Arterial Sleeve Lobectomy: Does Pulmonary Artery Reconstruction Type Impact Lung Function?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Preoperative Evaluation
2.2. Surgical Procedure
2.3. Follow Up
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics and Overall Survival
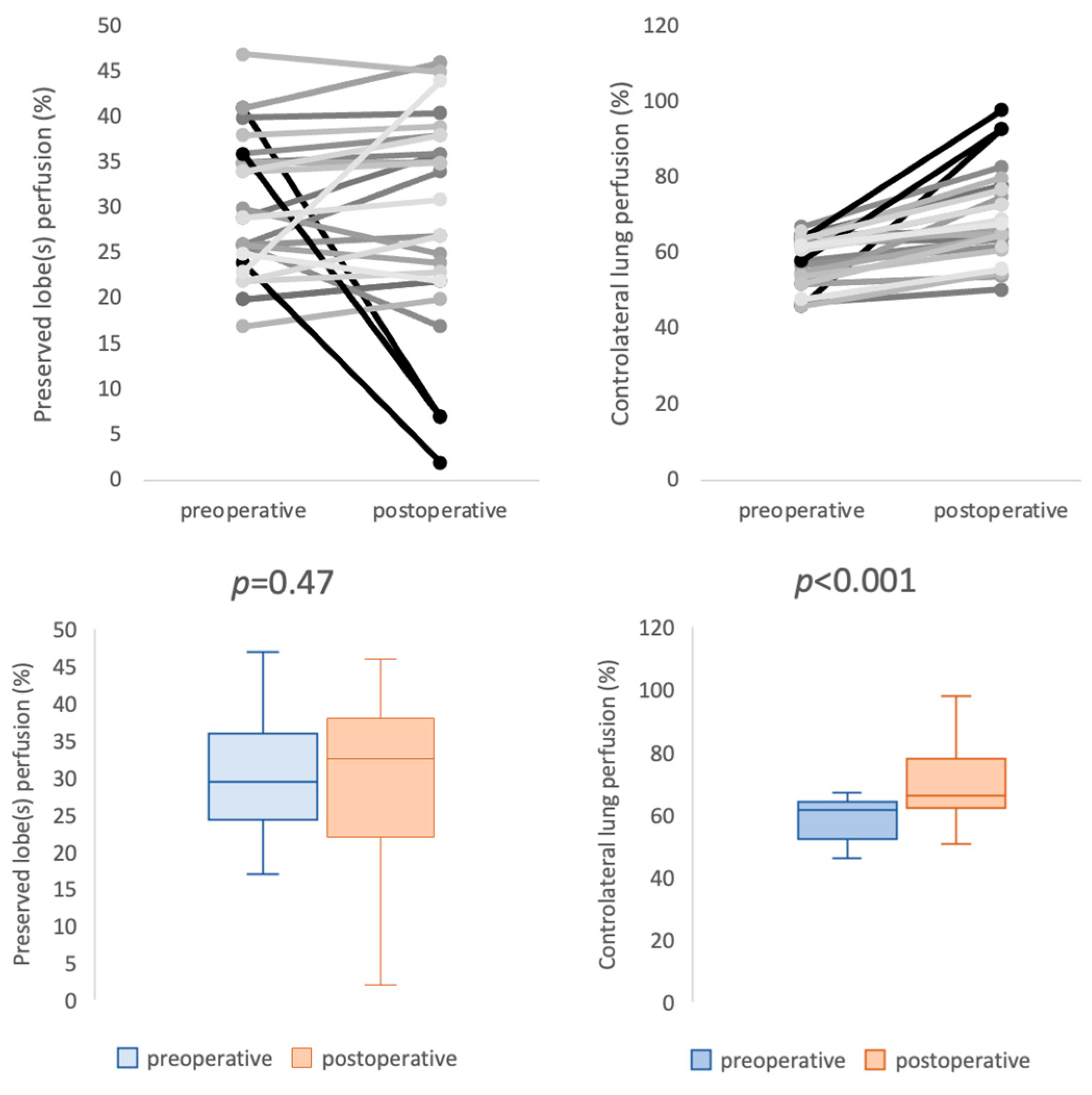
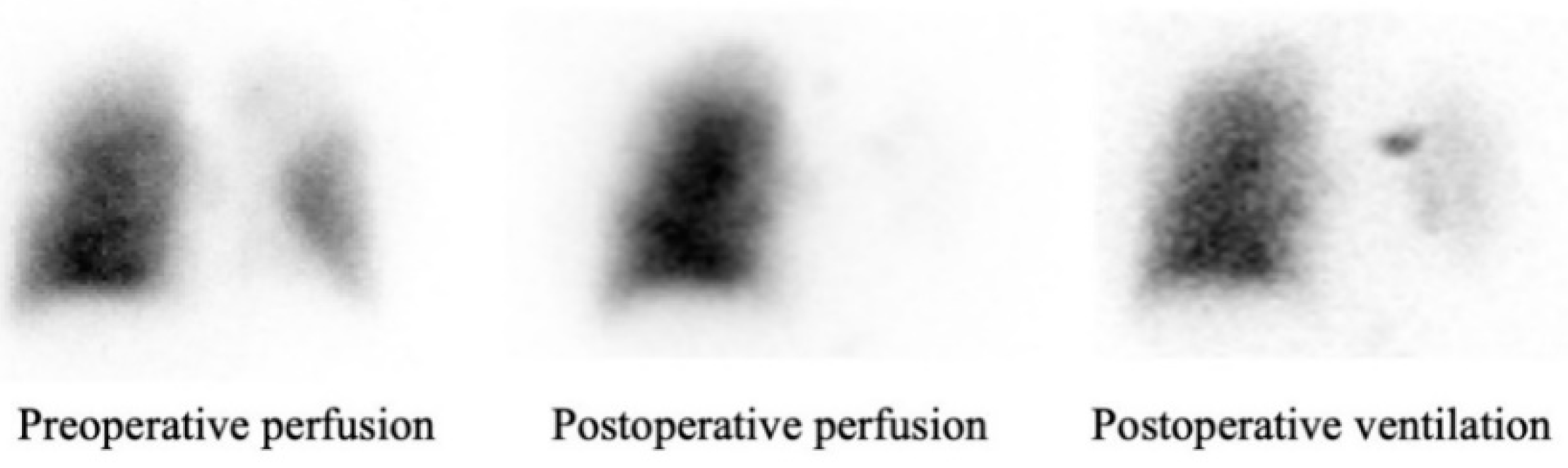
3.2. Patency and Perfusion Results
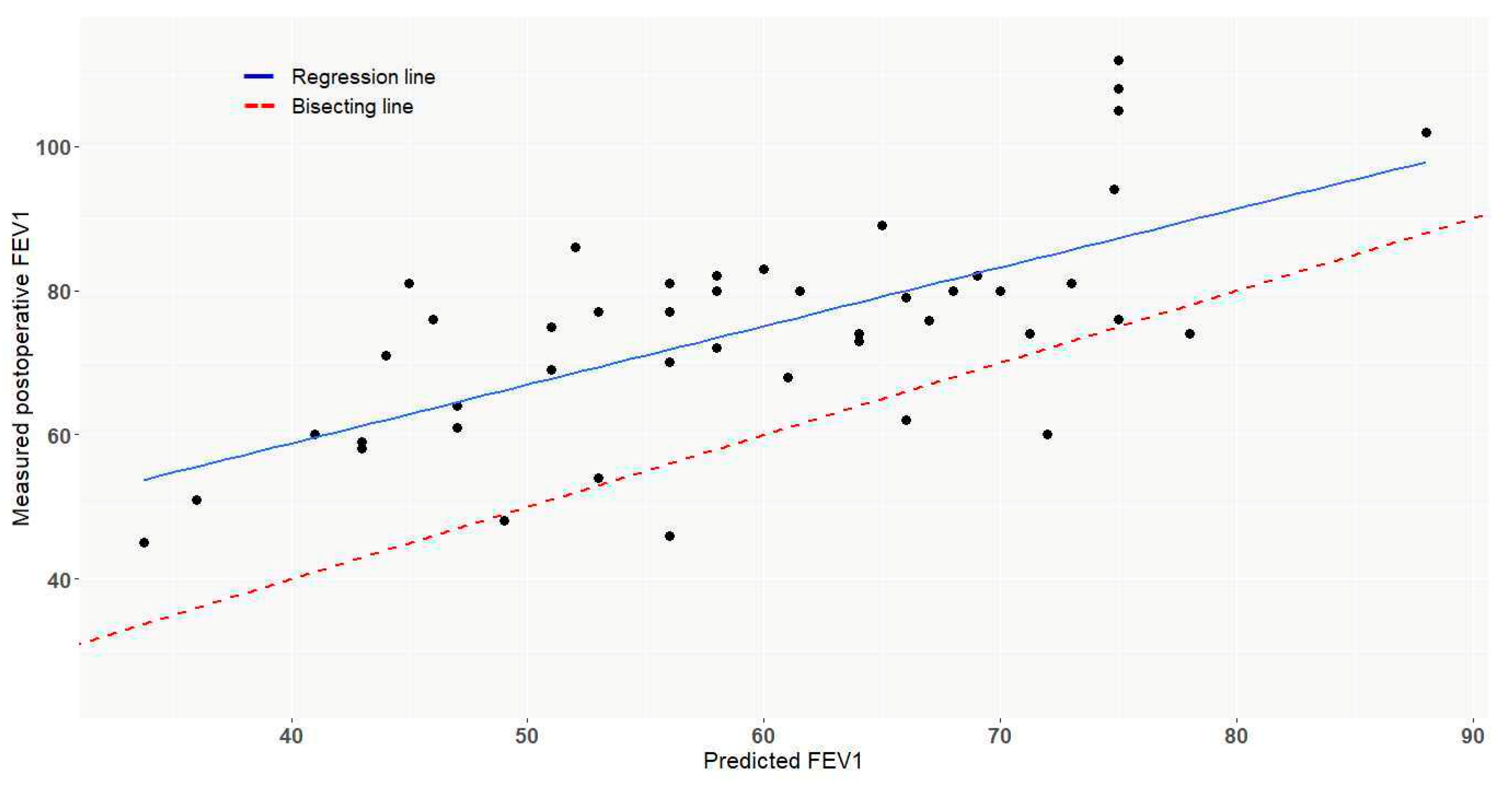
3.3. Functional Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PA | Pulmonary artery |
| FEV1 | First-second forced expiratory volume |
| MPO FEV1 | Measured postoperative FEV1 |
| PPO FEV1 | Predicted postoperative FEV1 |
| PFT | Pulmonary function test |
| PET/CTFDG | Fluorodeoxyglucose positron emission tomography |
| DLCO | Diffusing capacity of the lung for carbon monoxide |
References
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef]
- Brunelli, A.; Charloux, A.; Bolliger, C.T.; Rocco, G.; Sculier, J.-P.; Varela, G.; Licker, M.; Ferguson, M.K.; Faivre-Finn, C.; Huber, R.M.; et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur. Respir. J. 2009, 34, 17–41. [Google Scholar] [CrossRef]
- Chunwei, F.; Weiji, W.; Xinguan, Z.; Ni Qingzen, N.; Xiangmin, J.; Qingzhen, Z. Evaluations of bronchoplasty and pulmonary artery reconstruction for bronchogenic carcinoma. Eur. J. Cardio-Thoracic Surg. 2003, 23, 209–213. [Google Scholar] [CrossRef]
- Shrager, J.B.; Lambright, E.S.; McGrath, C.M.; Wahl, P.M.; Deeb, E.M.; Friedberg, J.S.; Kaiser, L.R. Lobectomy with tangential pulmonary artery resection without regard to pulmonary function. Ann. Thorac. Surg. 2000, 70, 234–239. [Google Scholar] [CrossRef]
- Ma, Z.; Dong, A.; Fan, J.; Cheng, H. Does sleeve lobectomy concomitant with or without pulmonary artery reconstruction (double sleeve) have favorable results for non-small cell lung cancer compared with pneumonectomy? A meta-analysis. Eur. J. Cardio-Thoracic Surg. 2007, 32, 20–28. [Google Scholar] [CrossRef]
- Ferguson, M.K.; Lehman, A.G. Sleeve lobectomy or pneumonectomy: Optimal management strategy using decision analysis techniques. Ann. Thorac. Surg. 2003, 76, 1782–1788. [Google Scholar] [CrossRef]
- Schiavon, M.; Comacchio, G.M.; Mammana, M.; Faccioli, E.; Stocca, F.; Gregori, D.; Lorenzoni, G.; Zuin, A.; Nicotra, S.; Pasello, G.; et al. Lobectomy With Artery Reconstruction and Pneumonectomy for Non-Small Cell Lung Cancer: A Propensity Score Weighting Study. Ann. Thorac. Surg. 2021, 112, 1805–1813. [Google Scholar] [CrossRef]
- Yang, M.; Zhong, Y.; Deng, J.; She, Y.; Zhang, L.; Wang, Y.; Zhao, M.; Hu, X.; Xie, D.; Chen, C. Comparison of Bronchial Sleeve Lobectomy With Pulmonary Arterioplasty Versus Pneumonectomy. Ann. Thorac. Surg. 2021, 113, 934–941. [Google Scholar] [CrossRef]
- Maurizi, G.; D’Andrilli, A.; Anile, M.; Ciccone, A.M.; Ibrahim, M.; Venuta, F.; Rendina, E.A. Sleeve Lobectomy Compared with Pneumonectomy after Induction Therapy for Non–Small-Cell Lung Cancer. J. Thorac. Oncol. 2013, 8, 637–643. [Google Scholar] [CrossRef]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S.; ESMO Guidelines Committee. Early and Locally Advanced Non-Small-Cell Lung Cancer (NSCLC): ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef]
- Brunelli, A.; Kim, A.W.; Berger, K.I.; Addrizzo-Harris, D.J. Physiologic Evaluation of the Patient With Lung Cancer Being Considered for Resectional Surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e166S–e190S. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Maurizi, G.; D’andrilli, A.; Venuta, F.; Rendina, E.A. Reconstruction of the bronchus and pulmonary artery. J. Thorac. Dis. 2016, 8, S168–S180. [Google Scholar] [CrossRef]
- Berthet, J.P.; Paradela, M.; Jimenez, M.J.; Molins, L.; Gómez-Caro, A. Extended Sleeve Lobectomy: One More Step Toward Avoiding Pneumonectomy in Centrally Located Lung Can-cer. Ann. Thorac. Surg. 2013, 96, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Berthet, J.-P.; Boada, M.; Paradela, M.; Molins, L.; Matecki, S.; Marty-Ané, C.-H.; Gómez-Caro, A. Pulmonary sleeve resection in locally advanced lung cancer using cryopreserved allograft for pulmonary artery replacement. J. Thorac. Cardiovasc. Surg. 2013, 146, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Alifano, M.; Cusumano, G.; Strano, S.; Magdeleinat, P.; Bobbio, A.; Giraud, F.; Lebeau, B.; Régnard, J.-F. Lobectomy with pulmonary artery resection: Morbidity, mortality, and long-term survival. J. Thorac. Cardiovasc. Surg. 2009, 137, 1400–1405. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cerfolio, R.J.; Bryant, A.S. Surgical Techniques and Results for Partial or Circumferential Sleeve Resection of the Pulmonary Artery for Patients with Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2007, 83, 1971–1977. [Google Scholar] [CrossRef]
- Venuta, F.; Ciccone, A.M. Reconstruction of the Pulmonary Artery. Semin. Thorac. Cardiovasc. Surg. 2006, 18, 104–108. [Google Scholar] [CrossRef]
- Vannucci, J.; Matricardi, A.; Potenza, R.; Ragusa, M.; Puma, F.; Cagini, L. Lobectomy with angioplasty: Which is the best technique for pulmonary artery reconstruction? J. Thorac. Dis. 2018, 10, S1892–S1898. [Google Scholar] [CrossRef]
- Galetta, D.; Borri, A.; Gasparri, R.; Petrella, F.; Spaggiari, L. Surgical Techniques and Long-Term Results of Pulmonary Artery Reconstruction in Patients With Lung Cancer. Ann. Thorac. Surg. 2015, 100, 1196–1202. [Google Scholar] [CrossRef]
- Rendina, A.E.; Venuta, F.; De Giacomo, T.; Ciccone, A.M.; Moretti, M.; Ruvolo, G.; Coloni, G.F. Sleeve resection and prosthetic reconstruction of the pulmonary artery for lung cancer. Ann. Thorac. Surg. 1999, 68, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Schirren, J.; Bölükbas, S.; Bergmann, T.; Fisseler-Eckhoff, A.; Trainer, S.; Beqiri, S. Prospective Study on Perioperative Risks and Functional Results in Bronchial and Bronchovascular Sleeve Resections. Thorac. Cardiovasc. Surg. 2009, 57, 35–41. [Google Scholar] [CrossRef]
- D’andrilli, A.; Ibrahim, M.; Venuta, F.; De Giacomo, T.; Coloni, G.F.; Rendina, E.A. Glutaraldehyde Preserved Autologous Pericardium for Patch Reconstruction of the Pulmonary Artery and Superior Vena Cava. Ann. Thorac. Surg. 2005, 80, 357–358. [Google Scholar] [CrossRef] [PubMed]
- D’andrilli, A.; Maurizi, G.; Ciccone, A.M.; Andreetti, C.; Ibrahim, M.; Menna, C.; Vanni, C.; Venuta, F.; Rendina, A.E. Long-segment pulmonary artery resection to avoid pneumonectomy: Long-term results after prosthetic replacement. Eur. J. Cardio-Thoracic Surg. 2018, 53, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Galetta, D.; Veronesi, G.; Leo, F.; Spaggiari, L. Pulmonary artery reconstruction by a custom-made heterologous pericardial conduit in the treatment of lung cancer. Lung Cancer 2006, 53, 241–243. [Google Scholar] [CrossRef]
- Shiraishi, T.; Hiratsuka, M.; Miyahara, S.; Waseda, R.; Sato, T.; Iwasaki, A. Pulmonary artery “conduit” reconstruction using bovine pericardium following long-segment sleeve resection: A unique “in situ tailor-made” sewing method. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 411–413. [Google Scholar] [CrossRef]
- Goldhaber, S.Z. Pulmonary embolism. Lancet 2004, 363, 1295–1305. [Google Scholar] [CrossRef]
- Danielsbacka, J.S.; Olsén, M.F.; Hansson, P.-O.; Mannerkorpi, K. Lung function, functional capacity, and respiratory symptoms at discharge from hospital in patients with acute pulmonary embolism: A cross-sectional study. Physiother. Theory Pr. 2018, 34, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, S.; Yoshimasu, T.; Hirai, T.; Hirai, I.; Maebeya, S.; Bessho, T.; Naito, Y. Exercise Capacity of Thoracotomy Patients in the Early Postoperative Period. Chest 2000, 118, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Nagamatsu, Y.; Maeshiro, K.; Kimura, N.Y.; Nishi, T.; Shima, I.; Yamana, H.; Shirouzu, K. Long-term recovery of exercise capacity and pulmonary function after lobectomy. J. Thorac. Cardiovasc. Surg. 2007, 134, 1273–1278. [Google Scholar] [CrossRef]
- Wang, J.-S.; Abboud, R.T.; Wang, L.-M. Effect of Lung Resection on Exercise Capacity and on Carbon Monoxide Diffusing Capacity During Exercise. Chest 2006, 129, 863–872. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brunelli, A.; Xiumé, F.; Refai, M.; Salati, M.; Marasco, R.; Sciarra, V.; Sabbatini, A. Evaluation of Expiratory Volume, Diffusion Capacity, and Exercise Tolerance Following Major Lung Resection. Chest 2007, 131, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Bolliger, C.; Jordan, P.; Soler, M.; Stulz, P.; Tamm, M.; Wyser, C.; Gonon, M.; Perruchoud, A. Pulmonary function and exercise capacity after lung resection. Eur. Respir. J. 1996, 9, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Deslauriers, J.; Ugalde, P.; Miro, S.; Ferland, S.; Bergeron, S.; Lacasse, Y.; Provencher, S. Adjustments in cardiorespiratory function after pneumonectomy: Results of the pneumonectomy project. J. Thorac. Cardiovasc. Surg. 2011, 141, 7–15. [Google Scholar] [CrossRef]

| Characteristics | ||
|---|---|---|
| Gender (n/%) | ||
| Male | 33 | 69% |
| Female | 15 | 31% |
| Age (median/IQR) | 67 (55–71) | |
| Smoking status (n/%) | ||
| Never smoked | 4 | 8% |
| Past smoker | 38 | 79% |
| Current smoker | 6 | 13% |
| Pack-year > 30 | 29 | 60% |
| IMC (Mean, kg·m−1) | 24 | |
| Comorbidity | ||
| Cancer | 10 | 21% |
| Diabetes | 4 | 8% |
| COPD | 12 | 25% |
| Cardiac disease | 13 | 27% |
| Pulmonary function test | ||
| Preoperative FEV1 (L, %) | 2.2 (1.8–2.6) | 80% (69–89) |
| Measured postoperative FEV1 (L, %) | 1.9 (1.6–2.3) | 75% (63–81) |
| Predicted postoperative FEV1 (L, %) | 1.1 (0.95–2.6) | 58% (50–69) |
| Preoperative DLCO (mL/mmHg/Mi, %) | 14.5 (12.1–17.8) | 63% (53–79) |
| Postoperative DLCO (mL/mmHg/Mi, %) | 14.6 (11.9–17.4) | 52% (62–71) |
| Neoadjuvant therapy (n/%) | 16 | 33% |
| Adjuvant therapy (n/%) | 27 | 56% |
| Surgical resection (n/%) | ||
| Right upper lobectomy | 6 | 13% |
| Middle lobectomy | 1 | 2% |
| Upper bilobectomy | 2 | 4% |
| Left upper lobectomy | 34 | 71% |
| Left lower lobectomy | 5 | 10% |
| Histology (n/%) | ||
| Squamous cell carcinoma | 27 | 56% |
| Adenocarcinoma | 18 | 38% |
| Neuroendocrine carcinoma | 2 | 4% |
| Undifferentiated carcinoma | 1 | 2% |
| cT status (n/%) | ||
| T1 | 6 | 13% |
| T2 | 15 | 31% |
| T3 | 13 | 27% |
| T4 | 11 | 23% |
| Unknown | 3 | 6% |
| cN status (n/%) | ||
| N0 | 21 | 44% |
| N1 | 14 | 29% |
| N2 | 10 | 21% |
| Unknown | 3 | 6% |
| pT status (n/%) | ||
| T1 | 6 | 13% |
| T2 | 12 | 25% |
| T3 | 19 | 40% |
| T4 | 7 | 14% |
| Unknown | 4 | 8% |
| pN status (n/%) | ||
| N0 | 19 | 40% |
| N1 | 24 | 50% |
| N2 | 3 | 6% |
| Unknown | 2 | 4% |
| Double sleeve (n/%) | 17 | 35% |
| PA reconstruction technique (n/%) | ||
| Direct angioplasty | 9 | 19% |
| Patch angioplasty | 14 | 29% |
| Biologic | 13 | 27% |
| Prosthetic | 1 | 2% |
| End-to-end anastomosis | 7 | 15% |
| Bypass | 18 | 37% |
| Prosthetic | 6 | 12% |
| Cryopreserved arterial allograft | 11 | 23% |
| Custom-made xenopericardial conduit | 1 | 2% |
| Variable | Number of Patients (n = 45) | p-Value in Univariate Analysis | p-Value in Multivariate Analysis |
|---|---|---|---|
| Direct angioplasty | 9 | 0.43 | 0.09 |
| End-to-end anastomosis | 7 | 0.16 | 0.07 |
| Biological patch angioplasty | 13 | 0.28 | 0.12 |
| Prosthetic bypass | 6 | 0.06 | 0.03 |
| Arterial allograft bypass | 11 | 0.42 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, A.; Solovei, L.; Marty-Ané, C.; Bourdin, A.; Canaud, L.; Alric, P.; Hireche, K. Arterial Sleeve Lobectomy: Does Pulmonary Artery Reconstruction Type Impact Lung Function? Cancers 2023, 15, 4971. https://doi.org/10.3390/cancers15204971
Nguyen A, Solovei L, Marty-Ané C, Bourdin A, Canaud L, Alric P, Hireche K. Arterial Sleeve Lobectomy: Does Pulmonary Artery Reconstruction Type Impact Lung Function? Cancers. 2023; 15(20):4971. https://doi.org/10.3390/cancers15204971
Chicago/Turabian StyleNguyen, Aude, Laurence Solovei, Charles Marty-Ané, Arnaud Bourdin, Ludovic Canaud, Pierre Alric, and Kheira Hireche. 2023. "Arterial Sleeve Lobectomy: Does Pulmonary Artery Reconstruction Type Impact Lung Function?" Cancers 15, no. 20: 4971. https://doi.org/10.3390/cancers15204971
APA StyleNguyen, A., Solovei, L., Marty-Ané, C., Bourdin, A., Canaud, L., Alric, P., & Hireche, K. (2023). Arterial Sleeve Lobectomy: Does Pulmonary Artery Reconstruction Type Impact Lung Function? Cancers, 15(20), 4971. https://doi.org/10.3390/cancers15204971






