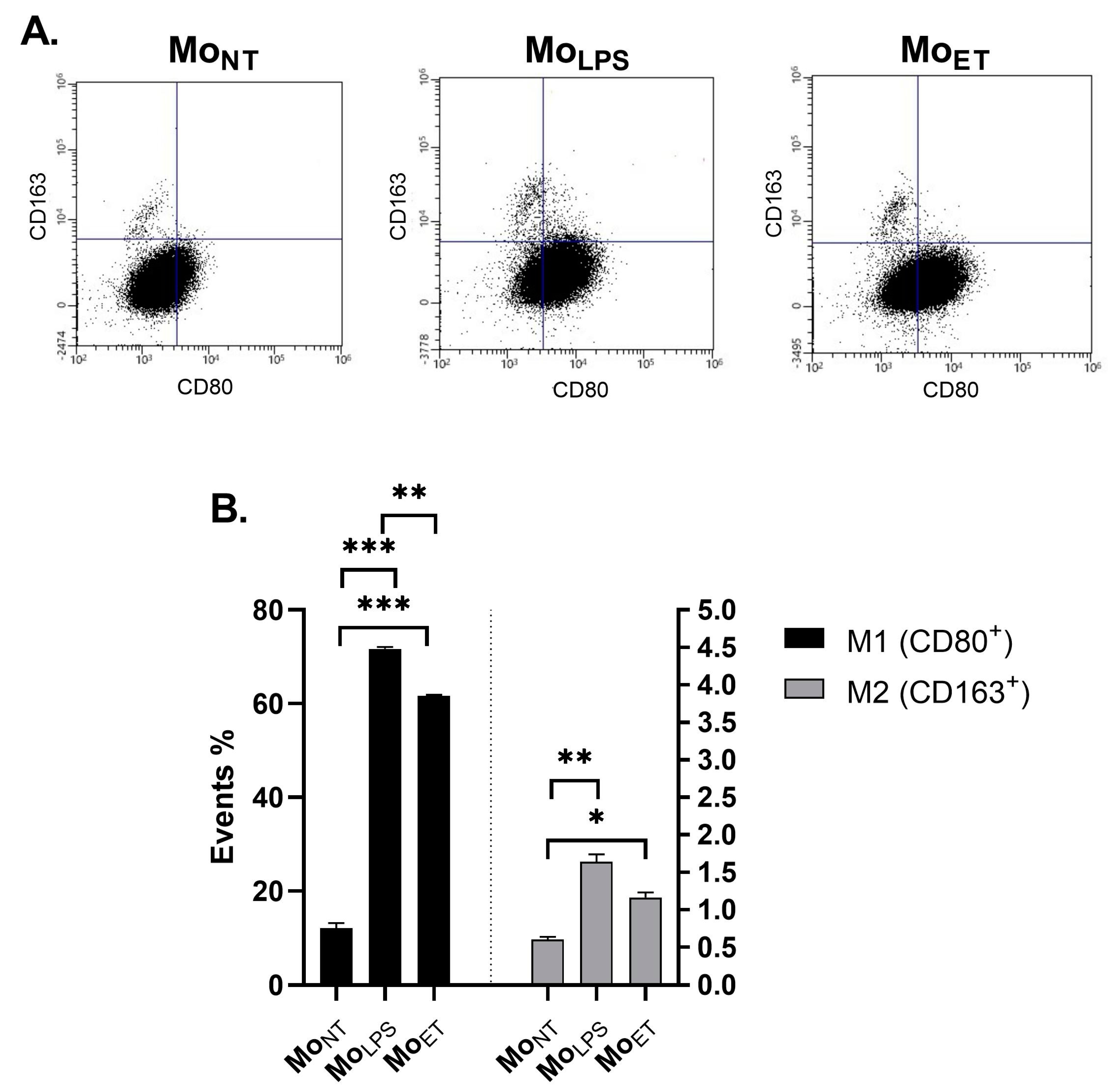
3.7. Macrophages Maintain the M1 Phenotype Even after Prolonged Exposure to LPS
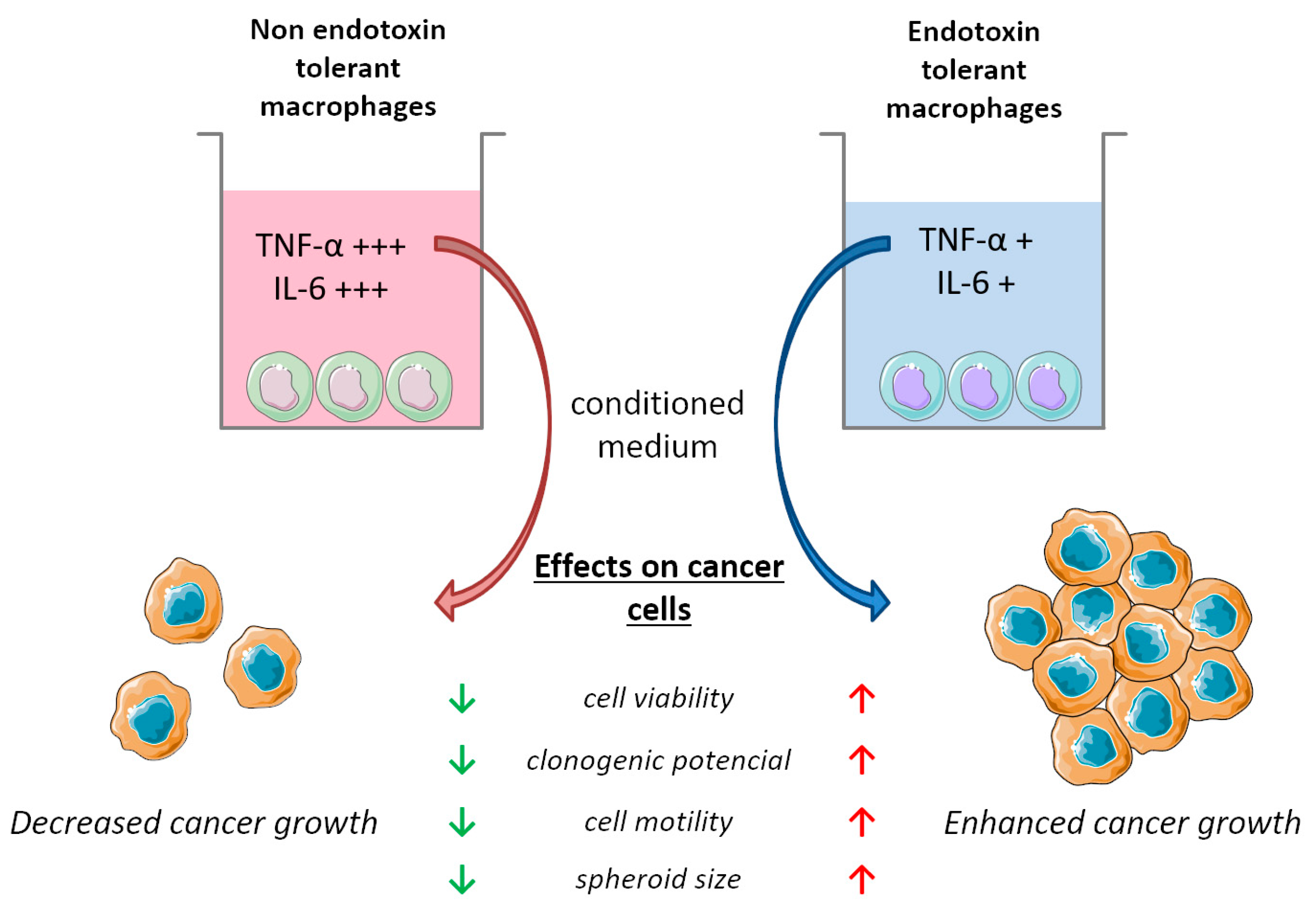
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.K.; Srivastava, S.K.; Poosarla, T.; Dyess, D.L.; Holliday, N.P.; Singh, A.P.; Singh, S. Inflammation, Immunosuppressive Microenvironment and Breast Cancer: Opportunities for Cancer Prevention and Therapy. Ann. Transl. Med. 2019, 7, 593. [Google Scholar] [CrossRef] [PubMed]
- Mangone, L.; Marinelli, F.; Bisceglia, I.; Braghiroli, M.B.; Damato, A.; Pinto, C. Five-Year Relative Survival by Stage of Breast and Colon Cancers in Northern Italy. Front. Oncol. 2022, 12, 982461. [Google Scholar] [CrossRef] [PubMed]
- Noy, R.; Pollard, J.W. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, S.; Zhang, M.; Zhen, L.; Pang, D.; Zhang, Q.; Li, Z. High-Infiltration of Tumor-Associated Macrophages Predicts Unfavorable Clinical Outcome for Node-Negative Breast Cancer. PLoS ONE 2013, 8, e76147. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Luo, Y. Targeting Macrophages in Cancer Immunotherapy. Signal Transduct. Target. Ther. 2021, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, M.; Maeda, K.; Nagahara, H.; Fukuoka, T.; Nakao, S.; Matsutani, S.; Hirakawa, K.; Ohira, M. The Peripheral Monocyte Count Is Associated with the Density of Tumor-Associated Macrophages in the Tumor Microenvironment of Colorectal Cancer: A Retrospective Study. BMC Cancer 2017, 17, 404. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Wang, Q.; Zhang, X. Tumor-Recruited M2 Macrophages Promote Gastric and Breast Cancer Metastasis via M2 Macrophage-Secreted CHI3L1 Protein. J. Hematol. Oncol. 2017, 10, 36. [Google Scholar] [CrossRef]
- Ruffell, B.; Chang-Strachan, D.; Chan, V.; Rosenbusch, A.; Ho, C.M.T.; Pryer, N.; Daniel, D.; Hwang, E.S.; Rugo, H.S.; Coussens, L.M. Macrophage IL-10 Blocks CD8+ T Cell-Dependent Responses to Chemotherapy by Suppressing IL-12 Expression in Intratumoral Dendritic Cells. Cancer Cell 2014, 26, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, C.-C. Inflammatory Response of Macrophages in Infection. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Michel, O. Role of Lipopolysaccharide (LPS) in Asthma and Other Pulmonary Conditions. J. Endotoxin Res. 2003, 9, 293–300. [Google Scholar] [CrossRef]
- Virca, G.D.; Kim, S.Y.; Glaser, K.B.; Ulevitch, R.J. Lipopolysaccharide Induces Hyporesponsiveness to Its Own Action in RAW 264.7 Cells. J. Biol. Chem. 1989, 264, 21951–21956. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage Plasticity, Polarization, and Function in Health and Disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Hongkuan, F.; Cook, J.A. Review: Molecular Mechanisms of Endotoxin Tolerance. J. Endotoxin Res. 2004, 10, 71–84. [Google Scholar] [CrossRef]
- Roth, J.; McClellan, J.L.; Kluger, M.J.; Zeisberger, E. Attenuation of Fever and Release of Cytokines after Repeated Injections of Lipopolysaccharide in Guinea-Pigs. J. Physiol. 1994, 477, 177–185. [Google Scholar] [CrossRef]
- Seeley, J.J.; Ghosh, S. Molecular Mechanisms of Innate Memory and Tolerance to LPS. J. Leukoc. Biol. 2017, 101, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Bae, J.; Lee, J.H.; Park, Y.J.; Lee, H.A.R.; Mun, S.; Kim, Y.; Yune, C.J.; Chung, T.N.; Kim, K. Serial Change of Endotoxin Tolerance in a Polymicrobial Sepsis Model. Int. J. Mol. Sci. 2022, 23, 6581. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Karl, I.E. The Pathophysiology and Treatment of Sepsis. N. Engl. J. Med. 2003, 348, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M. Endotoxins and Other Sepsis Triggers. Contrib. Nephrol. 2010, 167, 14–24. [Google Scholar] [PubMed]
- Jędrzejewski, T.; Sobocińska, J.; Pawlikowska, M.; Dzialuk, A.; Wrotek, S. Dual Effect of the Extract from the Fungus Coriolus Versicolor on Lipopolysaccharide-Induced Cytokine Production in RAW 264.7 Macrophages Depending on the Lipopolysaccharide Concentration. J. Inflamm. Res. 2022, 15, 3599–3611. [Google Scholar] [CrossRef] [PubMed]
- Broad, A.; Jones, D.; Kirby, J. Toll-Like Receptor (TLR) Response Tolerance: A Key Physiological “Damage Limitation” Effect and an Important Potential Opportunity for Therapy. Curr. Med. Chem. 2006, 13, 2487–2502. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.-M.; Pitton, C.; Fitting, C. Endotoxin Tolerance Is Not a LPS-Specific Phenomenon: Partial Mimicry with IL-1, IL-10 and TGFβ. J. Endotoxin Res. 1994, 1, 21–29. [Google Scholar] [CrossRef]
- Burkart, V.; Kim, Y.-E.; Hartmann, B.; Ghiea, I.; Syldath, U.; Kauer, M.; Fingberg, W.; Hanifi-Moghaddam, P.; Müller, S.; Kolb, H. Cholera Toxin B Pretreatment of Macrophages and Monocytes Diminishes Their Proinflammatory Responsiveness to Lipopolysaccharide. J. Immunol. 2002, 168, 1730–1737. [Google Scholar] [CrossRef]
- Medvedev, A.E.; Henneke, P.; Schromm, A.; Lien, E.; Ingalls, R.; Fenton, M.J.; Golenbock, D.T.; Vogel, S.N. Induction of Tolerance to Lipopolysaccharide and Mycobacterial Components in Chinese Hamster Ovary/CD14 Cells Is Not Affected by Overexpression of Toll-like Receptors 2 or 4. J. Immunol. 2001, 167, 2257–2267. [Google Scholar] [CrossRef]
- Macarthur, M.; Hold, G.L.; El-Omar, E.M. Inflammation and Cancer II. Role of Chronic Inflammation and Cytokine Gene Polymorphisms in the Pathogenesis of Gastrointestinal Malignancy. Am. J. Physiol.-Gastrointest. Liver Physiol. 2004, 286, G515–G520. [Google Scholar] [CrossRef] [PubMed]
- Andreani, V.; Gatti, G.; Simonella, L.; Rivero, V.; Maccioni, M. Activation of Toll-like Receptor 4 on Tumor Cells In Vitro Inhibits Subsequent Tumor Growth In Vivo. Cancer Res. 2007, 67, 10519–10527. [Google Scholar] [CrossRef] [PubMed]
- Lenters, V.; Basinas, I.; Beane-Freeman, L.; Boffetta, P.; Checkoway, H.; Coggon, D.; Portengen, L.; Sim, M.; Wouters, I.M.; Heederik, D.; et al. Endotoxin Exposure and Lung Cancer Risk: A Systematic Review and Meta-Analysis of the Published Literature on Agriculture and Cotton Textile Workers. Cancer Causes Control 2010, 21, 523–555. [Google Scholar] [CrossRef] [PubMed]
- Gillen, J.; Ondee, T.; Gurusamy, D.; Issara-Amphorn, J.; Manes, N.P.; Yoon, S.H.; Leelahavanichkul, A.; Nita-Lazar, A. LPS Tolerance Inhibits Cellular Respiration and Induces Global Changes in the Macrophage Secretome. Biomolecules 2021, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.E.; Kopydlowski, K.M.; Vogel, S.N. Inhibition of Lipopolysaccharide-Induced Signal Transduction in Endotoxin-Tolerized Mouse Macrophages: Dysregulation of Cytokine, Chemokine, and Toll-like Receptor 2 and 4 Gene Expression. J. Immunol. 2000, 164, 5564–5574. [Google Scholar] [CrossRef] [PubMed]
- Nomura, F.; Akashi, S.; Sakao, Y.; Sato, S.; Kawai, T.; Matsumoto, M.; Nakanishi, K.; Kimoto, M.; Miyake, K.; Takeda, K.; et al. Cutting Edge: Endotoxin Tolerance in Mouse Peritoneal Macrophages Correlates with Down-Regulation of Surface Toll-Like Receptor 4 Expression. J. Immunol. 2000, 164, 3476–3479. [Google Scholar] [CrossRef]
- Xiang, Q.; Wen, L.; Liu, M.; Zhang, Y.; Qu, J.; Tian, J. Endotoxin Tolerance of RAW264.7 Correlates with P38-Dependent Up-Regulation of Scavenger Receptor-A. J. Int. Med. Res. 2009, 37, 491–502. [Google Scholar] [CrossRef] [PubMed]
- León, P. Interleukin 1 and Its Relationship to Endotoxin Tolerance. Arch. Surg. 1992, 127, 146. [Google Scholar] [CrossRef] [PubMed]
- Erroi, A.; Fantuzzi, G.; Mengozzi, M.; Sironi, M.; Orencole, S.F.; Clark, B.D.; Dinarello, C.A.; Isetta, A.; Gnocchi, P.; Giovarelli, M. Differential Regulation of Cytokine Production in Lipopolysaccharide Tolerance in Mice. Infect. Immun. 1993, 61, 4356–4359. [Google Scholar] [CrossRef]
- Piszczek, P.; Radtke, A.; Ehlert, M.; Jędrzejewski, T.; Sznarkowska, A.; Sadowska, B.; Bartmański, M.; Erdoğan, Y.K.; Ercan, B.; Jedrzejczyk, W. Comprehensive Evaluation of the Biological Properties of Surface-Modified Titanium Alloy Implants. J. Clin. Med. 2020, 9, 342. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M. The Many Faces of Macrophage Activation. J. Leukoc. Biol. 2003, 73, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Pena, O.M.; Pistolic, J.; Raj, D.; Fjell, C.D.; Hancock, R.E.W. Endotoxin Tolerance Represents a Distinctive State of Alternative Polarization (M2) in Human Mononuclear Cells. J. Immunol. 2011, 186, 7243–7254. [Google Scholar] [CrossRef]
- Rajaiah, R.; Perkins, D.J.; Polumuri, S.K.; Zhao, A.; Keegan, A.D.; Vogel, S.N. Dissociation of Endotoxin Tolerance and Differentiation of Alternatively Activated Macrophages. J. Immunol. 2013, 190, 4763–4772. [Google Scholar] [CrossRef] [PubMed]
- López-Collazo, E.; del Fresno, C. Pathophysiology of Endotoxin Tolerance: Mechanisms and Clinical Consequences. Crit. Care 2013, 17, 242. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, S.; Ross, K.N.; Lander, E.S.; Golub, T.R. A Molecular Signature of Metastasis in Primary Solid Tumors. Nat. Genet. 2003, 33, 49–54. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Johansson, M.; Coussens, L.M. Immune Cells as Mediators of Solid Tumor Metastasis. Cancer Metastasis Rev. 2008, 27, 11–18. [Google Scholar] [CrossRef]
- Reynolds, D.S.; Tevis, K.M.; Blessing, W.A.; Colson, Y.L.; Zaman, M.H.; Grinstaff, M.W. Breast Cancer Spheroids Reveal a Differential Cancer Stem Cell Response to Chemotherapeutic Treatment. Sci. Rep. 2017, 7, 10382. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The Third Dimension Bridges the Gap between Cell Culture and Live Tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Huang, Q.; Huang, Y.; Zheng, W.; Hua, J.; Yang, S.; Zhuang, J.; Wang, J.; Ye, J. Lipopolysaccharide Increases the Release of VEGF-C That Enhances Cell Motility and Promotes Lymphangiogenesis and Lymphatic Metastasis through the TLR4- NF-ΚB/JNK Pathways in Colorectal Cancer. Oncotarget 2016, 7, 73711–73724. [Google Scholar] [CrossRef] [PubMed]
- Gulubova, M.; Ananiev, J.; Yovchev, Y.; Julianov, A.; Karashmalakov, A.; Vlaykova, T. The Density of Macrophages in Colorectal Cancer Is Inversely Correlated to TGF-Β1 Expression and Patients’ Survival. J. Mol. Histol. 2013, 44, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Peng, R.-Q.; Wu, X.-J.; Xia, Q.; Hou, J.-H.; Ding, Y.; Zhou, Q.-M.; Zhang, X.; Pang, Z.-Z.; Wan, D.-S.; et al. The Density of Macrophages in the Invasive Front Is Inversely Correlated to Liver Metastasis in Colon Cancer. J. Transl. Med. 2010, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Aras, S.; Zaidi, M.R. TAMeless Traitors: Macrophages in Cancer Progression and Metastasis. Br. J. Cancer 2017, 117, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, K.; Kozłowski, H.M.; Jędrzejewski, T.; Sobocińska, J.; Maciejewski, B.; Dzialuk, A.; Wrotek, S. Endotoxin Tolerance Creates Favourable Conditions for Cancer Development. Cancers 2023, 15, 5113. https://doi.org/10.3390/cancers15205113
Roy K, Kozłowski HM, Jędrzejewski T, Sobocińska J, Maciejewski B, Dzialuk A, Wrotek S. Endotoxin Tolerance Creates Favourable Conditions for Cancer Development. Cancers. 2023; 15(20):5113. https://doi.org/10.3390/cancers15205113
Chicago/Turabian StyleRoy, Konkonika, Henryk Mikołaj Kozłowski, Tomasz Jędrzejewski, Justyna Sobocińska, Bartosz Maciejewski, Artur Dzialuk, and Sylwia Wrotek. 2023. "Endotoxin Tolerance Creates Favourable Conditions for Cancer Development" Cancers 15, no. 20: 5113. https://doi.org/10.3390/cancers15205113
APA StyleRoy, K., Kozłowski, H. M., Jędrzejewski, T., Sobocińska, J., Maciejewski, B., Dzialuk, A., & Wrotek, S. (2023). Endotoxin Tolerance Creates Favourable Conditions for Cancer Development. Cancers, 15(20), 5113. https://doi.org/10.3390/cancers15205113





