Systematic Review of the Survival Outcomes of Neoadjuvant Chemotherapy in Women with Malignant Ovarian Germ Cell Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Approach to a Systematic Literature Review
2.3. Eligibility Criteria, Information Sources, and Search Strategy
2.4. Study Selection
2.5. Data Extraction
2.6. Analysis of Outcome Measures and Assessment of Bias Risk
2.7. Sensitivity Analysis
2.8. Meta-Analysis Plan
2.9. Statistical Analysis
3. Results
3.1. Results of the Systematic Review
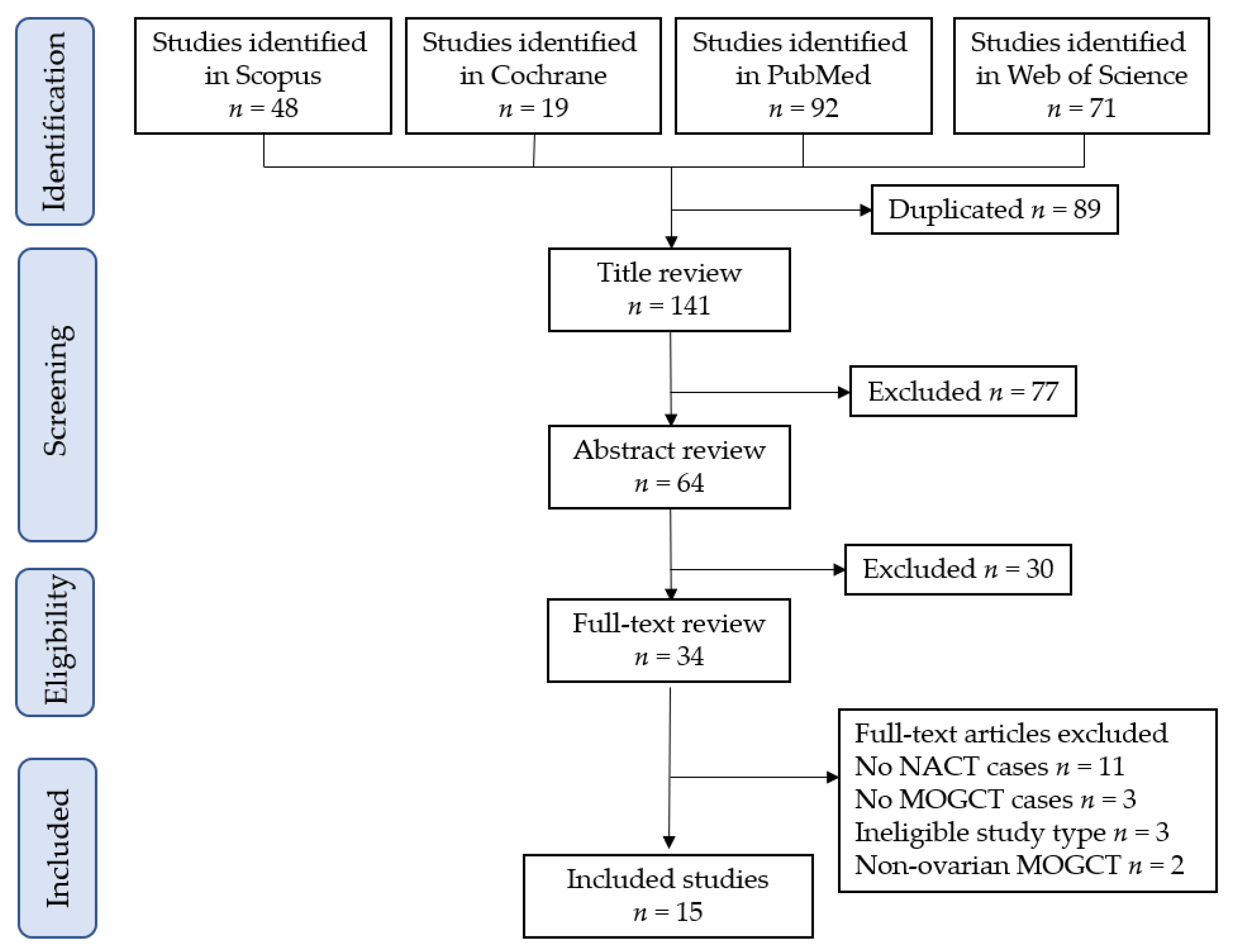
3.1.1. Study Selection
3.1.2. Study Characteristics
3.1.3. Risk of Bias in Included Studies
3.2. Results of the Meta-Analysis
3.2.1. Overview of Included Studies
3.2.2. NACT Rate, Regimen, and Cycles
3.2.3. Comparator Studies
Primary Outcomes: Response Rate
Co-Primary and Secondary Outcomes: Survival Outcomes
Additional Outcomes
3.2.4. Non-Comparator Studies
Primary Outcomes: Response Rate
Co-Primary and Secondary Outcomes: Survival Outcomes
Additional Outcomes: Rates of Hysterectomy, Resumed Menstruation, and Pregnancy
4. Discussion
4.1. Principal Findings
4.2. Strengths and Limitations
4.3. Comparison with Existing Literature
4.3.1. Primary Outcomes: Response Rate
4.3.2. Co-Primary and Secondary Outcomes: OS and DFS
4.3.3. Additional Outcomes
5. Conclusions
5.1. Implications for Practice
5.2. Implications for Clinical Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.; Frazier, A.L.; Shaikh, F. Germ Cell Tumors in Adolescents and Young Adults. J. Oncol. Pract. 2019, 15, 433–441. [Google Scholar] [CrossRef]
- Rogers, D.; Menias, C.; Shaaban, A. Malignant Germ Cell Tumors of the Ovary: Clinical and Imaging Features. Radiol. Clin. N. Am. 2023, 61, 579–594. [Google Scholar] [CrossRef]
- Calzas Rodríguez, J.; Carmen Juarez Morales, M.D.; Casero, M.A. Death by bleomycin pulmonary toxicity in ovarian dysgerminoma with pathologic complete response to chemotherapy. A case report. Respir. Med. Case Rep. 2016, 18, 48–50. [Google Scholar] [CrossRef][Green Version]
- Amirthalingam, V.; Sharma, T.D.; Rai, P.C.; Singh, T.T.; Devi, K.P. Malignant mixed germ cell tumor of ovary presenting as advanced disease in an adolescent girl. Eur. J. Gynaecol. Oncol. 2016, 37, 750–752. [Google Scholar]
- Puangthong, U.; Pongpirul, K. Chemotherapy-induced acute psychosis in a patient with malignant germ cell tumour. BMJ Case Rep. 2015, 2015, bcr2014208982. [Google Scholar] [CrossRef] [PubMed]
- Ovarian Cancer. NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network. Available online: https://www.nccn.org/professionals/physician_gls/ (accessed on 15 July 2023).
- Lu, Y.; Yang, J.; Cao, D.; Huang, H.; Wu, M.; You, Y.; Chen, J.; Lang, J.; Shen, K. Role of neoadjuvant chemotherapy in the management of advanced ovarian yolk sac tumor. Gynecol. Oncol. 2014, 134, 78–83. [Google Scholar] [CrossRef]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.; van der Burg, M.E.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef]
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.C.; Kitchener, H.; Lopes, T.; Luesley, D.; Perren, T.; Bannoo, S.; Mascarenhas, M.; et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet 2015, 386, 249–257. [Google Scholar] [CrossRef]
- Onda, T.; Satoh, T.; Ogawa, G.; Saito, T.; Kasamatsu, T.; Nakanishi, T.; Mizutani, T.; Takehara, K.; Okamoto, A.; Ushijima, K.; et al. Comparison of survival between primary debulking surgery and neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers in phase III randomised trial. Eur. J. Cancer 2020, 130, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Ferrandina, M.G.; Vizzielli, G.; Pasciuto, T.; Fanfani, F.; Gallotta, V.; Margariti, P.A.; Chiantera, V.; Costantini, B.; Gueli Alletti, S.; et al. Randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer (SCORPION-NCT01461850). Int. J. Gynecol. Cancer 2020, 30, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, M.; Matsuzaki, S.; Serada, S.; Matsuo, K.; Mizuta-Odani, C.; Jitsumori, M.; Nakae, R.; Matsuzaki, S.; Nakagawa, S.; Hiramatsu, K.; et al. CD70 antibody-drug conjugate: A potential novel therapeutic agent for ovarian cancer. Cancer Sci. 2021, 112, 3655–3668. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.D.; McNeish, I.A.; Cook, A.D.; James, E.C.; Lord, R.; Dark, G.; Glasspool, R.M.; Krell, J.; Parkinson, C.; Poole, C.J.; et al. Objective responses to first-line neoadjuvant carboplatin-paclitaxel regimens for ovarian, fallopian tube, or primary peritoneal carcinoma (ICON8): Post-hoc exploratory analysis of a randomised, phase 3 trial. Lancet Oncol. 2021, 22, 277–288. [Google Scholar] [CrossRef]
- Shafa, A.; Watkins, A.B.; McGree, M.E.; Weroha, S.J.; Wahner Hendrickson, A.E.; Block, M.S.; Langstraat, C.L.; McBane, R.D., 2nd; Bakkum-Gamez, J.N.; Kumar, A. Incidence of venous thromboembolism in patients with advanced stage ovarian cancer undergoing neoadjuvant chemotherapy: Is it time for thromboprophylaxis? Gynecol. Oncol. 2023, 176, 36–42. [Google Scholar] [CrossRef]
- Perrone, A.M.; Coada, C.A.; Ravegnini, G.; De Leo, A.; Damiano, G.; De Crescenzo, E.; Tesei, M.; Di Costanzo, S.; Genovesi, L.; Rubino, D.; et al. Post-operative residual disease and number of cycles of neoadjuvant chemotherapy in advanced epithelial ovarian carcinoma. Int. J. Gynecol. Cancer 2023, 33. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Ueda, Y.; Matsuzaki, S.; Sakaguchi, H.; Kakuda, M.; Lee, M.; Takemoto, Y.; Hayashida, H.; Maeda, M.; Kakubari, R.; et al. Relationship between Abnormal Placenta and Obstetric Outcomes: A Meta-Analysis. Biomedicines 2023, 11, 1522. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Nagase, Y.; Ueda, Y.; Lee, M.; Matsuzaki, S.; Maeda, M.; Takiuchi, T.; Kakigano, A.; Mimura, K.; Endo, M.; et al. The association of endometriosis with placenta previa and postpartum hemorrhage: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2021, 3, 100417. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Nagase, Y.; Takiuchi, T.; Kakigano, A.; Mimura, K.; Lee, M.; Matsuzaki, S.; Ueda, Y.; Tomimatsu, T.; Endo, M.; et al. Antenatal diagnosis of placenta accreta spectrum after in vitro fertilization-embryo transfer: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 9205. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Lee, M.; Nagase, Y.; Jitsumori, M.; Matsuzaki, S.; Maeda, M.; Takiuchi, T.; Kakigano, A.; Mimura, K.; Ueda, Y.; et al. A systematic review and meta-analysis of obstetric and maternal outcomes after prior uterine artery embolization. Sci. Rep. 2021, 11, 16914. [Google Scholar] [CrossRef] [PubMed]
- Paez, A. Gray literature: An important resource in systematic reviews. J. Evid. Based Med. 2017, 10, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Danna, S.M.; Graham, E.; Burns, R.J.; Deschenes, S.S.; Schmitz, N. Association between Depressive Symptoms and Cognitive Function in Persons with Diabetes Mellitus: A Systematic Review. PLoS ONE 2016, 11, e0160809. [Google Scholar] [CrossRef]
- ROBINS-I Detailed Guidance. 2016. Available online: https://www.riskofbias.info/welcome/home/current-version-of-robins-i/robins-i-detailed-guidance-2016 (accessed on 20 June 2023).
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane. 2022. Available online: www.training.cochrane.org/handbook (accessed on 20 June 2023).
- Agrawal, A.; Podder, A.R. Neoadjuvant Chemotherapy Versus Maximal Cytoreduction for Malignant Germ Cell Tumors of the Ovary. Indian J. Gynecol. Oncol. 2022, 21, 10. [Google Scholar] [CrossRef]
- Newton, C.; Murali, K.; Ahmad, A.; Hockings, H.; Graham, R.; Liberale, V.; Sarker, S.J.; Ledermann, J.; Berney, D.M.; Shamash, J.; et al. A multicentre retrospective cohort study of ovarian germ cell tumours: Evidence for chemotherapy de-escalation and alignment of paediatric and adult practice. Eur. J. Cancer 2019, 113, 19–27. [Google Scholar] [CrossRef]
- Agarwal, R.; Rajanbabu, A.; Keechilattu, P.; Nair, I.R.; Vijaykumar, D.K.; Unnikrishnan, U.G. A retrospective analysis of the pattern of care and survival in patients with malignant ovarian germ cell tumors. South Asian J. Cancer 2019, 8, 35–40. [Google Scholar] [CrossRef]
- Divya, S.; Syamala, O.; Rani, G.U.; Thanka, J.; Sundaram, S. Malignant Ovarian Tumors in Adolescents: A Case Series. J. South Asian Fed. Obstet. Gynaecol. 2019, 11, 331–335. [Google Scholar]
- Lakshmanan, M.; Gupta, S.; Kumar, V.; Akhtar, N.; Chaturvedi, A.; Misra, S.; Jain, K.; Garg, S. Germ Cell Tumor Ovary: An Institutional Experience of Treatment and Survival Outcomes. Indian J. Surg. Oncol. 2018, 9, 215–219. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Zhang, R.; Wu, L.Y.; Li, B.; Li, S.M. Neoadjuvant Bleomycin, Etoposide, and Cisplatin (BEP) Chemotherapy in the Treatment of Extensively Advanced Yolk Sac Tumors: A Single Center Experience. Int. J. Gynecol. Cancer 2018, 28, 713–720. [Google Scholar] [CrossRef]
- Talukdar, S.; Kumar, S.; Bhatla, N.; Mathur, S.; Thulkar, S.; Kumar, L. Neo-adjuvant chemotherapy in the treatment of advanced malignant germ cell tumors of ovary. Gynecol. Oncol. 2014, 132, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, A.; Gupta, S.; Bagga, R.; Saha, S.C.; Gainder, S.; Dhaliwal, L.K.; Patel, F.; Dey, P.; Nijhawan, R. Advanced germ cell malignancies of the ovary: Should neo-adjuvant chemotherapy be the first line of treatment? J. Obstet. Gynaecol. 2010, 30, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Bafna, U.D.; Umadevi, K.; Kumaran, C.; Nagarathna, D.S.; Shashikala, P.; Tanseem, R. Germ cell tumors of the ovary: Is there a role for aggressive cytoreductive surgery for nondysgerminomatous tumors? Int. J. Gynecol. Cancer 2001, 11, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Baranzelli, M.C.; Bouffet, E.; Quintana, E.; Portas, M.; Thyss, A.; Patte, C. Non-seminomatous ovarian germ cell tumours in children. Eur. J. Cancer 2000, 36, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Ohno, Y.; Shibata, D.; Niwa, Y.; Suzuki, Y.; Kajiyama, H.; Kitagawa, T.; Wakahara, Y.; Kakihara, M.; Arii, Y. Primary chemotherapy in strongly suspected yolk sac tumor of the ovary. Int. J. Clin. Oncol. 1999, 4, 48–51. [Google Scholar] [CrossRef]
- Matsuo, K.; Matsuzaki, S.; Maeda, M.; Rau, A.R.; Yoshihara, K.; Tamura, R.; Shimada, M.; Machida, H.; Mikami, M.; Klar, M.; et al. Uptake and Outcomes of Neoadjuvant Chemotherapy Among US Patients With Less Common Epithelial Ovarian Carcinomas. JAMA Netw. Open 2023, 6, e2318602. [Google Scholar] [CrossRef]
- Hinchcliff, E.; Rauh-Hain, J.A.; Clemmer, J.T.; Diver, E.; Hall, T.; Stall, J.; Growdon, W.; Clark, R.; Schorge, J. Racial disparities in survival in malignant germ cell tumors of the ovary. Gynecol. Oncol. 2016, 140, 463–469. [Google Scholar] [CrossRef]
- Nasioudis, D.; Chapman-Davis, E.; Frey, M.K.; Caputo, T.A.; Holcomb, K. Management and prognosis of ovarian yolk sac tumors; an analysis of the National Cancer Data Base. Gynecol. Oncol. 2017, 147, 296–301. [Google Scholar] [CrossRef]
- Faure Conter, C.; Xia, C.; Gershenson, D.; Hurteau, J.; Covens, A.; Pashankar, F.; Krailo, M.; Billmire, D.; Patte, C.; Fresneau, B.; et al. Ovarian Yolk Sac Tumors; Does Age Matter? Int. J. Gynecol. Cancer 2018, 28, 77–84. [Google Scholar] [CrossRef]
- Mitranovici, M.I.; Chiorean, D.M.; Mureșan, M.C.; Buicu, C.F.; Moraru, R.; Moraru, L.; Cotoi, T.C.; Cotoi, O.S.; Toru, H.S.; Apostol, A.; et al. Diagnosis and Management of Dysgerminomas with a Brief Summary of Primitive Germ Cell Tumors. Diagnostics 2022, 12, 3105. [Google Scholar] [CrossRef]
- Moraru, L.; Mitranovici, M.I.; Chiorean, D.M.; Coroș, M.; Moraru, R.; Oală, I.E.; Turdean, S.G. Immature Teratoma: Diagnosis and Management-A Review of the Literature. Diagnostics 2023, 13, 1516. [Google Scholar] [CrossRef] [PubMed]
- Nasioudis, D.; Mastroyannis, S.A.; Latif, N.A.; Ko, E.M. Trends in the surgical management of malignant ovarian germcell tumors. Gynecol. Oncol. 2020, 157, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Nasioudis, D.; Frey, M.K.; Chapman-Davis, E.; Caputo, T.A.; Holcomb, K. Fertility-preserving surgery for advanced stage ovarian germ cell tumors. Gynecol. Oncol. 2017, 147, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Sessa, C.; Schneider, D.T.; Planchamp, F.; Baust, K.; Braicu, E.I.; Concin, N.; Godzinski, J.; McCluggage, W.G.; Orbach, D.; Pautier, P.; et al. ESGO-SIOPE guidelines for the management of adolescents and young adults with non-epithelial ovarian cancers. Lancet. Oncol. 2020, 21, e360–e368. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Morice, P.; Lorusso, D.; Prat, J.; Oaknin, A.; Pautier, P.; Colombo, N. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv1–iv18. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Friedlander, M.; Backes, F.J.; Harter, P.; O’Connor, D.M.; de la Motte Rouge, T.; Lorusso, D.; Maenpaa, J.; Kim, J.W.; Tenney, M.E.; et al. Gynecologic Cancer Intergroup (GCIG) consensus review for ovarian germ cell tumors. Int. J. Gynecol. Cancer 2014, 24, S48–S54. [Google Scholar] [CrossRef]
- Management of Female Malignant Ovarian Germ Cell Tumours. Available online: https://www.rcog.org.uk/media/spph3igq/sip_52.pdf (accessed on 15 July 2023).
- Colombo, P.E.; Labaki, M.; Fabbro, M.; Bertrand, M.; Mourregot, A.; Gutowski, M.; Saint-Aubert, B.; Quenet, F.; Rouanet, P.; Mollevi, C. Impact of neoadjuvant chemotherapy cycles prior to interval surgery in patients with advanced epithelial ovarian cancer. Gynecol. Oncol. 2014, 135, 223–230. [Google Scholar] [CrossRef]
- Lauby, A.; Colomban, O.; Corbaux, P.; Peron, J.; Van Wagensveld, L.; Gertych, W.; Bakrin, N.; Descargues, P.; Lopez, J.; Kepenekian, V.; et al. The Increasing Prognostic and Predictive Roles of the Tumor Primary Chemosensitivity Assessed by CA-125 Elimination Rate Constant K (KELIM) in Ovarian Cancer: A Narrative Review. Cancers 2021, 14, 98. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- Quesada, S.; Thomas, Q.D.; Colombo, P.E.; Fiteni, F. Optimal First-Line Medico-Surgical Strategy in Ovarian Cancers: Are We There Yet? Cancers 2023, 15, 3556. [Google Scholar] [CrossRef]
- Nugawela, D.; Gorringe, K.L. Targeted therapy for mucinous ovarian carcinoma: Evidence from clinical trials. Int. J. Gynecol. Cancer 2023, 33, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Serada, S.; Morimoto, A.; Ueda, Y.; Yoshino, K.; Kimura, T.; Naka, T. Annexin A4 is a promising therapeutic target for the treatment of platinum-resistant cancers. Expert Opin. Ther. Targets 2014, 18, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Enomoto, T.; Serada, S.; Yoshino, K.; Nagamori, S.; Morimoto, A.; Yokoyama, T.; Kim, A.; Kimura, T.; Ueda, Y.; et al. Annexin A4-conferred platinum resistance is mediated by the copper transporter ATP7A. Int. J. Cancer 2014, 134, 1796–1809. [Google Scholar] [CrossRef]
- Grisham, R.N.; Manning-Geist, B.L.; Chui, M.H. The highs and lows of serous ovarian cancer. Cancer 2023, 129, 2613–2620. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Yoshino, K.; Ueda, Y.; Matsuzaki, S.; Kakuda, M.; Okazawa, A.; Egawa-Takata, T.; Kobayashi, E.; Kimura, T. Potential targets for ovarian clear cell carcinoma: A review of updates and future perspectives. Cancer Cell Int. 2015, 15, 117. [Google Scholar] [CrossRef]
- Cobb, L.; Gershenson, D. Novel therapeutics in low-grade serous ovarian cancer. Int. J. Gynecol. Cancer 2023, 33, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Borella, F.; Mitidieri, M.; Cosma, S.; Benedetto, C.; Bertero, L.; Fucina, S.; Ray-Coquard, I.; Carapezzi, A.; Ferraioli, D. Update on Prognostic and Predictive Markers in Mucinous Ovarian Cancer. Cancers 2023, 15, 1172. [Google Scholar] [CrossRef]
- Ghirardi, V.; Fagotti, A.; Ansaloni, L.; Valle, M.; Roviello, F.; Sorrentino, L.; Accarpio, F.; Baiocchi, G.; Piccini, L.; De Simone, M.; et al. Diagnostic and Therapeutic Pathway of Advanced Ovarian Cancer with Peritoneal Metastases. Cancers 2023, 15, 407. [Google Scholar] [CrossRef]
- You, B.; Robelin, P.; Tod, M.; Louvet, C.; Lotz, J.P.; Abadie-Lacourtoisie, S.; Fabbro, M.; Desauw, C.; Bonichon-Lamichhane, N.; Kurtz, J.E.; et al. CA-125 ELIMination Rate Constant K (KELIM) Is a Marker of Chemosensitivity in Patients with Ovarian Cancer: Results from the Phase II CHIVA Trial. Clin. Cancer Res. 2020, 26, 4625–4632. [Google Scholar] [CrossRef]
- Kessous, R.; Wissing, M.D.; Piedimonte, S.; Abitbol, J.; Kogan, L.; Laskov, I.; Yasmeen, A.; Salvador, S.; Lau, S.; Gotlieb, W.H. CA-125 reduction during neoadjuvant chemotherapy is associated with success of cytoreductive surgery and outcome of patients with advanced high-grade ovarian cancer. Acta Obstet. Gynecol. Scand. 2020, 99, 933–940. [Google Scholar] [CrossRef]
- Morimoto, A.; Nagao, S.; Kogiku, A.; Yamamoto, K.; Miwa, M.; Wakahashi, S.; Ichida, K.; Sudo, T.; Yamaguchi, S.; Fujiwara, K. A preoperative low cancer antigen 125 level (≤25.8 mg/dL) is a useful criterion to determine the optimal timing of interval debulking surgery following neoadjuvant chemotherapy in epithelial ovarian cancer. Jpn. J. Clin. Oncol. 2016, 46, 517–521. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Total | Age ‡ | II-IV | NACT | Rate (all) | Rate (adv) | Regimen † | Cycles |
|---|---|---|---|---|---|---|---|---|---|
| Agrawal A [28] | 2023 | 31 | 19 (11–48) | -- | 2 | 6.5% | -- | TC, BEP (1,1) | 4 |
| Newton C [29] | 2019 | 138 | 23.6 (8–76) | 49 (35.5%) | 16 | 11.6% | 32.7% | -- | -- |
| Agarwal R [30] | 2019 | 48 | 20.5 (9–45) | 17 (35.4%) | 11 | 22.9% | 64.7% | -- | 3–4 |
| Divya S [31] | 2019 | 10 | 20.5 (13–21) | -- | 2 | 25% | -- | BEC (2) | -- |
| Lakshmanan M [32] | 2018 | 39 | 22 (11–65) | 33 (84.6%) | 27 | 69.2% | 81.8% | BEP (27) | -- |
| Zhang GY [33] | 2018 | 58 | -- | -- | 18 | 31.0% | -- | BEP (18) | 1–3 |
| Lu Y [9] | 2014 | 53 | -- | 53 (100%) | 21 | 39.6% | 39.6% | Cis-based | 1–3 |
| Talukdar S [34] | 2014 | 66 | -- | 66 (100%) | 23 | 34.8% | 34.8% | BEP (23) | 4 |
| Bafna UD [36] | 2001 | 33 | 17 (4–32) | -- | 4 | 12.1% | -- | BEP (4) | 2–3 |
| Baranzelli MC [37] | 2000 | 49 | -- | 48 (98.0%) | 12 | 24.5% | 25.0% | Pt-based | -- |
| Response Rate (Stage III–IV) | NACT | PDS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Year | N | RR | CR | PR | N | RR | CR | PR | |
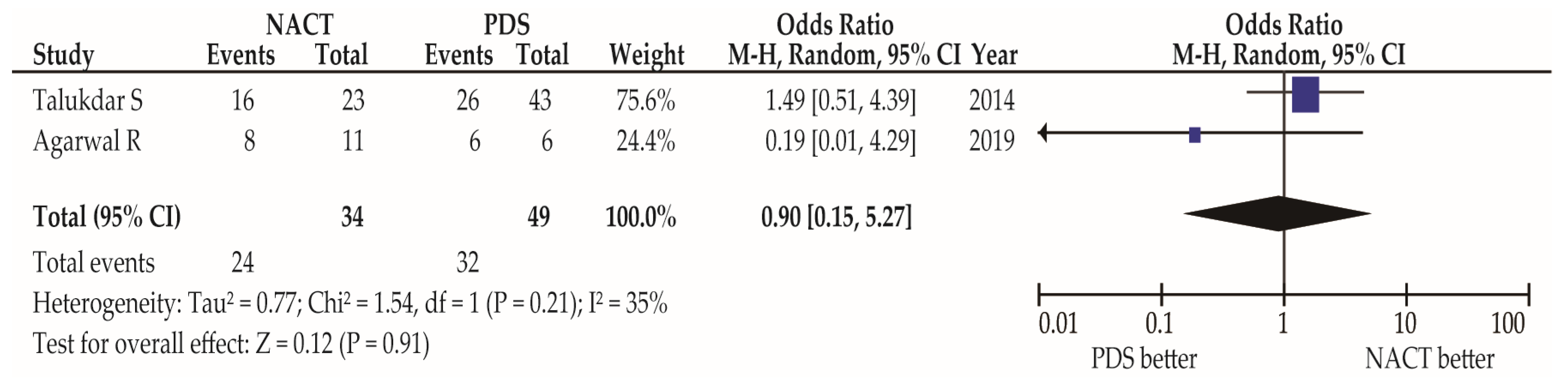
| Agarwal R [30] | 2019 | 11 | >72.7% | 8 | -- | 6 | 100% | 6 | -- |
| Talukdar S [34] | 2014 | 23 | 91.30% | 16 | 5 | 43 | 76.70% | 26 | 7 |
| Survival rate | Year | N | NACT | N | PDS | ||||
| Agarwal R [30] | 2019 | 11 | 5-yr OS: 100% | 6 | 5-yr OS: 100% | ||||
| 5-yr DFS: 100% | 5-yr DFS: 100% | ||||||||
| Lu Y [9] | 2014 | 21 | 5-yr OS: 95.3% $ | 32 | 5-yr OS: 96.9% $ | ||||
| 5-yr DFS: 95.3% $ | 5-yr DFS: 96.9% $ | ||||||||
| Talukdar S [34] | 2014 | 23 | 5-yr OS: 87.0% | 43 | 5-yr OS: 70.0% | ||||
| 5-yr DFS: 87.0% | 5-yr DFS: 70.0% | ||||||||
| Tumor size > 20 cm | Year | N | NACT | N | PDS | ||||
| Lu Y [9] | 2014 | 16 | 3-yr DFS: 80.0% $ | 4 | 3-yr DFS: 25.0% $ | ||||
| Recurrence rate | Year | N | NACT | N | PDS | ||||
| Lakshmanan M [32] | 2018 | 27 | 7 (25.9%) | 11 | 1 (9.1%) $ | ||||
| IDS | Year | N | NACT | N | PDS | ||||
| Lu Y [9] | 2014 | # similar surgical outcomes except for intraoperative blood loss. | |||||||
| Resumed menstruation | Year | N | NACT | N | PDS | ||||
| Agarwal R [30] | 2019 | 11 | 11 (100%) | 4 | 4 (100%) | ||||
| Talukdar S [34] | 2014 | 18 | 18 (100%) | 30 | 17 (56.6%) | ||||
| AE of chemo (G3/4) | Year | N | NACT | N | PDS | ||||
| Talukdar S [34] | 2014 | ||||||||
| Overall | ## similar adverse effects between the two groups. | ||||||||
| Leukopenia * | 23 | 6 (26.8%) | 43 | 11 (25.5%) | |||||
| Thrombocytopenia * | 23 | 1 (4.3%) | 43 | 6 (14.0%) | |||||
| Pulmonary toxicity * | 23 | 0 (0%) $ | 43 | 0 (0%) $ | |||||
| Author | Year | Total | NACT | RR | CR | PR | PD |
|---|---|---|---|---|---|---|---|
| Agarwal R [30] | 2019 | 48 | 11 | >72.7% | 8 | -- | 0 $ |
| Lakshmanan M [32] | 2018 | 39 | 27 | 100% | 6 | 21 | 0 |
| Zhang GY [33] | 2018 | 58 | 18 | 94.4% | 0 | 17 | 0 |
| Lu Y [9] | 2014 | 53 | 21 | >14.3% | 3 # | -- | 0 $ |
| Talukdar S [34] | 2014 | 66 | 23 | 91.3% | 16 | 5 | 1 |
| Bafna UD [36] | 2001 | 33 | 4 | 100% | 0 | 4 $ | 0 $ |
| Author | Year | NACT | Hist # | OS | DFS |
|---|---|---|---|---|---|
| Agarwal R [30] | 2019 | 11 | Dys, MG, IT, YST | 100% | 100% |
| Zhang GY [33] | 2018 | 18 | YST | 94.4% | 94.4% |
| Lu Y [9] | 2014 | 21 | YST | 95.3% $ | 95.3% $ |
| Talukdar S [34] | 2014 | 23 | Dys, YST, MG | 87.0% | 87.0% |
| Author | Year | Total | Stage | II-IV | NACT | Hyst | Mens | Preg |
|---|---|---|---|---|---|---|---|---|
| Agrawal A [28] | 2023 | 31 | I-IV | -- | 2 | 1/2 | 1/1 | -- |
| Agarwal R [30] | 2019 | 48 | I-IV | 17 | 11 | 0/11 | 11/11 | -- |
| Lakshmanan M [32] | 2018 | 39 | I-III | 33 | 27 | 22/27 | -- | -- |
| Zhang GY [33] | 2018 | 58 | III-IV | -- | 18 | 1/18 | -- | 6/6 |
| Talukdar S [34] | 2014 | 66 | III-IV | 66 | 23 | 3/21 | 18/18 | 10/10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakaguchi-Mukaida, H.; Matsuzaki, S.; Ueda, Y.; Matsuzaki, S.; Kakuda, M.; Lee, M.; Deguchi, S.; Sakata, M.; Maeda, M.; Kakubari, R.; et al. Systematic Review of the Survival Outcomes of Neoadjuvant Chemotherapy in Women with Malignant Ovarian Germ Cell Tumors. Cancers 2023, 15, 4470. https://doi.org/10.3390/cancers15184470
Sakaguchi-Mukaida H, Matsuzaki S, Ueda Y, Matsuzaki S, Kakuda M, Lee M, Deguchi S, Sakata M, Maeda M, Kakubari R, et al. Systematic Review of the Survival Outcomes of Neoadjuvant Chemotherapy in Women with Malignant Ovarian Germ Cell Tumors. Cancers. 2023; 15(18):4470. https://doi.org/10.3390/cancers15184470
Chicago/Turabian StyleSakaguchi-Mukaida, Hitomi, Shinya Matsuzaki, Yutaka Ueda, Satoko Matsuzaki, Mamoru Kakuda, Misooja Lee, Satoki Deguchi, Mina Sakata, Michihide Maeda, Reisa Kakubari, and et al. 2023. "Systematic Review of the Survival Outcomes of Neoadjuvant Chemotherapy in Women with Malignant Ovarian Germ Cell Tumors" Cancers 15, no. 18: 4470. https://doi.org/10.3390/cancers15184470
APA StyleSakaguchi-Mukaida, H., Matsuzaki, S., Ueda, Y., Matsuzaki, S., Kakuda, M., Lee, M., Deguchi, S., Sakata, M., Maeda, M., Kakubari, R., Hisa, T., Mabuchi, S., & Kamiura, S. (2023). Systematic Review of the Survival Outcomes of Neoadjuvant Chemotherapy in Women with Malignant Ovarian Germ Cell Tumors. Cancers, 15(18), 4470. https://doi.org/10.3390/cancers15184470