Sodium Butyrate Induces CRC Cell Ferroptosis via the CD44/SLC7A11 Pathway and Exhibits a Synergistic Therapeutic Effect with Erastin
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Cell Culture
2.3. MTT
2.4. Cell Morphology Observation and Photography
2.5. PI Staining and Fluorescence Mapping
2.6. Ferroptosis PCR Array Analysis and Real-Time Fluorescence Quantitative PCR
2.7. Population Public Database Analysis
2.8. Western Blotting
2.9. Immunofluorescence and Laser Confocal Imaging
2.10. Animal Tissue Immunohistochemistry and Prussian Staining
2.11. GSH Detection
2.12. Fe2+ Staining
2.13. Lipid ROS Detection
2.14. Isobolographic Analysis
2.15. Statistical Methods
3. Results
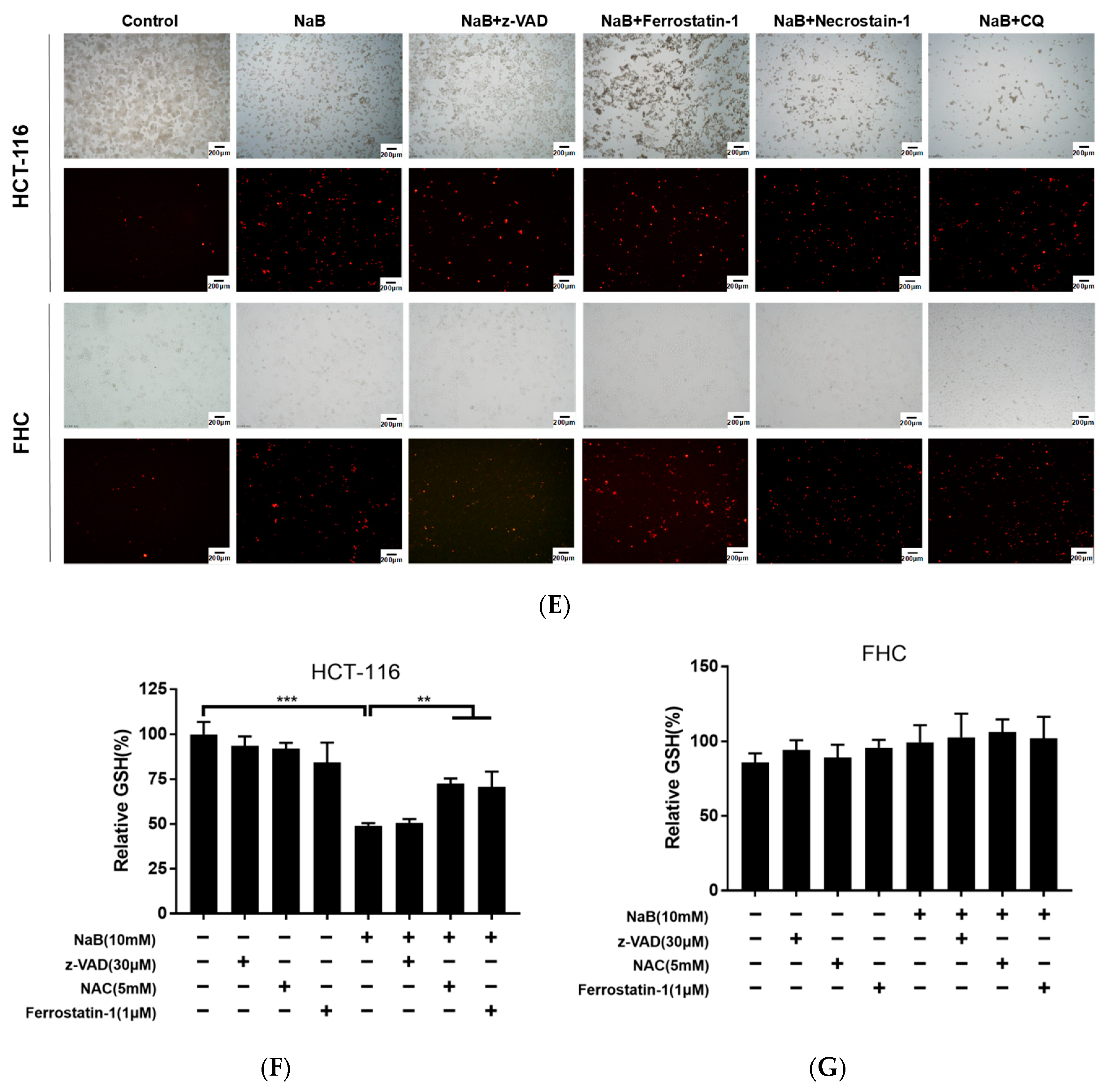
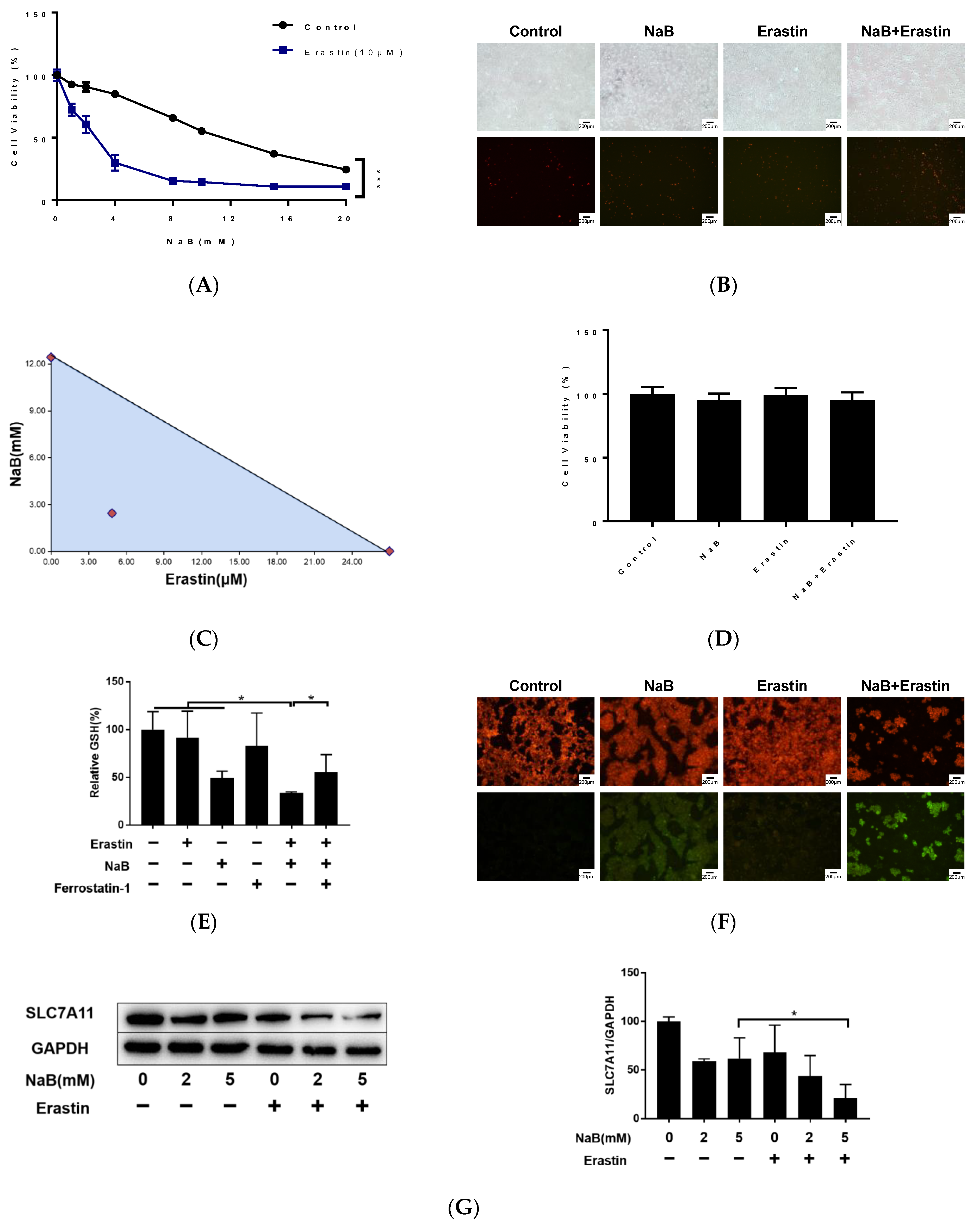
3.1. NaB Induces CRC Cell Death through Apoptosis and Ferroptosis
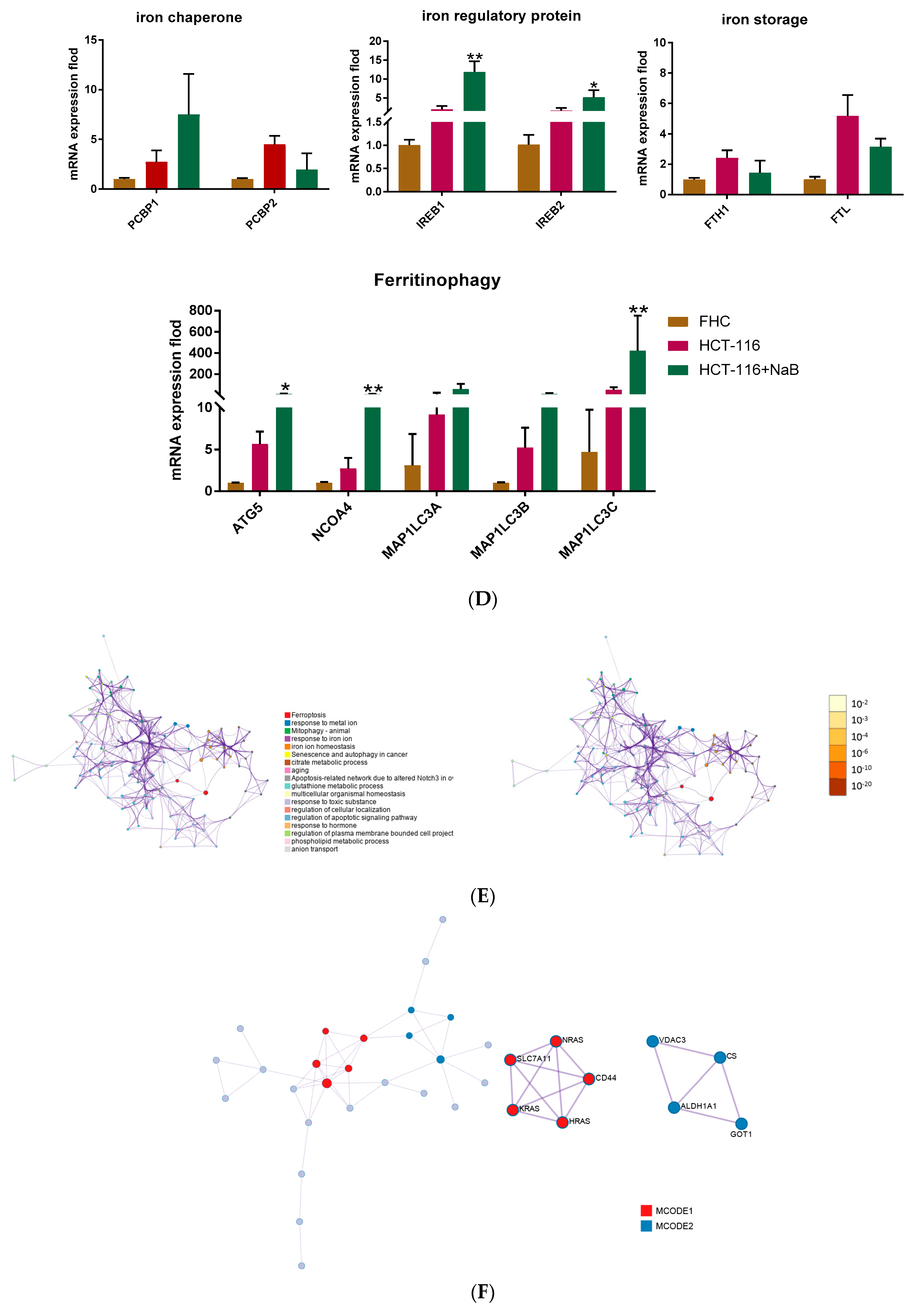
3.2. Ferroptosis-Related qPCR Array Analysis Identified CD44/SLC7A11 as a Potential Effector Molecular of NaB-Induced Ferroptosis
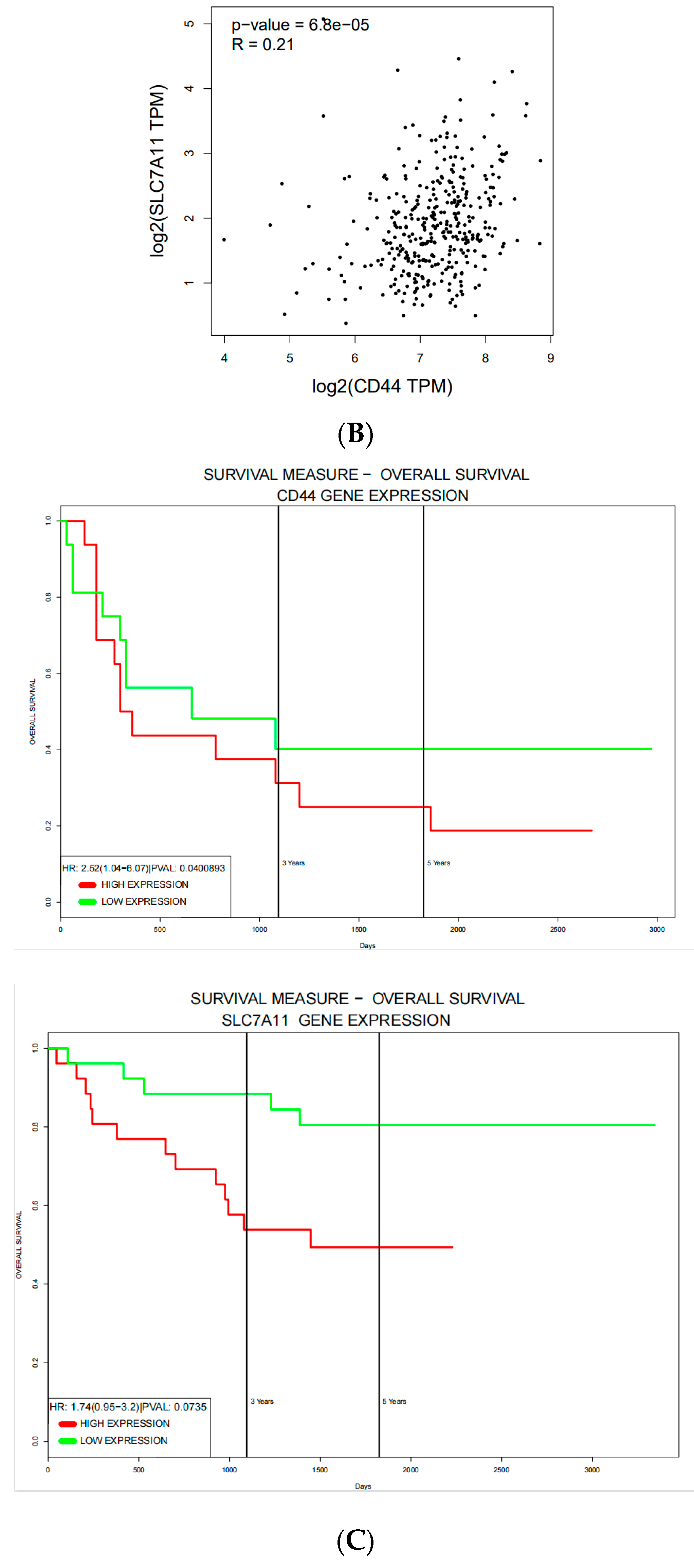
3.3. CD44 and SLC7A11 Are Highly Expressed and Positively Correlated in Human CRC
3.4. NaB Inhibits CD44/SLC7A11 Expression In Vivo and In Vitro
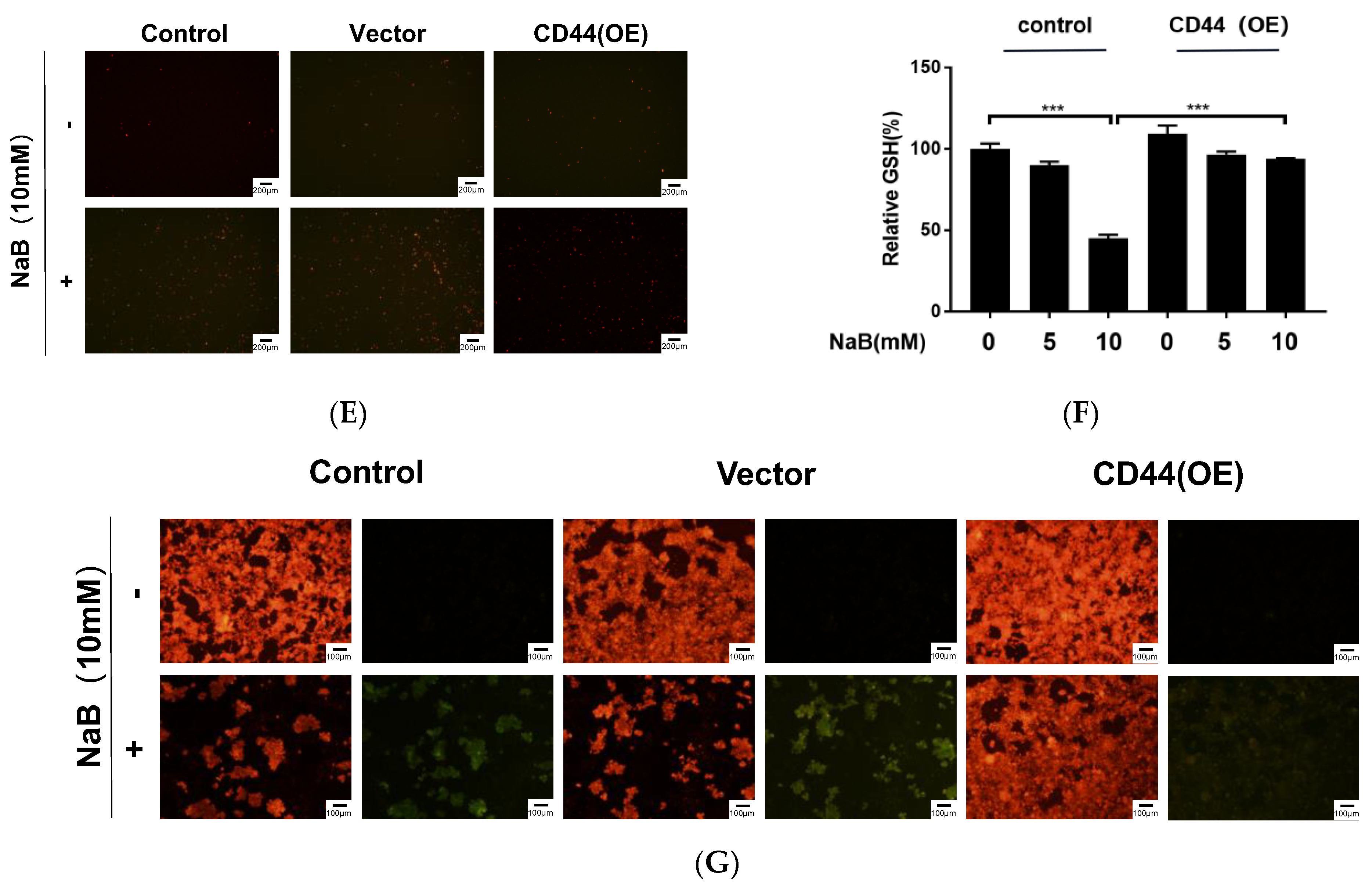
3.5. CD44 Overexpression Inhibits NaB-Induced Ferroptosis in CRC Cells
3.6. Erastin and NaB Synergistically Induce CRC Cell Ferroptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: A global perspective. Proc. Nutr. Soc. 2008, 67, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.A.; Day, N.E.; Luben, R.; Ferrari, P.; Slimani, N.; Norat, T.; Clavel-Chapelon, F.; Kesse, E.; Nieters, A.; Boeing, H.; et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): An observational study. Lancet 2003, 361, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, S.J.D.; Li, J.V.; Lahti, L.; Ou, J.; Carbonero, F.; Mohammed, K.; Posma, J.M.; Kinross, J.; Wahl, E.; Ruder, E.; et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 2015, 6, 6342. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Bultman, S.J. Molecular pathways: Gene-environment interactions regulating dietary fiber induction of proliferation and apoptosis via butyrate for cancer prevention. Clin. Cancer Res. 2014, 20, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Krysko, D.V.; Conrad, M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer 2019, 19, 405–414. [Google Scholar] [CrossRef]
- Hassannia, B.; Vandenabeele, P.; Vanden Berghe, T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann, A.J.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Lu, Y.; Chen, D.; Tian, T.; Mo, H.-Y.; Wei, X.-L.; Liao, J.-W.; Wang, F.; Zeng, Z.-L.; Pelicano, H.; et al. Redox Regulation of Stem-like Cells Though the CD44v-xCT Axis in Colorectal Cancer: Mechanisms and Therapeutic Implications. Theranostics 2016, 6, 1160–1175. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; Nagano, O.; Yae, T.; Tamada, M.; Motohara, T.; Oshima, H.; Oshima, M.; Ikeda, T.; Asaba, R.; Yagi, H.; et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011, 19, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bian, Z.; Zhang, Q.; Xiao, Z.; Cao, Y.; Sun, X.; Qin, Y.; Mao, L.; Chu, X.; Liao, W.; et al. Sodium butyrate inhibits colitis-associated colorectal cancer through preventing the gut microbiota dysbiosis and reducing the expression of NLRP3 and IL-1β. J. Funct. Foods 2021, 87, 104862. [Google Scholar] [CrossRef]
- Huang, R.; Pei, L.; Liu, Q.; Chen, S.; Dou, H.; Shu, G.; Yuan, Z.-X.; Lin, J.; Peng, G.; Zhang, W.; et al. Isobologram Analysis: A Comprehensive Review of Methodology and Current Research. Front. Pharmacol. 2019, 10, 1222. [Google Scholar] [CrossRef]
- Fan, F.; Liu, P.; Bao, R.; Chen, J.; Zhou, M.; Mo, Z.; Ma, Y.; Liu, H.; Zhou, Y.; Cai, X.; et al. A Dual PI3K/HDAC Inhibitor Induces Immunogenic Ferroptosis to Potentiate Cancer Immune Checkpoint Therapy. Cancer Res. 2021, 81, 6233–6245. [Google Scholar] [CrossRef]
- Oliveira, T.; Hermann, E.; Lin, D.; Chowanadisai, W.; Hull, E.; Montgomery, M. HDAC inhibition induces EMT and alterations in cellular iron homeostasis to augment ferroptosis sensitivity in SW13 cells. Redox Biol. 2021, 47, 102149. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, L.; Gao, Y.; Yao, F.; Marti, T.; Schmid, R.; Peng, R.-W. Pharmacotranscriptomic Analysis Reveals Novel Drugs and Gene Networks Regulating Ferroptosis in Cancer. Cancers 2020, 12, 3273. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Collins, L.B.; Wali, A.; Bigler, R.; Sun, W.; Bultman, S.J. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol. Cell 2012, 48, 612–626. [Google Scholar] [CrossRef]
- Chapkin, R.S.; Navarro, S.L.; Hullar, M.A.J.; Lampe, J.W. Diet and Gut Microbes Act Coordinately to Enhance Programmed Cell Death and Reduce Colorectal Cancer Risk. Dig. Dis. Sci. 2020, 65, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Schreiber, S.L.; Conrad, M. Persister cancer cells: Iron addiction and vulnerability to ferroptosis. Mol. Cell 2022, 82, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Salnikow, K. Role of iron in cancer. Semin. Cancer Biol. 2021, 76, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Samaniego, F.; Chin, J.; Iwai, K.; Rouault, T.; Klausner, R.D. Molecular characterization of a second iron-responsive element binding protein, iron regulatory protein 2. Structure, function, and post-translational regulation. J. Biol. Chem. 1994, 269, 30904–30910. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Xie, Y.; Song, X.; Sun, X.; Lotze, M.T.; Zeh, H.J., 3rd; Kang, R.; Tang, D. Autophagy promotes ferroptosis by degradation of ferritin. J. Biol. Chem. 1994, 269, 30904–30910. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Chen, M.; Wang, S.; Hsu, C.; Yin, P.-H.; Yeh, T.-S.; Lee, H.-C.; Tseng, L.-M. CHAC1 degradation of glutathione enhances cystine-starvation-induced necroptosis and ferroptosis in human triple negative breast cancer cells via the GCN2-eIF2α-ATF4 pathway. Oncotarget 2017, 8, 114588–114602. [Google Scholar] [CrossRef]
- Liu, T.; Jiang, L.; Tavana, O.; Gu, W. The Deubiquitylase OTUB1 Mediates Ferroptosis via Stabilization of SLC7A11. Cancer Res. 2019, 79, 1913–1924. [Google Scholar] [CrossRef]
- Chen, G.; Benthani, F.A.; Wu, J.; Liang, D.; Bian, Z.X.; Jiang, X. Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death Differ. 2020, 27, 242–254. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, B.; Zhong, C.; Xu, K.; Wang, Z.; Hofman, P.; Nagano, T.; Legras, A.; Breadner, D.; Ricciuti, B.; et al. Targeting histone deacetylase enhances the therapeutic effect of Erastin-induced ferroptosis in -activating mutant lung adenocarcinoma. Transl. Lung Cancer Res. 2021, 10, 1857–1872. [Google Scholar] [CrossRef]
- Mao, C.; Liu, X.; Zhang, Y.; Lei, G.; Yan, Y.; Lee, H.; Koppula, P.; Wu, S.; Zhuang, L.; Fang, B.; et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 2021, 593, 586–590. [Google Scholar] [CrossRef] [PubMed]








| HUMAM | 1 | 2 | 3 | 4 | 5 | 6 |
| A | IREB1 | ATG5 | CDO1 | DPP4 | GCLM | HARS |
| B | ACSL4 | ATP5G3 | CHAC1 | ELAVL1 | GLS2 | HEPH |
| C | AKR1B1 | BBC3 | CISD1 | EMC2 | GOT1 | HFE |
| D | AKR1B10 | BECN1 | CISD2 | EPRS | GPX4 | HMOX1 |
| E | AKR1C1 | BRAF | CP | FTH1 | GSS | HMOX2 |
| F | ALDH1A1 | BRD4 | CS | FTL | GSTA1 | HRAS |
| G | ALOX15 | CA9 | CYBB | FTMT | GSTP1 | HSF1 |
| H | ATF4 | CARS1 | DMT1 | GCLC | HAMP | HSPB1 |
| HUMAM | 7 | 8 | 9 | 10 | 11 | 12 |
| A | IREB2 | NCOA4 | PCBP1 | SAT2 | STEAP3 | VDAC2 |
| B | KEAP1 | NFE2L2 | PCBP2 | SLC1A5 | STIM1 | VDAC3 |
| C | KRAS | NOX1 | PPARG | SLC39A14 | TF | MAP1LC3C |
| D | LOX | NOX3 | PRDX6 | SLC39A8 | TFR1 | PANX2 |
| E | LPCAT3 | NOX4 | PRNP | SLC3A2 | TFR2 | SAT1 |
| F | MAP1LC3A | NQO1 | PTGES2 | SLC40A1 | TP53 | SQSTM1 |
| G | MAP1LC3B | NRAS | RPL8 | SLC7A11 | TXNRD1 | USP7 |
| H | ACTB | GAPDH | HPRT1 | 18s | CD44 | FSP1 |
| Forward Sequence | Reverse Sequence | |
|---|---|---|
| CD44 | CCAGGCAACTCCTAGTAGTACAACG | CGAATGGGAGTCTTCTTTGGGT |
| SLC7A11 | TTGGAGCCCTGTCCTATGC | CGAGCAGTTCCACCCAGAC |
| GAPDH | GCTCAGACACCATGGGGAAG | TGTAGTTGAGGTCAATGAAGGGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, Z.; Sun, X.; Liu, L.; Qin, Y.; Zhang, Q.; Liu, H.; Mao, L.; Sun, S. Sodium Butyrate Induces CRC Cell Ferroptosis via the CD44/SLC7A11 Pathway and Exhibits a Synergistic Therapeutic Effect with Erastin. Cancers 2023, 15, 423. https://doi.org/10.3390/cancers15020423
Bian Z, Sun X, Liu L, Qin Y, Zhang Q, Liu H, Mao L, Sun S. Sodium Butyrate Induces CRC Cell Ferroptosis via the CD44/SLC7A11 Pathway and Exhibits a Synergistic Therapeutic Effect with Erastin. Cancers. 2023; 15(2):423. https://doi.org/10.3390/cancers15020423
Chicago/Turabian StyleBian, Zhongbo, Xiaodie Sun, Lulin Liu, Yong Qin, Qiuyu Zhang, Huahuan Liu, Lianzhi Mao, and Suxia Sun. 2023. "Sodium Butyrate Induces CRC Cell Ferroptosis via the CD44/SLC7A11 Pathway and Exhibits a Synergistic Therapeutic Effect with Erastin" Cancers 15, no. 2: 423. https://doi.org/10.3390/cancers15020423
APA StyleBian, Z., Sun, X., Liu, L., Qin, Y., Zhang, Q., Liu, H., Mao, L., & Sun, S. (2023). Sodium Butyrate Induces CRC Cell Ferroptosis via the CD44/SLC7A11 Pathway and Exhibits a Synergistic Therapeutic Effect with Erastin. Cancers, 15(2), 423. https://doi.org/10.3390/cancers15020423





