Imaging in Upper Tract Urothelial Carcinoma: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Detection and Diagnosis
2.1. UTUC Staging
2.2. Computed Tomography
2.3. MRI
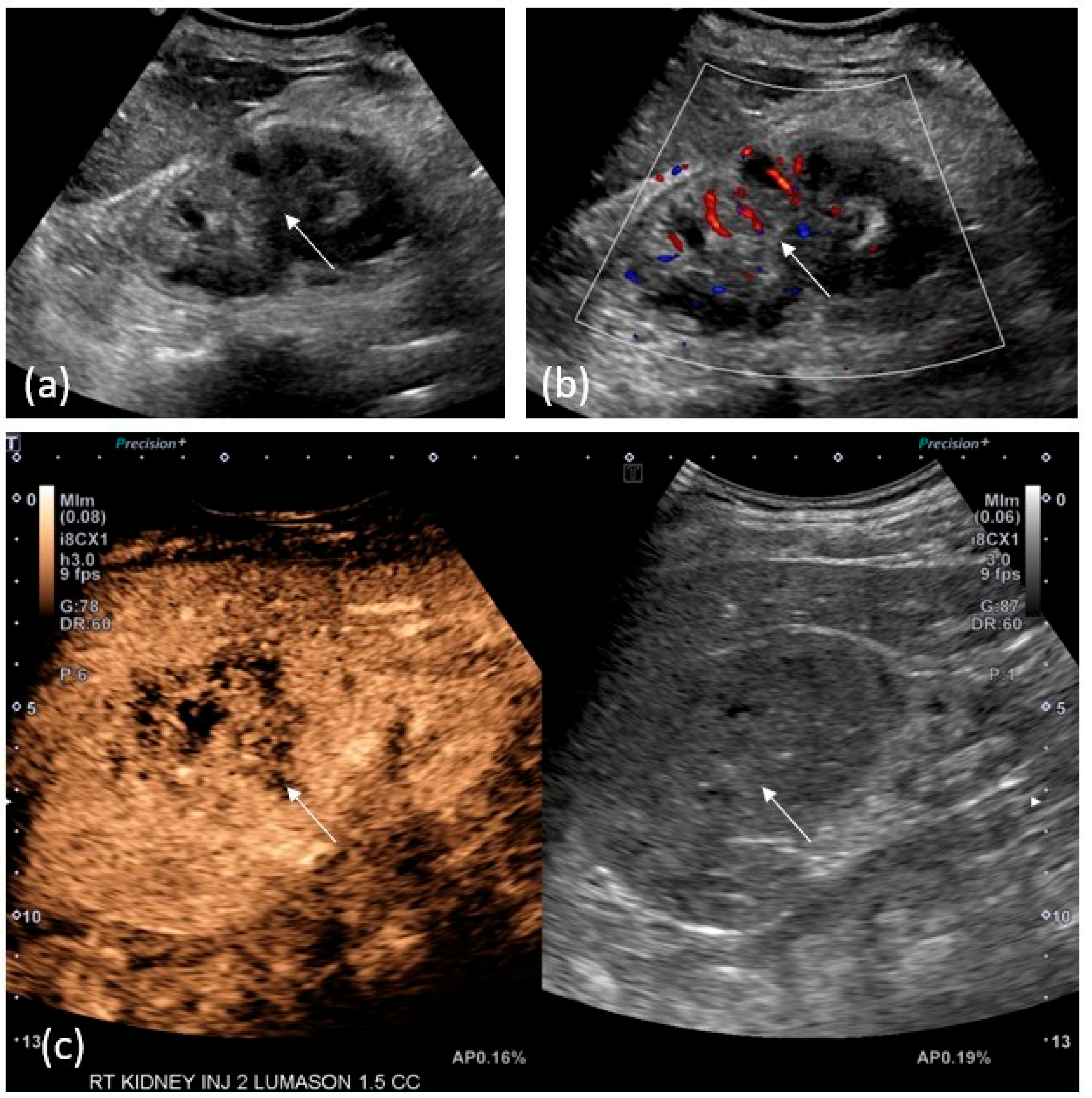
2.4. US/CEUS
2.5. PET/CT
3. AI and Multiomics in the Classification and Prognostication of Upper Urothelial Tract Urothelial Carcinoma
4. Image-Guided Percutaneous Biopsy
5. Surveillance
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rouprêt, M.; Seisen, T.; Birtle, A.J.; Capoun, O.; Compérat, E.M.; Dominguez-Escrig, J.L.; Andersson, I.G.; Liedberg, F.; Mariappan, P.; Mostafid, A.H.; et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2023 Update. Eur. Urol. 2023, 84, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.P.; Fishman, E.K. Upper and lower tract urothelial imaging using computed tomography urography. Urol. Clin. N. Am. 2018, 45, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.; Fishman, E.K.; Sheth, S. Upper urinary tract urothelial carcinoma on multidetector CT: Spectrum of disease. Abdom. Imaging 2019, 44, 3874–3885. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.; Grossman, H.B.; Droller, M.; Schmidbauer, J.; Hermann, G.; Drăgoescu, O.; Ray, E.; Fradet, Y.; Karl, A.; Burgués, J.P.; et al. Photodynamic diagnosis of non–muscle-invasive bladder cancer with hexaminolevulinate cystoscopy: A meta-analysis of detection and recurrence based on raw data. Eur. Urol. 2013, 64, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Kawashima, A.; Glockner, J.F.; Hartman, R.P.; Kim, B.; King, B.F. MR urography for suspected upper tract urothelial carcinoma. Eur. Radiol. 2008, 19, 912–923. [Google Scholar] [CrossRef] [PubMed]
- Cheaib, J.G.; Claus, L.E.; Patel, H.D.; Kates, M.R.; Matoso, A.; Hahn, N.M.; Bivalacqua, T.J.; Hoffman-Censits, J.H.; Pierorazio, P.M. Site of metastatic recurrence impacts prognosis in patients with high-grade upper tract urothelial carcinoma. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 74.e9–74.e16. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; Chiujdea, S.; Vartolomei, M.D.; Bove, P.; Porreca, A.; Busetto, G.M.; del Giudice, F.; Antonelli, A.; Foschi, N.; Racioppi, M.; et al. Advanced Age Impacts Survival After Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma. Clin. Genitourin. Cancer 2023, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vikram, R.; Sandler, C.M.; Ng, C.S. Imaging and Staging of Transitional Cell Carcinoma: Part 2, Upper Urinary Tract. Am. J. Roentgenol. 2009, 192, 1488–1493. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Alessandrino, F.; Ghaith, O.; Williams, K.; Sonpavde, G.P.; Silverman, S.G.; Shinagare, A.B. Advanced urothelial cancer: A radiology update. Abdom. Radiol. 2019, 44, 3858–3873. [Google Scholar] [CrossRef]
- Imokawa, S.; Uehara, M.; Uto, T.; Sagisaka, S.; Sato, J.; Yasuda, K.; Matsushita, K.; Oi, S.; Tanioka, F.; Suda, T.; et al. Pulmonary metastasis from urothelial carcinoma showing progressive multiple cystic lesions. Am. J. Respir. Crit. Care Med. 2013, 188, 1267–1268. [Google Scholar] [CrossRef] [PubMed]
- Galluzzo, A.; Danti, G.; Bicci, E.; Mastrorosato, M.; Bertelli, E.; Miele, V. The role of Dual-Energy CT in the study of urinary tract tumours: Review of recent literature. Semin. Ultrasound CT MRI 2023, 44, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Bicci, E.; Mastrorosato, M.; Danti, G.; Lattavo, L.; Bertelli, E.; Cozzi, D.; Pradella, S.; Agostini, S.; Miele, V. Dual-Energy CT applications in urinary tract cancers: An update. Tumori J. 2022, 109, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.A.; Sadigh, G.; Kelly, A.M.; Cronin, P. Comparative effectiveness of imaging modalities for the diagnosis of upper and lower urinary tract malignancy: A critically appraised topic. Acad. Radiol. 2012, 19, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Gomez, J.; Udare, A.; Shanbhogue, K.P.; Schieda, N. Update on MR urography (MRU): Technique and clinical applications. Abdom. Radiol. 2019, 44, 3800–3810. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, O.J.; McLaughlin, P.; Maher, M.M. MR Urography. Am. J. Roentgenol. 2010, 195, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Iancu, A.S.; Colin, P.; Puech, P.; Villers, A.; Ouzzane, A.; Fantoni, J.C.; Leroy, X.; Lemaitre, L. Significance of ADC value for detection and characterization of urothelial carcinoma of upper urinary tract using diffusion-weighted MRI. World J. Urol. 2012, 31, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Lonati, C.; Simeone, C.; Suardi, N.; Spiess, P.E.; Necchi, A.; Moschini, M. Genitourinary manifestations of Lynch syndrome in the urological practice. Asian J. Urol. 2022, 9, 443–450. [Google Scholar] [CrossRef]
- Seguel, A.P.; Pérez, M.M.; González, T.R.; Gómez, D.; del Río, J.V.; Benítez, G.M. Evaluation of upper tract urothelial carcinomas by contrast-enhanced ultrasound. Radiol. (Eng. Ed.) 2018, 60, 496–503. [Google Scholar] [CrossRef]
- Xue, L.-Y.; Lu, Q.; Huang, B.-J.; Li, C.-L.; Yan, C.-J.; Wen, J.-X.; Wang, W.-P. Evaluation of renal urothelial carcinoma by contrast-enhanced ultrasonography. Eur. J. Radiol. 2013, 82, e151–e157. [Google Scholar] [CrossRef]
- Drudi, F.M.; Di Candio, G.; Di Leo, N.; Malpassini, F.; Gnecchi, M.; Cantisani, V.; Iori, F.; Liberatore, M. Contrast-enhanced ultrasonography in the diagnosis of upper urinary tract urothelial cell carcinoma: A preliminary study. Ultraschall Med.—Eur. J. Ultrasound 2012, 34, 30–37. [Google Scholar] [CrossRef]
- Tanaka, H.; Yoshida, S.; Komai, Y.; Sakai, Y.; Urakami, S.; Yuasa, T.; Yamamoto, S.; Masuda, H.; Koizumi, M.; Kohno, A.; et al. Clinical value of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in upper tract urothelial carcinoma: Impact on detection of metastases and patient management. Urol. Int. 2015, 96, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Voskuilen, C.S.; Schweitzer, D.; Jensen, J.B.; Nielsen, A.M.; Joniau, S.; Muilwijk, T.; Necchi, A.; Azizi, M.; Spiess, P.E.; Briganti, A.; et al. Diagnostic value of 18F-fluorodeoxyglucose positron emission tomography with computed tomography for lymph node staging in patients with upper tract urothelial carcinoma. Eur. Urol. Oncol. 2020, 3, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Zattoni, F.; Incerti, E.; Moro, F.D.; Moschini, M.; Castellucci, P.; Panareo, S.; Picchio, M.; Fallanca, F.; Briganti, A.; Gallina, A.; et al. 18F-FDG PET/CT and urothelial carcinoma: Impact on management and prognosis—A multicenter retrospective study. Cancers 2019, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Sassa, N.; Kato, K.; Abe, S.; Iwano, S.; Ito, S.; Ikeda, M.; Shimamoto, K.; Yamamoto, S.; Yamamoto, T.; Gotoh, M.; et al. Evaluation of 11C-choline PET/CT for primary diagnosis and staging of urothelial carcinoma of the upper urinary tract: A pilot study. Eur. J. Nucl. Med. 2014, 41, 2232–2241. [Google Scholar] [CrossRef] [PubMed]
- Gofrit, O.N.; Mishani, E.; Orevi, M.; Klein, M.; Freedman, N.; Pode, D.; Shapiro, A.; Katz, R.; Libson, E.; Chisin, R. Contribution of 11C-Choline Positron Emission Tomography/Computerized Tomography to Preoperative Staging of Advanced Transitional Cell Carcinoma. J. Urol. 2006, 176, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.L.; Tripathi, S.K.; Gomella, L.G.; Salmanoglu, E.; Kim, S.; Kelly, W.K.; Keith, S.W.; Intenzo, C.; McCue, P.; Hoffman-Censits, J.; et al. Imaging urothelial bladder cancer: A VPAC PET targeted approach. Can. J. Urol. 2021, 28, 10596–10602. [Google Scholar] [PubMed]
- Orevi, M.; Klein, M.; Mishani, E.; Chisin, R.; Freedman, N.; Gofrit, O.N. 11C-Acetate PET/CT in Bladder Urothelial Carcinoma: Intraindividual Comparison with: 11: C-Choline. Clin. Nucl. Med. 2012, 37, e67–e72. [Google Scholar] [CrossRef]
- He, Y.; Gao, W.; Ying, W.; Feng, N.; Wang, Y.; Jiang, P.; Gong, Y.; Li, X. A Novel Preoperative Prediction Model Based on Deep Learning to Predict Neoplasm T Staging and Grading in Patients with Upper Tract Urothelial Carcinoma. J. Clin. Med. 2022, 11, 5815. [Google Scholar] [CrossRef]
- Angeloni, M.; van Doeveren, T.; Lindner, S.; Volland, P.; Schmelmer, J.; Foersch, S.; Matek, C.; Stoehr, R.; Geppert, C.I.; Heers, H.; et al. A deep-learning workflow to predict upper tract urothelial cancer subtypes supporting the prioritization of patients for molecular testing. medRxiv 2023. [Google Scholar] [CrossRef]
- Liu, J.; Wu, P.; Lai, S.; Wang, J.; Hou, H.; Zhang, Y. Prognostic models for upper urinary tract urothelial carcinoma patients after radical nephroureterectomy based on a novel systemic immune-inflammation score with machine learning. BMC Cancer 2023, 23, 574. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Dong, W.; Xiong, S.; Jin, D.; Zhou, H.; Zhang, L.; Xie, L.; Deng, Y.; Xu, R.; Fan, B. Machine learning models combining computed tomography semantic features and selected clinical variables for accurate prediction of the pathological grade of bladder cancer. Front. Oncol. 2023, 13, 1166245. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Min, K.; Ikram, W.; Tatton, R.W.; Riaz, I.B.; Silva, A.C.; Bryce, A.H.; Moore, C.; Ho, T.H.; Sonpavde, G.; et al. Performing Automatic Identification and Staging of Urothelial Carcinoma in Bladder Cancer Patients Using a Hybrid Deep-Machine Learning Approach. Cancers 2023, 15, 1673. [Google Scholar] [CrossRef] [PubMed]
- Slywotzky, C.; Maya, M. Needle tract seeding of transitional cell carcinoma following fine-needle aspiration of a renal mass. Abdom. Imaging 1994, 19, 174–176. [Google Scholar] [CrossRef]
- Huang, S.Y.; Ahrar, K.; Gupta, S.; Wallace, M.J.; Ensor, J.E.; Krishnamurthy, S.; Matin, S.F. Safety and diagnostic accurary of percutaneous biopsy in upper tract urothelial carcinoma. BJUI Int. 2015, 115, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Messina, E.; Pisciotti, M.L.; Pecoraro, M.; Borrelli, A.; Del Giudice, F.; Panebianco, V. The use of MRI in urothelial carcinoma. Curr. Opin. Urol. 2022, 32, 536–544. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsikitas, L.A.; Hopstone, M.D.; Raman, A.; Duddalwar, V. Imaging in Upper Tract Urothelial Carcinoma: A Review. Cancers 2023, 15, 5040. https://doi.org/10.3390/cancers15205040
Tsikitas LA, Hopstone MD, Raman A, Duddalwar V. Imaging in Upper Tract Urothelial Carcinoma: A Review. Cancers. 2023; 15(20):5040. https://doi.org/10.3390/cancers15205040
Chicago/Turabian StyleTsikitas, Lucas A., Michelle Diamond Hopstone, Alex Raman, and Vinay Duddalwar. 2023. "Imaging in Upper Tract Urothelial Carcinoma: A Review" Cancers 15, no. 20: 5040. https://doi.org/10.3390/cancers15205040
APA StyleTsikitas, L. A., Hopstone, M. D., Raman, A., & Duddalwar, V. (2023). Imaging in Upper Tract Urothelial Carcinoma: A Review. Cancers, 15(20), 5040. https://doi.org/10.3390/cancers15205040





