Association of Fibrinolytic Potential and Risk of Mortality in Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
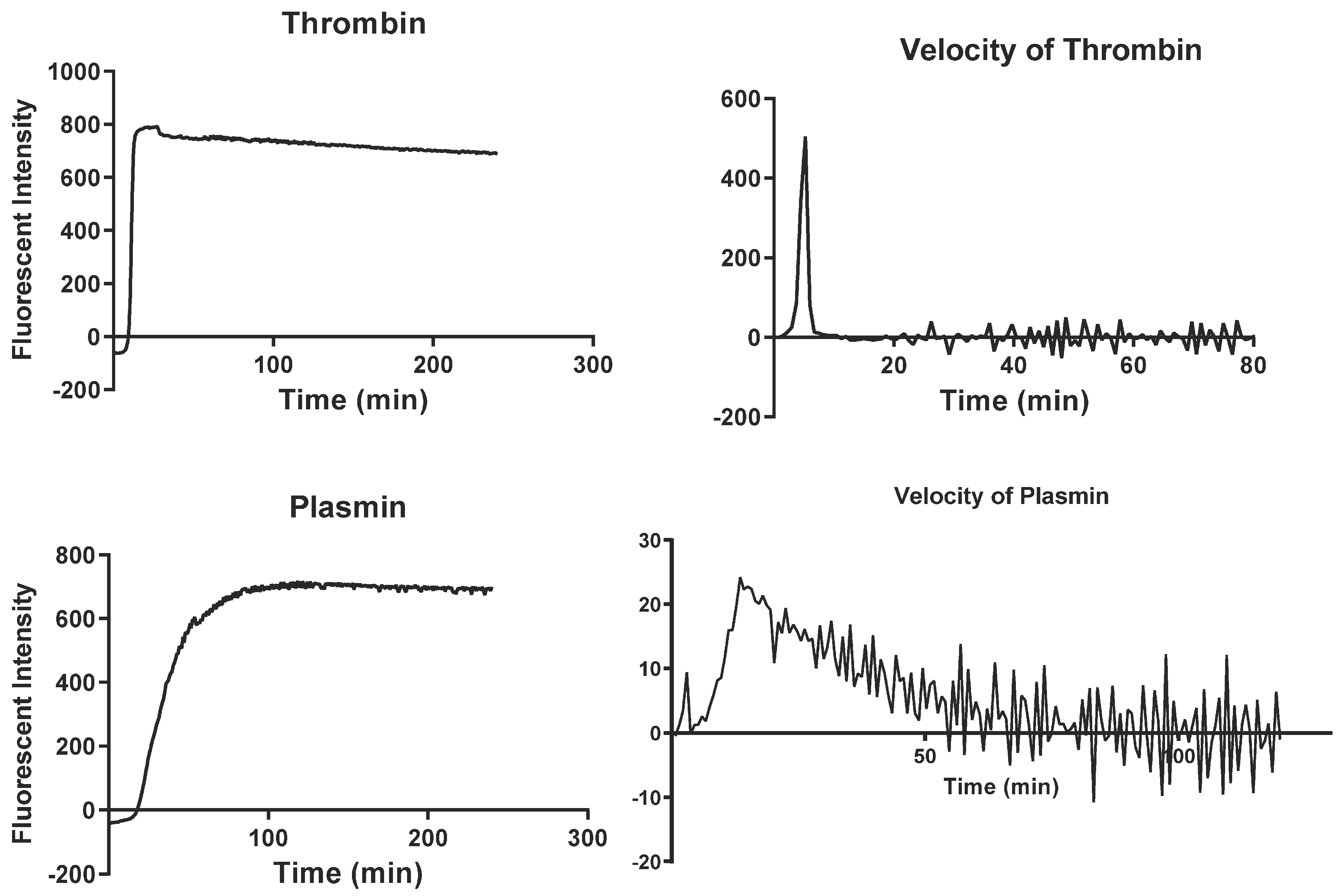
2.2. Simultaneous Thrombin and Plasmin Generation Assay
2.3. Statistical Analysis
3. Results
3.1. Cancer Group vs. Control Group
3.2. Non-Death Cancer Group vs. Death Cancer Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Cancer Site | Cancer (N) | Death (N) | Death (%) |
| Brain | 6 | 4 | 66.67 |
| Pancreas | 5 | 3 | 60.00 |
| Stomach | 23 | 12 | 52.17 |
| Skin | 6 | 3 | 50.00 |
| Testis | 2 | 1 | 50.00 |
| Esophagus | 13 | 6 | 46.15 |
| Lung | 21 | 8 | 38.10 |
| Liver | 9 | 3 | 33.33 |
| Myeloma | 15 | 5 | 33.33 |
| Intestine | 10 | 3 | 30.00 |
| Prostate | 8 | 2 | 25.00 |
| Lymphoma | 46 | 9 | 19.57 |
| Head and neck | 21 | 4 | 19.05 |
| Kidney | 7 | 1 | 14.29 |
| Uterus | 10 | 1 | 10.00 |
| Rectum | 21 | 2 | 9.52 |
| Colon | 16 | 1 | 6.25 |
Appendix B
| Variable | HR (95% IC) | p-Value |
| Age | 1.01 (0.99;1.03) | 0.208 |
| Male gender | 1.86 (1.07;3.23) | 0.027 |
| BMI (Kg/m2) | 0.93 (0.88;0.98) | 0.005 |
| Heart Disease | 2.29 (1.08;4.87) | 0.031 |
| Nephoropathy | 1.61 (0.64;4.10) | 0.315 |
| Smoking/ex-smoker | 2.19 (1.27;3.76) | 0.005 |
Appendix C
| HR (95% IC) | p-Value | |
| Tumor Site—TEV Group of Risk | ||
| High risk tumor | 2.81 (1.62;4.87) | <0.001 |
| Tumor site—Mortality group risk | ||
| High risk tumor | 2.05 (1.18;3.56) | 0.011 |
| Stage | ||
| Cancer stage I | ref | <0.001 |
| Cancer stage II | 0.22 (0.05;1.07) | 0.061 |
| Cancer stage III | 1.91 (0.77;4.77) | 0.166 |
| Cancer stage IV | 4.48 (1.90;10.57) | <0.001 |
| Chemotherapy protocol | ||
| Without chemeotherapy | ref | 0.006 |
| Chemeotherapy based on platin | 0.41 (0.23;0.73) | 0.002 |
| Chemeotherapy without platin | 0.48 (0.25;0.93) | 0.030 |
References
- Ministério da Saúde, INCA. Estimativa 2020: Incidência de Câncer no Brasil; INCA: Rio de Janeiro, Brazil, 2019; 120p.
- Posch, F.; Hofer, S.; Thaler, J.; Hell, L.; Königsbrügge, O.; Grilz, E.; Mauracher, L.M.; Gebhart, J.; Marosi, C.; Jilma, B.; et al. Ex vivo properties of plasma clot formation and lysis in patients with cancer at risk for venous thromboembolism, arterial thrombosis, and death. Transl. Res. 2020, 215, 41–56. [Google Scholar] [CrossRef]
- Palumbo, J.S.; Kombrinck, K.W.; Drew, A.F.; Grimes, T.S.; Kiser, J.H.; Degen, J.L.; Bugge, T.H. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood 2000, 96, 3302–3309. [Google Scholar] [CrossRef]
- Valente-Acosta, B.; Flores-García, M.; González-Zárate, G.; Gerson-Cwilich, R.; Maldonado-Méndez, M.; Juárez-Vega, G.; Anglés-Cano, E.; Peña-Díaz, A. Fibrinolytic Activity of Circulating Microvesicles Is Associated with Progression of Breast Cancer. Tohoku J. Exp. Med. 2020, 250, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Zorio, E.; Gilabert-Estellés, J.; España, F.; Ramón, L.A.; Cosín, R.; Estellés, A. Fibrinolysis: The key to new pathogenetic mechanisms. Curr. Med. Chem. 2008, 15, 923–929. [Google Scholar] [CrossRef]
- Ilich, A.; Bokarev, I.; Key, N.S. Global assays of fibrinolysis. Int. J. Lab. Hematol. 2017, 39, 441–447. [Google Scholar] [CrossRef]
- Heissig, B.; Dhahri, D.; Eiamboonsert, S.; Salama, Y.; Shimazu, H.; Munakata, S.; Hattori, K. Role of mesenchymal stem cell-derived fibrinolytic factor in tissue regeneration and cancer progression. Cell. Mol. Life Sci. 2015, 72, 4759–4770. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, S.D.; Ornstein, D.L.; Zacharski, L.R. Cancer treatment with inhibitors of urokinase-type plasminogen activator and plasmin. Expert Opin. Investig. Drugs 2000, 9, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Draxler, D.F.; Sashindranath, M.; Medcalf, R.L. Plasmin: A Modulator of Immune Function. Semin. Thromb. Hemost. 2017, 43, 143–153. [Google Scholar] [CrossRef]
- Law, R.H.; Abu-Ssaydeh, D.; Whisstock, J.C. New insights into the structure and function of the plasminogen/plasmin system. Curr. Opin. Struct. Biol. 2013, 23, 836–841. [Google Scholar] [CrossRef]
- Didiasova, M.; Wujak, L.; Wygrecka, M.; Zakrzewicz, D. From plasminogen to plasmin: Role of plasminogen receptors in human cancer. Int. J. Mol. Sci. 2014, 15, 21229–21252. [Google Scholar] [CrossRef]
- Berger, D.H. Plasmin/plasminogen system in colorectal cancer. World J. Surg. 2002, 26, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Schuliga, M. The inflammatory actions of coagulant and fibrinolytic proteases in disease. Mediators Inflamm. 2015, 2015, 437695. [Google Scholar] [CrossRef]
- Tan, X.; Egami, H.; Nozawa, F.; Abe, M.; Baba, H. Analysis of the invasion-metastasis mechanism in pancreatic cancer: Involvement of plasmin(ogen) cascade proteins in the invasion of pancreatic cancer cells. Int. J. Oncol. 2006, 28, 369–374. [Google Scholar] [CrossRef]
- Heissig, B.; Eiamboonsert, S.; Salama, Y.; Shimazu, H.; Dhahri, D.; Munakata, S.; Tashiro, Y.; Hattori, K. Cancer therapy targeting the fibrinolytic system. Adv. Drug Deliv. Rev. 2016, 99 Pt B, 172–179. [Google Scholar] [CrossRef]
- Chapin, J.C.; Hajjar, K.A. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015, 29, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Abdol Razak, N.B.; Jones, G.; Bhandari, M.; Berndt, M.C.; Metharom, P. Cancer-Associated Thrombosis: An Overview of Mechanisms, Risk Factors, and Treatment. Cancers 2018, 10, 380. [Google Scholar] [CrossRef]
- Longstaff, C. Measuring fibrinolysis: From research to routine diagnostic assays. J. Thromb. Haemost. 2018, 16, 652–662. [Google Scholar] [CrossRef]
- Kolev, K.; Longstaff, C. Bleeding related to disturbed fibrinolysis. Br. J. Haematol. 2016, 175, 12–23. [Google Scholar] [CrossRef]
- Simpson, M.L.; Goldenberg, N.A.; Jacobson, L.J.; Bombardier, C.G.; Hathaway, W.E.; Manco-Johnson, M.J. Simultaneous thrombin and plasmin generation capacities in normal and abnormal states of coagulation and fibrinolysis in children and adults. Thromb. Res. 2011, 127, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Pieters, M.; Philippou, H.; Undas, A.; de Lange, Z.; Rijken, D.C.; Mutch, N.J.; Subcommittee on Factor XIII and Fibrinogen; Subcommittee on Fibrinolysis. An international study on the feasibility of a standardized combined plasma clot turbidity and lysis assay: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1007–1012. [Google Scholar] [CrossRef]
- Sheth, R.A.; Niekamp, A.; Quencer, K.B.; Shamoun, F.; Knuttinen, M.G.; Naidu, S.; Oklu, R. Thrombosis in cancer patients: Etiology, incidence, and management. Cardiovasc. Diagn. Ther. 2017, 7 (Suppl. S3), S178–S185. [Google Scholar] [CrossRef]
- Kvolik, S.; Jukic, M.; Matijevic, M.; Marjanovic, K.; Glavas-Obrovac, L. An overview of coagulation disorders in cancer patients. Surg Oncol. 2010, 19, e33–e46. [Google Scholar] [CrossRef]
- Gronostaj, K.; Richter, P.; Nowak, W.; Undas, A. Altered plasma fibrin clot properties in patients with digestive tract cancers: Links with the increased thrombin generation. Thromb Res. 2013, 131, 262–267. [Google Scholar] [CrossRef]
- Kwaan, H.C.; Weiss, I.; Tallman, M.S. The Role of Abnormal Hemostasis and Fibrinolysis in Morbidity and Mortality of Acute Promyelocytic Leukemia. Semin. Thromb. Hemost. 2019, 45, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.; Haim, N.; Brenner, B. Cancer and thrombosis revisited. Blood Rev. 2001, 15, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.C.; Tham, K.F.; Razvi, K.; Oei, P.L.; Lim, F.K.; Roy, A.C.; Prasad, R.N. Hemostatic and fibrinolytic status in patients with ovarian cancer and benign ovarian cysts: Could D-dimer and antithrombin III levels be included as prognostic markers for survival outcome? Clin. Appl. Thromb. Hemost. 2001, 7, 141–148. [Google Scholar] [CrossRef]
- Giaccherini, C.; Verzeroli, C.; Russo, L.; Gamba, S.; Tartari, C.J.; Bolognini, S.; Schieppati, F.; Ticozzi, C.; Sarmiento, R.; Celio, L.; et al. Thrombin Generation and D-Dimer for Prediction of Disease Progression and Mortality in Patients with Metastatic Gastrointestinal Cancer. Cancers 2022, 14, 4347. [Google Scholar] [CrossRef]
- Leiva, O.; Newcomb, R.; Connors, J.M.; Al-Samkari, H. Cancer and thrombosis: New insights to an old problem. J. Med. Vasc. 2020, 45, 6S8–6S16. [Google Scholar] [CrossRef] [PubMed]
- Wojtukiewicz, M.Z.; Sierko, E.; Klement, P.; Rak, J. The hemostatic system and angiogenesis in malignancy. Neoplasia 2001, 3, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Yapijakis, C.; Bramos, A.; Nixon, A.M.; Ragos, V.; Vairaktaris, E. The interplay between hemostasis and malignancy: The oral cancer paradigm. Anticancer Res. 2012, 32, 1791–1800. [Google Scholar] [PubMed]
- Danckwardt, S.; Hentze, M.W.; Kulozik, A.E. Pathologies at the nexus of blood coagulation and inflammation: Thrombin in hemostasis, cancer, and beyond. J. Mol. Med. 2013, 91, 1257–1271. [Google Scholar] [CrossRef] [PubMed]
- Moik, F.; Ay, C. Hemostasis and cancer: Impact of haemostatic biomarkers for the prediction of clinical outcomes in patients with cancer. J. Thromb. Haemost. 2022, 20, 2733–2745. [Google Scholar] [CrossRef]
- Repetto, O.; De Re, V. Coagulation and fibrinolysis in gastric cancer. Ann. N. Y. Acad. Sci. 2017, 1404, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Moik, F.; Prager, G.; Thaler, J.; Posch, F.; Wiedemann, S.; Schramm, T.; Englisch, C.; Mackman, N.; Pabinger, I.; Ay, C. Hemostatic Biomarkers and Venous Thromboembolism Are Associated with Mortality and Response to Chemotherapy in Patients with Pancreatic Cancer. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2837–2847. [Google Scholar] [CrossRef]
- Undas, A.; Zubkiewicz-Usnarska, L.; Helbig, G.; Woszczyk, D.; Kozińska, J.; Dmoszyńska, A.; Podolak-Dawidziak, M.; Kuliczkowski, K. Altered plasma fibrin clot properties and fibrinolysis in patients with multiple myeloma. Eur. J. Clin. Investig. 2014, 44, 557–566. [Google Scholar] [CrossRef]
- Van Geffen, M.; Loof, A.; Lap, P.; Boezeman, J.; Laros-van Gorkom, B.A.; Brons, P.; Verbruggen, B.; van Kraaij, M.; van Heerde, W.L. A novel hemostasis assay for the simultaneous measurement of coagulation and fibrinolysis. Hematology 2011, 16, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Kwaan, H.C.; Lindholm, P.F. Fibrin and Fibrinolysis in Cancer. Semin. Thromb. Hemost. 2019, 45, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Hyman, D.M.; Soff, G.A.; Kampel, L.J. Disseminated intravascular coagulation with excessive fibrinolysis in prostate cancer: A case series and review of the literature. Oncology 2011, 81, 119–125. [Google Scholar] [CrossRef]
- Collet, J.P.; Park, D.; Lesty, C.; Soria, J.; Soria, C.; Montalescot, G.; Weisel, J.W. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: Dynamic and structural approaches by confocal microscopy. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1354–1361. [Google Scholar] [CrossRef]
| Variable | Cancer Patients (N = 323) | Control Group (N = 148) | p-Value |
|---|---|---|---|
| Age (years) | 63 (52–71) | 52 (40–60) | <0.0001 1 |
| Male gender | 159 (49.2%) | 56 (37.8%) | 0.022 2 |
| Female gender | 164 (50.8%) | 92 (62.2%) | |
| BMI (Kg/m2) | 25.1 (21.9–29.3) N = 319 | 28.0 (25.1–31.2) N = 144 | <0.0001 1 |
| Smoker or ex-smoker | 133 (41.2%) | 27 (18.4%) N = 147 | <0.0001 2 |
| Alcoholism or ex-alcoholism | 42 (13.0%) | 2 (1.4%) | <0.0001 2 |
| Medications | / | / | / |
| Antihypertensive | 135 (41.8%) | 41 (27.7%) | 0.004 2 |
| Statin | 56 (17.3%) | 8 (5.4%) | 0.0003 2 |
| Diabetes medications | 45 (13.9%) | 14 (9.5%) | 0.2298 2 |
| AAS | 21 (6.5%) | 4 (2.7%) | 0.1198 2 |
| Tumor site | / | / | / |
| Bladder | 04 (1.24%) | - | - |
| Brain | 06 (1.86%) | - | - |
| Breast | 48 (14.86%) | - | - |
| Colon and Rectum | 38 (11.76%) | - | - |
| Esophagus | 13 (4.02%) | - | - |
| Kidney | 07 (2.17%) | - | - |
| Liver and bile duct | 12 (3.72%) | - | - |
| Lung | 21 (6.50%) | - | - |
| Lymphoma | 46 (14.24%) | - | - |
| Myeloma | 18 (5.57%) | - | - |
| Other sites | 65 (20.12%) | - | - |
| Ovary | 04 (1.24%) | - | - |
| Pancreas | 06 1.86%) | - | - |
| Stomach | 25 (7.74%) | - | - |
| Uterus | 10 (3.10%) | - | - |
| Cancer stage | / | / | / |
| Stage I | 47 (14.55%) | - | - |
| Stage II | 86 (26.63%) | - | - |
| Stage III | 101 (31.27%) | - | - |
| Stage IV | 89 (27.55%) | - | - |
| WBC (103/µL) | 7.5 (5.8–9.5) N = 315 | 6.1 (5.3–7.2) N = 146 | <0.0001 1 |
| Hemoglobin (g/dL) | 12.8 (10.8–13.9) N = 315 | 13.8 (13.0–15.1) N = 146 | <0.0001 1 |
| Platelets (103/µL) | 269 (204–344) N = 315 | 241 (208–271) N = 146 | 0.0001 1 |
| Parameters | Control Group N = 148 | Cancer Group N = 323 | p-Value |
|---|---|---|---|
| Plasmin (%NC) | / | / | / |
| MA | 102.8 (92.4–110.9) | 122.9 (112.1–135.7) | <0.0001 |
| T(MA) | 110.2 (97.9–136.9) | 107.9 (96.4–133.1) | 0.518 |
| Lag Time) | 150.0 (133.3–166.6) | 125.0 (107.1–146.6) | <0.0001 |
| AUC | 90.7 (76.9–103.1) | 111.7 (99.8–127.5) | <0.0001 |
| Vmax | 109.4 (86.5–159.8) | 174.6 (118.9–179.8) | <0.0001 |
| T(Vmax) | 319.4 (195.7–911.5) | 250.0 (140.9–686.4) | 0.007 |
| Thrombin (%NC) | / | / | / |
| MA | 102.4 (93.6–110.7) | 112.7 (104.3–123.3) | <0.0001 |
| T(MA) | 166.6 (139.1–204.4) | 143.7 (118.2–177.3) | <0.0001 |
| Lag Time | 140.0 (120.0–160.0) | 115.4 (88.9–150.0) | <0.0001 |
| AUC | 101.4 (92.5–113.1) | 119.1 (109.2–130.9) | <0.0001 |
| Vmax | 79.1 (60.5–110.0) | 97.5 (68.7–131.7) | 0.0003 |
| T(Vmax) | 140.0 (120.8–160.0) | 114.3 (90.9–145.4) | <0.0001 |
| Variable | DEATH | p-Value | |
|---|---|---|---|
| No N = 245 | Yes N = 68 | ||
| Age (years) | 62.0 (51.0–70.0) | 65.0 (59.0–73.0) | 0.0053 1 |
| Gender | / | / | / |
| Male | 107 (43.7%) | 46 (67.6%) | 0.0006 2 |
| Female | 138 (56.3%) | 22 (32.4%) | |
| BMI (Kg/m2) | 25.6 (22.8–29.7) N = 243 | 23.0 (20.3–27.5) N = 66 | 0.0004 1 |
| Comorbidities | / | / | / |
| Hypertension | 103 (42.0%) | 31 (45.6%) | 0.6780 2 |
| Diabetes | 38 (15.5%) | 12 (17.6%) | 0.7089 2 |
| Previous thrombosis | 16 (6.5%) | 6 (8.8%) | 0.5909 2 |
| Lung disease | 12 (4.9%) | 3 (4.4%) | 1.0000 2 |
| Heart disease | 13 (5.3%) | 9 (13.2%) | 0.0320 2 |
| Nephropathy | 4 (1.6%) | 5 (7.4%) | 0.0258 2 |
| Habits | / | / | / |
| Smoking/ex-smoker | 87 (35.5%) | 43 (63.2%) | <0.0001 2 |
| Alcoholic/ex-alcoholic | 33 (13.5%) | 8 (11.8%) | 0.8402 2 |
| Laboratory tests | / | / | / |
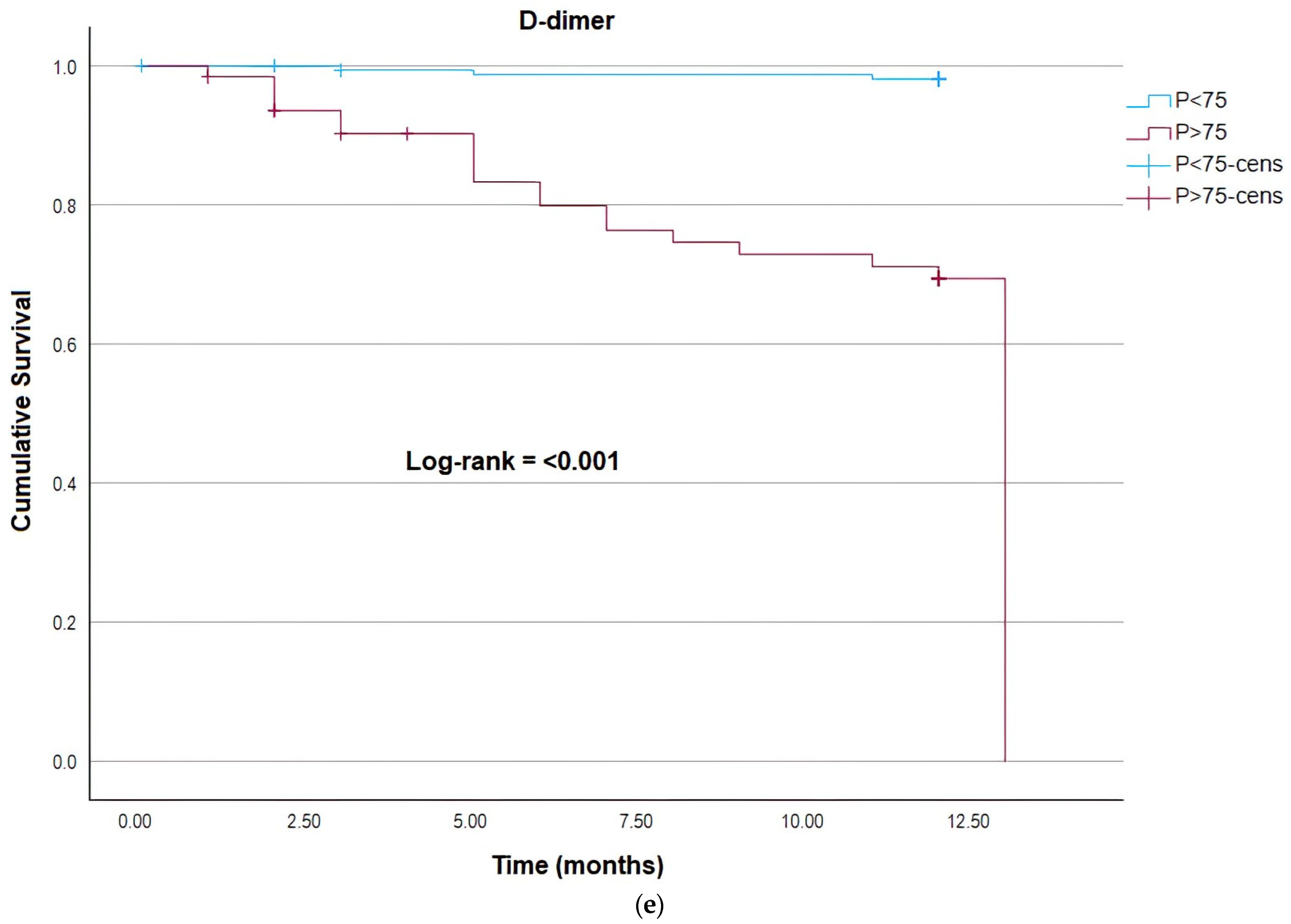
| D-dimer (ng/mL) | 493 (290.5–1018) N = 193 | 882 (421–2026) N = 48 | 0.0010 1 |
| P-selectin (ng/mL) | 76.3 (48.9–98.9) N = 174 | 82.9 (54.9–122.9) N = 52 | 0.2903 1 |
| Cancer stage | / | / | / |
| I | 38 (16.0%) | 7 (10.3%) | <0.0001 3 |
| II | 79 (32.0%) | 2 (2.9%) | |
| III | 79 (32.0%) | 20 (29.4%) | |
| IV | 49 (20.0%) | 39 (57.4%) | |
| Cause of death | / | / | / |
| Disease progression | / | 28 (41.2%) | / |
| infections/complications | / | 23 (33.8%) | / |
| thromboembolic complications | / | 5 (7.4%) | / |
| Unclear | / | 12 (17.6%) | / |
| Chemotherapy protocol | / | / | / |
| Platinum and/or gemcitabine-based chemotherapy | 75 (43.1%) N = 174 | 26 (61.9%) N = 42 | 0.0381 2 |
| Without platinum and/or gemcitabine-based chemotherapy | 99 (56.9%) N = 174 | 16 (38.1%) N = 42 | |
| Parameters | DEATH | p-Value | |
|---|---|---|---|
| No N = 245 | Yes N = 68 | ||
| Plasmin | / | / | / |
| MA | 122.8 (111.7–135.7) | 124.1 (115.6–137.5) | 0.086 |
| T(MA) | 107.9 (96.2–132.5) | 111.2 (96.9–146.4) | 0.639 |
| Lag Time | 123.1 (106.2–143.7) | 133.3 (114.5–153.3) | 0.067 |
| AUC | 111.6 (99.3–127.5) | 111.9 (101.5–127.6) | 0.673 |
| Vmax | 148.2 (122.1–183.1) | 129.3 (109.5–173.3) | 0.016 |
| T(Vmax) | 282.6 (143.5–795.8) | 178.0 (135.6–342.3) | 0.016 |
| Thrombin | / | / | / |
| MA | 111.8 (103.5–121.9) | 117.7 (108.6–130.0) | 0.001 |
| T(MA) | 142.8 (116.7–171.4) | 146.0 (123.9–204.8) | 0.138 |
| Lag Time | 115.4 (91.7–150.0) | 113.9 (88.9–161.9) | 0.613 |
| AUC | 117.6 (108.5–130.5) | 123.9 (110.9–133.0) | 0.018 |
| Vmax | 95.2 (68.8–130.2) | 107.1 (68.9–140.3) | 0.359 |
| T(Vmax) | 114.3 (90.9–144.4) | 114.3 (89.8–156.5) | 0.797 |
| Dependent Variable | Explanatory Variable | HR | 95%IC | p-Value | Wald |
|---|---|---|---|---|---|
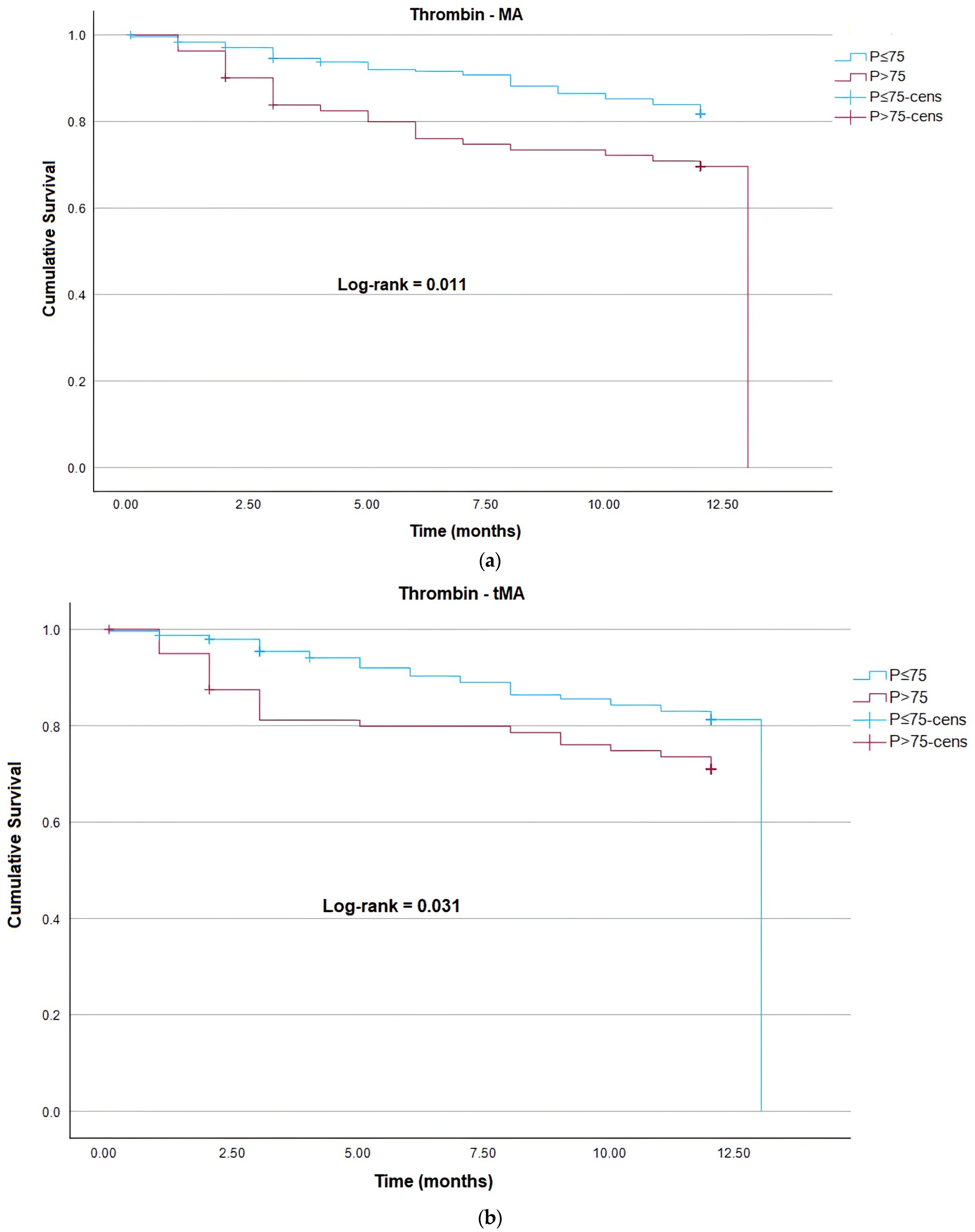
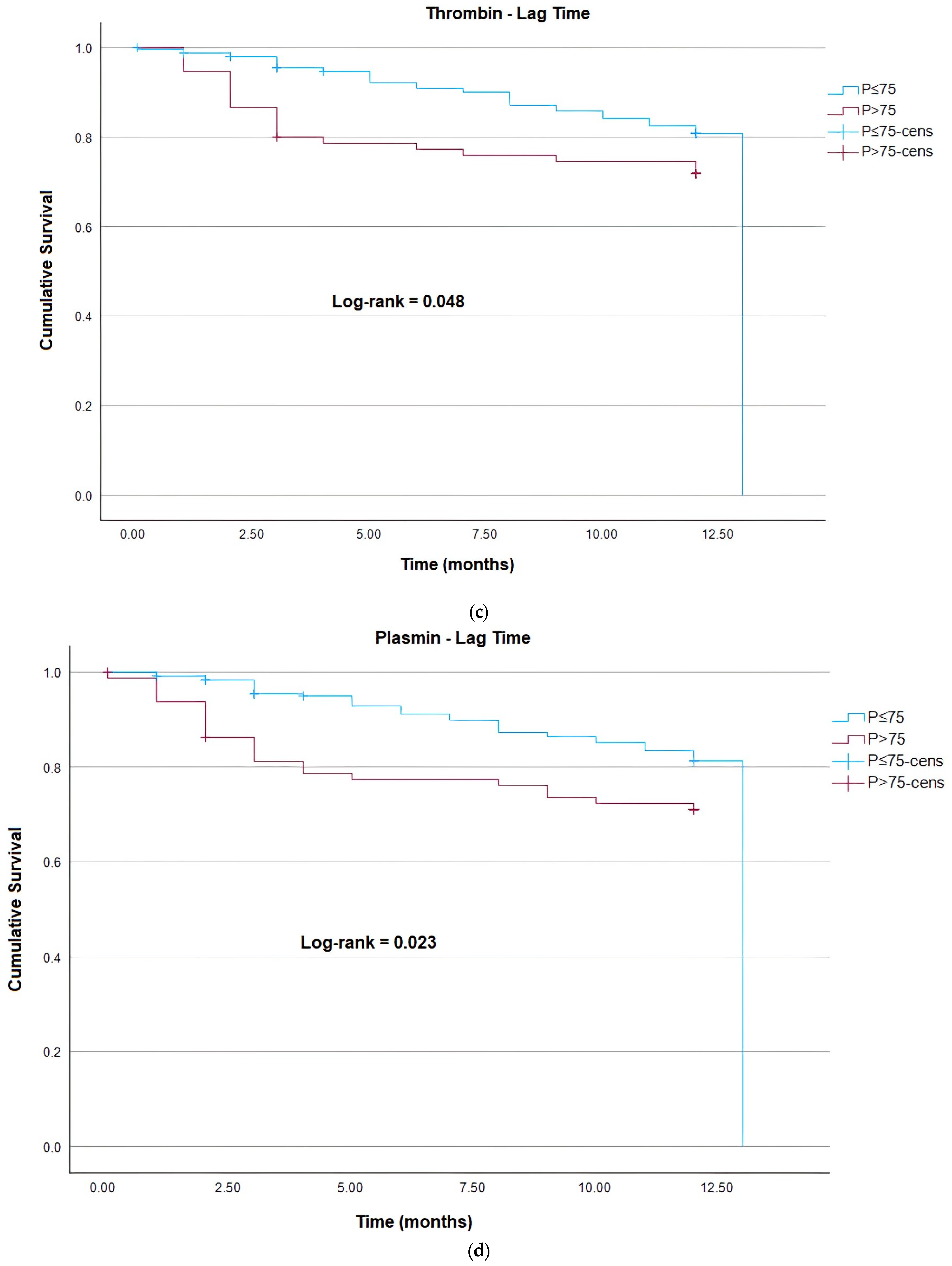
| DEATH | Thrombin MA | 1.02 | 1.01–1.04 | <0.001 | 11.78 |
| Thrombin tMA | 1.00 | 0.99–1.01 | 0.107 | 2.59 | |
| Thrombin Lag Time | 1.00 | 0.99–1.01 | 0.266 | 1.24 | |
| Thrombin AUC | 1.02 | 1.01–1.03 | 0.005 | 8.00 | |
| Thrombin Vmax | 1.00 | 0.99–1.01 | 0.392 | 0.73 | |
| Thrombin tVmax | 1.00 | 0.99–1.01 | 0.478 | 0.50 | |
| Plasmin MA | 1.01 | 0.99–1.02 | 0.101 | 2.69 | |
| Plasmin tMA | 1.01 | 0.99–1.01 | 0.408 | 0.69 | |
| Plasmin Lag Time | 1.01 | 1.00–1.01 | 0.065 | 3.41 | |
| Plasmin AUC | 1.01 | 0.99–1.01 | 0.721 | 0.13 | |
| Plasmin Vmax | 1.00 | 0.99–1.01 | 0.614 | 0.25 | |
| Plasmin tVmax | 1.00 | 0.99–1.00 | 0.027 | 4.92 |
| Model | Variable | HR (95% IC) | p-Value |
|---|---|---|---|
| Model 1—Death | Male gender | 1.78 (1.04;3.06) | 0.037 |
| BMI (Kg/m2) | 0.95 (0.90;1.00) | 0.040 | |
| Smoking/ex-smoker | 1.99 (1.15;3.45) | 0.014 | |
| High risk tumor | 2.00 (1.15;3.47) | 0.014 | |
| Cancer stage I | Ref. | <0.001 | |
| Cancer stage II | 0.14 (0.29;0.67) | 0.014 | |
| Cancer stage III | 0.93 (0.39;2.24) | 0.868 | |
| Cancer stage IV | 2.25 (0.97;5.19) | 0.058 | |
| Thrombin AUC | 1.02 (1.01;1.03) | 0.025 | |
| Model 2—Death | Male gender | 1.86 (1.08;3.21) | 0.025 |
| BMI (Kg/m2) | 0.95 (0.90;1.00) | 0.046 | |
| Smoking/ex-smoker | 1.91 (1.10;3.30) | 0.021 | |
| High risk tumor | 2.11 (1.21;3.69) | 0.009 | |
| Cancer stage I | Ref. | <0.001 | |
| Cancer stage II | 0.12 (0.25;0.60) | 0.009 | |
| Cancer stage III | 0.83 (0.34;2.03) | 0.689 | |
| Cancer stage IV | 2.05 (0.88;4.78) | 0.095 | |
| Thrombin MA | 1.02 (1.01;1.04) | 0.004 | |
| Model 3—Death | Male gender | 1.58 (0.93;2.71) | 0.094 |
| BMI (Kg/m2) | 0.95 (0.91;1.00) | 0.045 | |
| Smoking/ex-smoker | 1.97 (1.15;3.40) | 0.014 | |
| High risk tumor | 1.67 (0.96;2.89) | 0.069 | |
| Cancer stage I | Ref. | <0.001 | |
| Cancer stage II | 0.16 (0.32;0.75) | 0.021 | |
| Cancer stage III | 1.01 (0.42;2.42) | 0.990 | |
| Cancer stage IV | 2.83 (1.26;6.37) | 0.012 | |
| Plasmin tVmax | 1.00 (1.00;1.00) | 0.167 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gois, G.S.S.; Montalvão, S.A.L.; Anhaia, T.R.A.; Almeida, M.E.A.; Martinelli, B.M.; Fernandes, M.C.G.L.; Hubers, S.C.; Ferreira, M.R.M.; Ribeiro, D.D.; Teixeira, J.C.; et al. Association of Fibrinolytic Potential and Risk of Mortality in Cancer Patients. Cancers 2023, 15, 4408. https://doi.org/10.3390/cancers15174408
Gois GSS, Montalvão SAL, Anhaia TRA, Almeida MEA, Martinelli BM, Fernandes MCGL, Hubers SC, Ferreira MRM, Ribeiro DD, Teixeira JC, et al. Association of Fibrinolytic Potential and Risk of Mortality in Cancer Patients. Cancers. 2023; 15(17):4408. https://doi.org/10.3390/cancers15174408
Chicago/Turabian StyleGois, Gabriele Silva Souza, Silmara Aparecida Lima Montalvão, Thaizy Ramires Alencar Anhaia, Millene Evelyn Alves Almeida, Beatriz Moraes Martinelli, Maria Carmen Gonçalves Lopes Fernandes, Stephany Cares Hubers, Monique R. M. Ferreira, Daniel Dias Ribeiro, Júlio César Teixeira, and et al. 2023. "Association of Fibrinolytic Potential and Risk of Mortality in Cancer Patients" Cancers 15, no. 17: 4408. https://doi.org/10.3390/cancers15174408
APA StyleGois, G. S. S., Montalvão, S. A. L., Anhaia, T. R. A., Almeida, M. E. A., Martinelli, B. M., Fernandes, M. C. G. L., Hubers, S. C., Ferreira, M. R. M., Ribeiro, D. D., Teixeira, J. C., Carvalheira, J. B. C., Lima, C. S. P., Andreollo, N. A., Etchebehere, M., Zambon, L., Ferreira, U., Tincani, A. J., Martins, A. S., Coy, C. S. R., ... ADVENTH Cancer Group. (2023). Association of Fibrinolytic Potential and Risk of Mortality in Cancer Patients. Cancers, 15(17), 4408. https://doi.org/10.3390/cancers15174408





