Disruption of CCL2 in Mesenchymal Stem Cells as an Anti-Tumor Approach against Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Conditioned Media Preparation
2.2. In Vitro Cell Proliferation Analysis
2.3. Western Blot Analysis
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Differentiation of MSCs and Staining Assays
2.6. Flow Cytometry Analysis
2.7. Generation of CCL2-KO Cell Lines and Conditioned Media Preparation
2.8. siRNA Knockdown of the il1rn & ccl2 Expression
2.9. Migration Assay
2.10. Syngeneic Prostate Cancer Mouse Model
2.11. In Vitro Cytotoxicity Assay
2.12. Statistical Analysis
3. Results
3.1. Inhibition of Tumor Growth by CCL2 Knockout (KO) in a Syngeneic Prostate Cancer Model
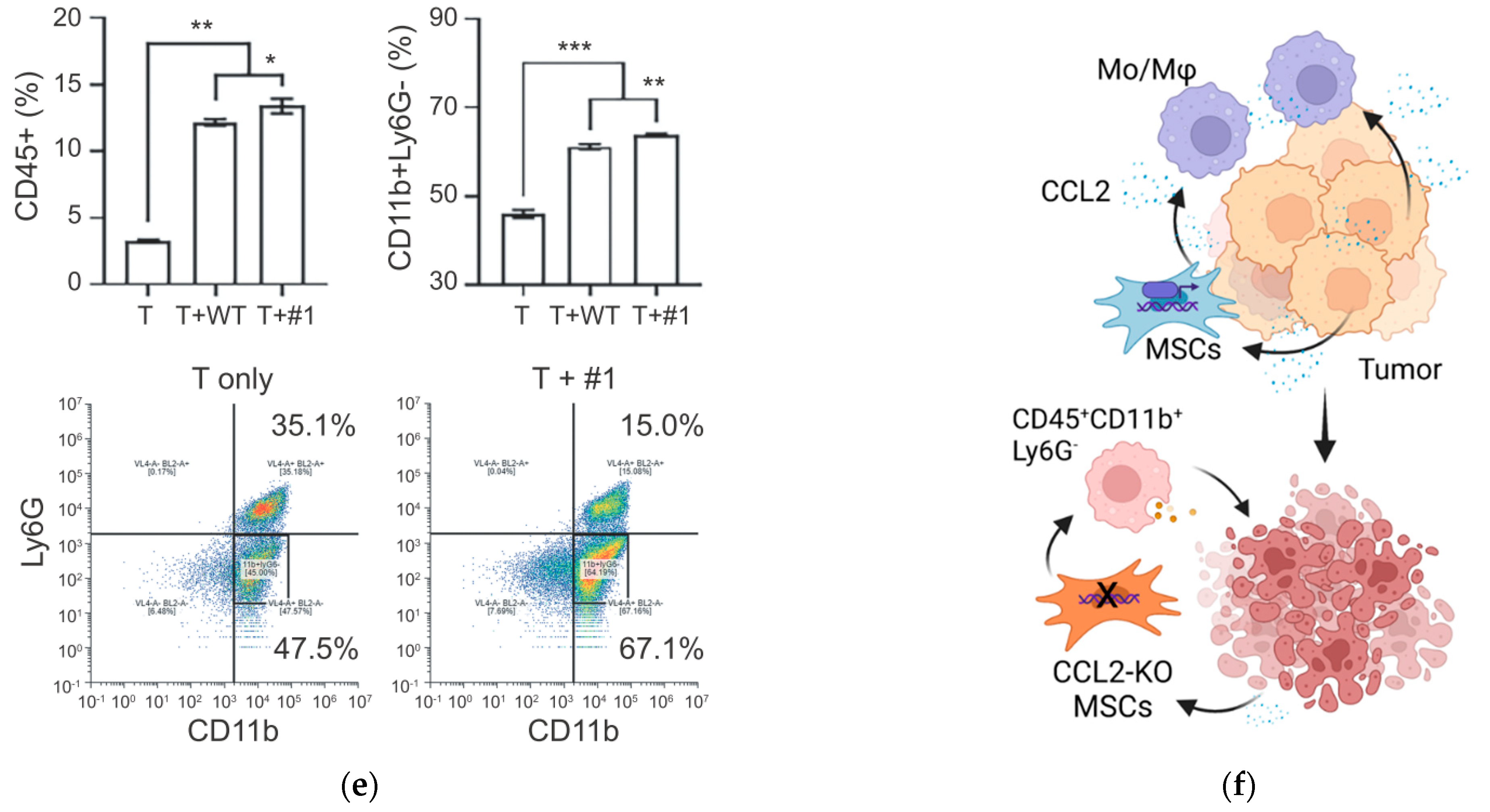
3.2. Tumor Cell-Derived CCL2 Is Crucial in Recruiting Mesenchymal Stem Cells (MSCs)
3.3. Inhibitory Effects of Condition Media Collected from the Bone Marrow-Enriched Mesenchymal Stem Cells (MSCs) with CCL2 Knockdown by Specific Small Interfering RNA (siRNA)
3.4. Establishment of CCL2 Knockout (KO) in Mesenchymal Stem Cells (MSCs)
3.5. Enhanced Anti-Tumor Effects of the CCL2 KO MSCs in a Syngeneic Prostate Cancer Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leuprolide Study Group. Leuprolide versus diethylstilbestrol for metastatic prostate cancer. N. Engl. J. Med. 1984, 311, 1281–1286. [Google Scholar] [CrossRef]
- Eisenberger, M.A.; Blumenstein, B.A.; Crawford, E.D.; Miller, G.; McLeod, D.G.; Loehrer, P.J.; Wilding, G.; Sears, K.; Culkin, D.J.; Thompson, I.M., Jr.; et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N. Engl. J. Med. 1998, 339, 1036–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linja, M.J.; Savinainen, K.J.; Saramaki, O.R.; Tammela, T.L.; Vessella, R.L.; Visakorpi, T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001, 61, 3550–3555. [Google Scholar] [PubMed]
- Chang, K.H.; Li, R.; Papari-Zareei, M.; Watumull, L.; Zhao, Y.D.; Auchus, R.J.; Sharifi, N. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 13728–13733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrader, A.J.; Boegemann, M.; Ohlmann, C.H.; Schnoeller, T.J.; Krabbe, L.M.; Hajili, T.; Jentzmik, F.; Stoeckle, M.; Schrader, M.; Herrmann, E.; et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur. Urol. 2014, 65, 30–36. [Google Scholar] [CrossRef]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef]
- Zhu, P.; Baek, S.H.; Bourk, E.M.; Ohgi, K.A.; Garcia-Bassets, I.; Sanjo, H.; Akira, S.; Kotol, P.F.; Glass, C.K.; Rosenfeld, M.G.; et al. Macrophage/cancer cell interactions mediate hormone resistance by a nuclear receptor derepression pathway. Cell 2006, 124, 615–629. [Google Scholar] [CrossRef] [Green Version]
- Erlandsson, A.; Carlsson, J.; Lundholm, M.; Falt, A.; Andersson, S.O.; Andren, O.; Davidsson, S. M2 macrophages and regulatory T cells in lethal prostate cancer. Prostate 2019, 79, 363–369. [Google Scholar] [CrossRef]
- Argyle, D.; Kitamura, T. Targeting Macrophage-Recruiting Chemokines as a Novel Therapeutic Strategy to Prevent the Progression of Solid Tumors. Front. Immunol. 2018, 9, 2629. [Google Scholar] [CrossRef] [Green Version]
- Izumi, K.; Fang, L.Y.; Mizokami, A.; Namiki, M.; Li, L.; Lin, W.J.; Chang, C. Targeting the androgen receptor with siRNA promotes prostate cancer metastasis through enhanced macrophage recruitment via CCL2/CCR2-induced STAT3 activation. EMBO Mol. Med. 2013, 5, 1383–1401. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Chen, W.Y.; Abou-Kheir, W.; Zeng, T.; Yin, J.J.; Bahmad, H.; Lee, Y.C.; Liu, Y.N. Androgen deprivation therapy-induced epithelial-mesenchymal transition of prostate cancer through downregulating SPDEF and activating CCL2. Biochim. Et Biophys. Acta 2018, 1864, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Yang, K.; Zhang, Q.; Yu, Y.; Meng, Q.; Mo, N.; Zhou, Y.; Yi, X.; Ma, C.; Lei, A.; et al. The role of mesenchymal stem cells in promoting the transformation of androgen-dependent human prostate cancer cells into androgen-independent manner. Sci. Rep. 2016, 6, 16993. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.; Kim, J.K.; Shiozawa, Y.; Wang, J.; Mishra, A.; Joseph, J.; Berry, J.E.; McGee, S.; Lee, E.; Sun, H.; et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat. Commun. 2013, 4, 1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whelan, D.S.; Caplice, N.M.; Clover, A.J.P. Mesenchymal stromal cell derived CCL2 is required for accelerated wound healing. Sci. Rep. 2020, 10, 2642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boomsma, R.A.; Geenen, D.L. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS ONE 2012, 7, e35685. [Google Scholar] [CrossRef] [Green Version]
- Giri, J.; Das, R.; Nylen, E.; Chinnadurai, R.; Galipeau, J. CCL2 and CXCL12 Derived from Mesenchymal Stromal Cells Cooperatively Polarize IL-10+ Tissue Macrophages to Mitigate Gut Injury. Cell Rep. 2020, 30, 1923–1934.e4. [Google Scholar] [CrossRef] [Green Version]
- Dwyer, R.M.; Potter-Beirne, S.M.; Harrington, K.A.; Lowery, A.J.; Hennessy, E.; Murphy, J.M.; Barry, F.P.; O’Brien, T.; Kerin, M.J. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 5020–5027. [Google Scholar] [CrossRef] [Green Version]
- Izumi, K.; Mizokami, A.; Lin, H.P.; Ho, H.M.; Iwamoto, H.; Maolake, A.; Natsagdorj, A.; Kitagawa, Y.; Kadono, Y.; Miyamoto, H.; et al. Serum chemokine (CC motif) ligand 2 level as a diagnostic, predictive, and prognostic biomarker for prostate cancer. Oncotarget 2016, 7, 8389–8398. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, H.; Izumi, K.; Nakagawa, R.; Toriumi, R.; Aoyama, S.; Shimada, T.; Kano, H.; Makino, T.; Kadomoto, S.; Yaegashi, H.; et al. Usefulness of serum CCL2 as prognostic biomarker in prostate cancer: A long-term follow-up study. Jpn. J. Clin. Oncol. 2022, 52, 1337–1344. [Google Scholar] [CrossRef]
- Iwamoto, H.; Izumi, K.; Nakagawa, R.; Toriumi, R.; Aoyama, S.; Kamijima, T.; Shimada, T.; Kano, H.; Makino, T.; Naito, R.; et al. Serum CCL2 Is a Prognostic Biomarker for Non-Metastatic Castration-Sensitive Prostate Cancer. Biomedicines 2022, 10, 2369. [Google Scholar] [CrossRef]
- Kirk, P.S.; Koreckij, T.; Nguyen, H.M.; Brown, L.G.; Snyder, L.A.; Vessella, R.L.; Corey, E. Inhibition of CCL2 signaling in combination with docetaxel treatment has profound inhibitory effects on prostate cancer growth in bone. Int. J. Mol. Sci. 2013, 14, 10483–10496. [Google Scholar] [CrossRef] [Green Version]
- Loberg, R.D.; Ying, C.; Craig, M.; Day, L.L.; Sargent, E.; Neeley, C.; Wojno, K.; Snyder, L.A.; Yan, L.; Pienta, K.J. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res. 2007, 67, 9417–9424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonapace, L.; Coissieux, M.M.; Wyckoff, J.; Mertz, K.D.; Varga, Z.; Junt, T.; Bentires-Alj, M. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature 2014, 515, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, B.M.; Alzahrani, A.M.; Abdel-Moneim, A.M.; Ditzel, N.; Kassem, M. A simple and reliable protocol for long-term culture of murine bone marrow stromal (mesenchymal) stem cells that retained their in vitro and in vivo stemness in long-term culture. Biol. Proced. Online 2019, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.C.; Chen, W.Y.; Lee, K.D.; Tsai, Y.C. Tumor-infiltrating Leukocytes Suppress Local Inflammation Via Interleukin-1 Receptor Antagonist in a Syngeneic Prostate Cancer Model. Biology 2020, 9, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzman, C.; Bagga, M.; Kaur, A.; Westermarck, J.; Abankwa, D. ColonyArea: An ImageJ plugin to automatically quantify colony formation in clonogenic assays. PLoS ONE 2014, 9, e92444. [Google Scholar] [CrossRef] [PubMed]
- Foster, B.A.; Gingrich, J.R.; Kwon, E.D.; Madias, C.; Greenberg, N.M. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997, 57, 3325–3330. [Google Scholar]
- Moore, R.J.; Owens, D.M.; Stamp, G.; Arnott, C.; Burke, F.; East, N.; Holdsworth, H.; Turner, L.; Rollins, B.; Pasparakis, M.; et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat. Med. 1999, 5, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.C.; Lee, K.D.; Tsai, Y.C. Roles of Interleukin-1 Receptor Antagonist in Prostate Cancer Progression. Biomedicines 2020, 8, 602. [Google Scholar] [CrossRef]
- Cheng, C.C.; Lian, W.S.; Hsiao, F.S.; Liu, I.H.; Lin, S.P.; Lee, Y.H.; Chang, C.C.; Xiao, G.Y.; Huang, H.Y.; Cheng, C.F.; et al. Isolation and characterization of novel murine epiphysis derived mesenchymal stem cells. PLoS ONE 2012, 7, e36085. [Google Scholar] [CrossRef]
- Ortiz, L.A.; Dutreil, M.; Fattman, C.; Pandey, A.C.; Torres, G.; Go, K.; Phinney, D.G. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc. Natl. Acad. Sci. USA 2007, 104, 11002–11007. [Google Scholar] [CrossRef] [PubMed]
- Luz-Crawford, P.; Djouad, F.; Toupet, K.; Bony, C.; Franquesa, M.; Hoogduijn, M.J.; Jorgensen, C.; Noel, D. Mesenchymal Stem Cell-Derived Interleukin 1 Receptor Antagonist Promotes Macrophage Polarization and Inhibits B Cell Differentiation. Stem. Cells 2016, 34, 483–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuire, J.J.; Frieling, J.S.; Lo, C.H.; Li, T.; Muhammad, A.; Lawrence, H.R.; Lawrence, N.J.; Cook, L.M.; Lynch, C.C. Mesenchymal stem cell-derived interleukin-28 drives the selection of apoptosis resistant bone metastatic prostate cancer. Nat. Commun. 2021, 12, 723. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Peault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Ahn, G.O.; Tseng, D.; Liao, C.H.; Dorie, M.J.; Czechowicz, A.; Brown, J.M. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc. Natl. Acad. Sci. USA 2010, 107, 8363–8368. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.C.; Khan, S.Q.; Kaneda, M.M.; Pathria, P.; Shepard, R.; Louis, T.L.; Anand, S.; Woo, G.; Leem, C.; Faridi, M.H.; et al. Integrin CD11b activation drives anti-tumor innate immunity. Nat. Commun. 2018, 9, 5379. [Google Scholar] [CrossRef] [Green Version]
- Jo, H.; Eom, Y.W.; Kim, H.S.; Park, H.J.; Kim, H.M.; Cho, M.Y. Regulatory Dendritic Cells Induced by Mesenchymal Stem Cells Ameliorate Dextran Sodium Sulfate-Induced Chronic Colitis in Mice. Gut Liver 2018, 12, 664–673. [Google Scholar] [CrossRef]
- Oh, S.H.; Choi, C.; Noh, J.E.; Lee, N.; Jeong, Y.W.; Jeon, I.; Shin, J.M.; Kim, J.H.; Kim, H.J.; Lee, J.M.; et al. Interleukin-1 receptor antagonist-mediated neuroprotection by umbilical cord-derived mesenchymal stromal cells following transplantation into a rodent stroke model. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Abdelaziz, M.H.; Abdelwahab, S.F.; Wan, J.; Cai, W.; Huixuan, W.; Jianjun, C.; Kumar, K.D.; Vasudevan, A.; Sadek, A.; Su, Z.; et al. Alternatively activated macrophages; a double-edged sword in allergic asthma. J. Transl. Med. 2020, 18, 58. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, Q.T.; Lee, K.-D.; Fan, Y.-C.; Lewis, B.S.; Deng, L.-W.; Tsai, Y.-C. Disruption of CCL2 in Mesenchymal Stem Cells as an Anti-Tumor Approach against Prostate Cancer. Cancers 2023, 15, 441. https://doi.org/10.3390/cancers15020441
Bui QT, Lee K-D, Fan Y-C, Lewis BS, Deng L-W, Tsai Y-C. Disruption of CCL2 in Mesenchymal Stem Cells as an Anti-Tumor Approach against Prostate Cancer. Cancers. 2023; 15(2):441. https://doi.org/10.3390/cancers15020441
Chicago/Turabian StyleBui, Quoc Thang, Kuan-Der Lee, Yu-Ching Fan, Branwen S. Lewis, Lih-Wen Deng, and Yuan-Chin Tsai. 2023. "Disruption of CCL2 in Mesenchymal Stem Cells as an Anti-Tumor Approach against Prostate Cancer" Cancers 15, no. 2: 441. https://doi.org/10.3390/cancers15020441
APA StyleBui, Q. T., Lee, K.-D., Fan, Y.-C., Lewis, B. S., Deng, L.-W., & Tsai, Y.-C. (2023). Disruption of CCL2 in Mesenchymal Stem Cells as an Anti-Tumor Approach against Prostate Cancer. Cancers, 15(2), 441. https://doi.org/10.3390/cancers15020441




