Immune Checkpoint Inhibitors in Advanced Cutaneous Squamous Cell Carcinoma: Real-World Experience from a Canadian Comprehensive Cancer Centre
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Cohort
2.2. Efficacy and Safety Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patients
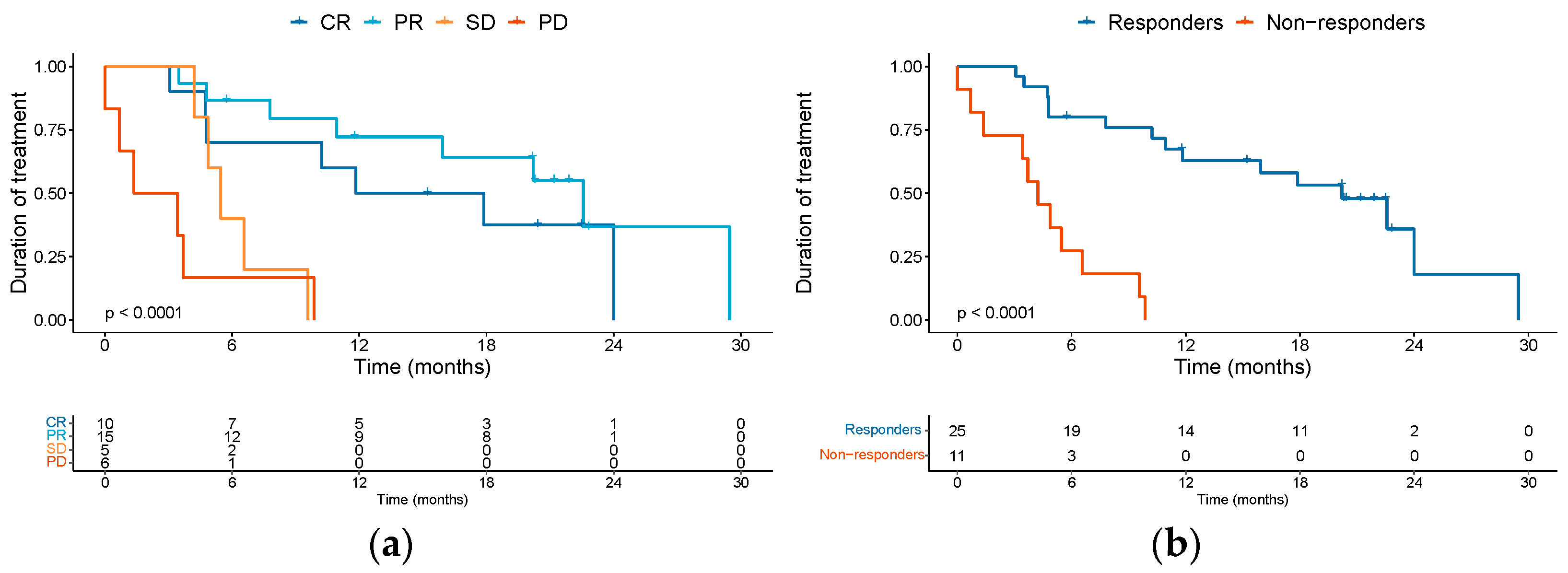
3.2. Effectiveness Outcomes
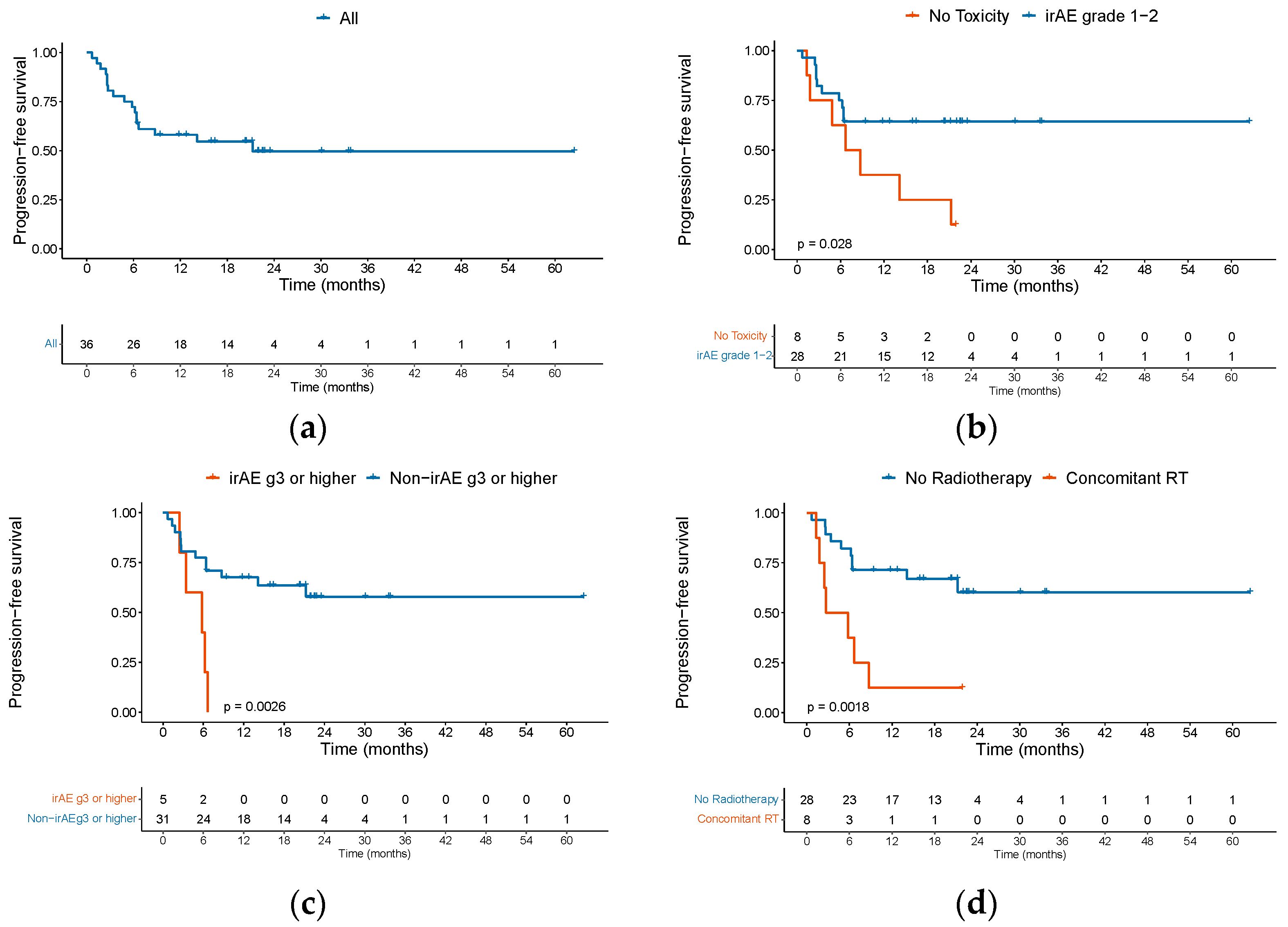
3.3. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the US Population, 2012. JAMA Dermatol. 2015, 151, 1081. [Google Scholar] [CrossRef] [PubMed]
- Brantsch, K.D.; Meisner, C.; Schonfisch, B.; Trilling, B.; Wehner-Caroli, J.; Rocken, M.; Breuninger, H. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: A prospective study. Lancet Oncol. 2008, 9, 713–720. [Google Scholar] [CrossRef] [PubMed]
- In, G.K.; Vaidya, P.; Filkins, A.; Hermel, D.J.; King, K.G.; Ragab, O.; Tseng, W.W.; Swanson, M.; Kokot, N.; Lang, J.E.; et al. PD-1 inhibition therapy for advanced cutaneous squamous cell carcinoma: A retrospective analysis from the University of Southern California. J. Cancer Res. Clin. Oncol. 2021, 147, 1803–1811. [Google Scholar] [CrossRef]
- Schmults, C.D.; Karia, P.S.; Carter, J.B.; Han, J.; Qureshi, A.A. Factors Predictive of Recurrence and Death From Cutaneous Squamous Cell Carcinoma. JAMA Dermatol. 2013, 149, 541. [Google Scholar] [CrossRef]
- Tam, S.; Yao, C.M.K.L.; Amit, M.; Gajera, M.; Luo, X.; Treistman, R.; Khanna, A.; Aashiq, M.; Nagarajan, P.; Bell, D.; et al. Association of Immunosuppression With Outcomes of Patients With Cutaneous Squamous Cell Carcinoma of the Head and Neck. JAMA Otolaryngol.—Head Neck Surg. 2020, 146, 128. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.; Quinn, A.; Stasko, T. Skin Cancer and Immunosuppression. Dermatol. Clin. 2019, 37, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Zelin, E.; Maronese, C.A.; Dri, A.; Toffoli, L.; Di Meo, N.; Nazzaro, G.; Zalaudek, I. Identifying Candidates for Immunotherapy among Patients with Non-Melanoma Skin Cancer: A Review of the Potential Predictors of Response. J. Clin. Med. 2022, 11, 3364. [Google Scholar] [CrossRef]
- Allegra, A.; Tonacci, A.; Musolino, C.; Pioggia, G.; Gangemi, S. Secondary Immunodeficiency in Hematological Malignancies: Focus on Multiple Myeloma and Chronic Lymphocytic Leukemia. Front. Immunol. 2021, 12, 738915. [Google Scholar] [CrossRef]
- Lanz, J.; Bouwes Bavinck, J.N.; Westhuis, M.; Quint, K.D.; Harwood, C.A.; Nasir, S.; Van-De-Velde, V.; Proby, C.M.; Ferrándiz, C.; Genders, R.E.; et al. Aggressive Squamous Cell Carcinoma in Organ Transplant Recipients. JAMA Dermatol. 2019, 155, 66. [Google Scholar] [CrossRef] [PubMed]
- Chockalingam, R.; Downing, C.; Tyring, S. Cutaneous Squamous Cell Carcinomas in Organ Transplant Recipients. J. Clin. Med. 2015, 4, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Crum-Cianflone, N.; Hullsiek, K.H.; Satter, E.; Marconi, V.; Weintrob, A.; Ganesan, A.; Barthel, R.V.; Fraser, S.; Agan, B.K. Cutaneous Malignancies Among HIV-Infected Persons. Arch. Intern. Med. 2009, 169, 1130. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, K.; Turner, R.; Dolev, J.C.; LeBoit, P.E.; Berger, T.G.; Maurer, T.A. Cutaneous malignancy and human immunodeficiency virus disease. J. Am. Acad. Dermatol. 2006, 54, 189–206, quiz 207-110. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Brodland, D.G.; Su, W.P. Skin cancers associated with acquired immunodeficiency syndrome. Mayo Clin. Proc. 1995, 70, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Colegio, O.R. Skin Cancers in Organ Transplant Recipients. Am. J. Transplant. 2017, 17, 2509–2530. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef]
- Hillen, U.; Leiter, U.; Haase, S.; Kaufmann, R.; Becker, J.; Gutzmer, R.; Terheyden, P.; Krause-Bergmann, A.; Schulze, H.J.; Hassel, J.; et al. Advanced cutaneous squamous cell carcinoma: A retrospective analysis of patient profiles and treatment patterns-Results of a non-interventional study of the DeCOG. Eur. J. Cancer 2018, 96, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Sadek, H.; Azli, N.; Wendling, J.L.; Cvitkovic, E.; Rahal, M.; Mamelle, G.; Guillaume, J.C.; Armand, J.P.; Avril, M.F. Treatment of advanced squamous cell carcinoma of the skin with cisplatin, 5-fluorouracil, and bleomycin. Cancer 1990, 66, 1692–1696. [Google Scholar] [CrossRef]
- Guthrie, T.H.; McElveen, L.J.; Porubsky, E.S.; Harmon, J.D. Cisplatin and doxorubicin. An effective chemotherapy combination in the treatment of advanced basal cell and squamous carcinoma of the skin. Cancer 1985, 55, 1629–1632. [Google Scholar] [CrossRef]
- Khansur, T.; Kennedy, A. Cisplatin and 5-fluorouracil for advanced locoregional and metastatic squamous cell carcinoma of the skin. Cancer 1991, 67, 2030–2032. [Google Scholar] [CrossRef]
- Foote, M.C.; McGrath, M.; Guminski, A.; Hughes, B.G.M.; Meakin, J.; Thomson, D.; Zarate, D.; Simpson, F.; Porceddu, S.V. Phase II study of single-agent panitumumab in patients with incurable cutaneous squamous cell carcinoma. Ann. Oncol. 2014, 25, 2047–2052. [Google Scholar] [CrossRef] [PubMed]
- Maubec, E.; Petrow, P.; Scheer-Senyarich, I.; Duvillard, P.; Lacroix, L.; Gelly, J.; Certain, A.; Duval, X.; Crickx, B.; Buffard, V.; et al. Phase II Study of Cetuximab As First-Line Single-Drug Therapy in Patients With Unresectable Squamous Cell Carcinoma of the Skin. J. Clin. Oncol. 2011, 29, 3419–3426. [Google Scholar] [CrossRef]
- Pickering, C.R.; Zhou, J.H.; Lee, J.J.; Drummond, J.A.; Peng, S.A.; Saade, R.E.; Tsai, K.Y.; Curry, J.L.; Tetzlaff, M.T.; Lai, S.Y.; et al. Mutational Landscape of Aggressive Cutaneous Squamous Cell Carcinoma. Clin. Cancer Res. 2014, 20, 6582–6592. [Google Scholar] [CrossRef] [PubMed]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef]
- Migden, M.R.; Khushalani, N.I.; Chang, A.L.S.; Lewis, K.D.; Schmults, C.D.; Hernandez-Aya, L.; Meier, F.; Schadendorf, D.; Guminski, A.; Hauschild, A.; et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: Results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020, 21, 294–305. [Google Scholar] [CrossRef]
- Rischin, D.; Migden, M.R.; Lim, A.M.; Schmults, C.D.; Khushalani, N.I.; Hughes, B.G.M.; Schadendorf, D.; Dunn, L.A.; Hernandez-Aya, L.; Chang, A.L.S.; et al. Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: Primary analysis of fixed-dosing, long-term outcome of weight-based dosing. J. Immunother. Cancer 2020, 8, e000775. [Google Scholar] [CrossRef]
- Rischin, D.; Khushalani, N.I.; Schmults, C.D.; Guminski, A.; Chang, A.L.S.; Lewis, K.D.; Lim, A.M.; Hernandez-Aya, L.; Hughes, B.G.M.; Schadendorf, D.; et al. Integrated analysis of a phase 2 study of cemiplimab in advanced cutaneous squamous cell carcinoma: Extended follow-up of outcomes and quality of life analysis. J. Immunother. Cancer 2021, 9, e002757. [Google Scholar] [CrossRef]
- Grob, J.J.; Gonzalez, R.; Basset-Seguin, N.; Vornicova, O.; Schachter, J.; Joshi, A.; Meyer, N.; Grange, F.; Piulats, J.M.; Bauman, J.R.; et al. Pembrolizumab Monotherapy for Recurrent or Metastatic Cutaneous Squamous Cell Carcinoma: A Single-Arm Phase II Trial (KEYNOTE-629). J. Clin. Oncol. 2020, 38, 2916–2925. [Google Scholar] [CrossRef]
- Hughes, B.G.M.; Munoz-Couselo, E.; Mortier, L.; Bratland, A.; Gutzmer, R.; Roshdy, O.; Gonzalez Mendoza, R.; Schachter, J.; Arance, A.; Grange, F.; et al. Pembrolizumab for locally advanced and recurrent/metastatic cutaneous squamous cell carcinoma (KEYNOTE-629 study): An open-label, nonrandomized, multicenter, phase II trial. Ann. Oncol. 2021, 32, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Maubec, E.; Boubaya, M.; Petrow, P.; Beylot-Barry, M.; Basset-Seguin, N.; Deschamps, L.; Grob, J.J.; Dreno, B.; Scheer-Senyarich, I.; Bloch-Queyrat, C.; et al. Phase II Study of Pembrolizumab As First-Line, Single-Drug Therapy for Patients With Unresectable Cutaneous Squamous Cell Carcinomas. J. Clin. Oncol. 2020, 38, 3051–3061. [Google Scholar] [CrossRef]
- Munhoz, R.R.; Nader-Marta, G.; De Camargo, V.P.; Queiroz, M.M.; Cury-Martins, J.; Ricci, H.; De Mattos, M.R.; De Menezes, T.A.F.; Machado, G.U.C.; Bertolli, E.; et al. A phase 2 study of first-line nivolumab in patients with locally advanced or metastatic cutaneous squamous-cell carcinoma. Cancer 2022, 128, 4223–4231. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Welponer, T.; Richtig, E.; Wolf, I.; Hoeller, C.; Hafner, C.; Nguyen, V.A.; Kofler, J.; Barta, M.; Koelblinger, P.; et al. Nivolumab for locally advanced and metastatic cutaneous squamous cell carcinoma (NIVOSQUACS study)—Phase II data covering impact of concomitant haematological malignancies. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1799–1810. [Google Scholar] [CrossRef]
- Haist, M.; Stege, H.; Lang, B.M.; Tsochataridou, A.; Salzmann, M.; Mohr, P.; Schadendorf, D.; Ugurel, S.; Placke, J.-M.; Weichenthal, M.; et al. Response to First-Line Treatment with Immune-Checkpoint Inhibitors in Patients with Advanced Cutaneous Squamous Cell Carcinoma: A Multicenter, Retrospective Analysis from the German ADOReg Registry. Cancers 2022, 14, 5543. [Google Scholar] [CrossRef] [PubMed]
- Hober, C.; Fredeau, L.; Pham-Ledard, A.; Boubaya, M.; Herms, F.; Celerier, P.; Aubin, F.; Beneton, N.; Dinulescu, M.; Jannic, A.; et al. Cemiplimab for Locally Advanced and Metastatic Cutaneous Squamous-Cell Carcinomas: Real-Life Experience from the French CAREPI Study Group. Cancers 2021, 13, 3547. [Google Scholar] [CrossRef]
- Guillaume, T.; Puzenat, E.; Popescu, D.; Aubin, F.; Nardin, C. Cemiplimab-rwlc in advanced cutaneous squamous cell carcinoma: Real-world experience in a French dermatology department. Br. J. Dermatol. 2021, 185, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Strippoli, S.; Fanizzi, A.; Quaresmini, D.; Nardone, A.; Armenio, A.; Figliuolo, F.; Filotico, R.; Fucci, L.; Mele, F.; Traversa, M.; et al. Cemiplimab in an Elderly Frail Population of Patients With Locally Advanced or Metastatic Cutaneous Squamous Cell Carcinoma: A Single-Center Real-Life Experience From Italy. Front. Oncol. 2021, 11, 686308. [Google Scholar] [CrossRef] [PubMed]
- Baggi, A.; Quaglino, P.; Rubatto, M.; Depenni, R.; Guida, M.; Ascierto, P.A.; Trojaniello, C.; Queirolo, P.; Saponara, M.; Peris, K.; et al. Real world data of cemiplimab in locally advanced and metastatic cutaneous squamous cell carcinoma. Eur. J. Cancer 2021, 157, 250–258. [Google Scholar] [CrossRef]
- Hanna, G.J.; Ruiz, E.S.; LeBoeuf, N.R.; Thakuria, M.; Schmults, C.D.; Decaprio, J.A.; Silk, A.W. Real-world outcomes treating patients with advanced cutaneous squamous cell carcinoma with immune checkpoint inhibitors (CPI). Br. J. Cancer 2020, 123, 1535–1542. [Google Scholar] [CrossRef]
- Salzmann, M.; Leiter, U.; Loquai, C.; Zimmer, L.; Ugurel, S.; Gutzmer, R.; Thoms, K.M.; Enk, A.H.; Hassel, J.C. Programmed cell death protein 1 inhibitors in advanced cutaneous squamous cell carcinoma: Real-world data of a retrospective, multicenter study. Eur. J. Cancer 2020, 138, 125–132. [Google Scholar] [CrossRef]
- Rios-Vinuela, E.; Alvarez, P.; Lavernia, J.; Serra-Guillen, C.; Requena, C.; Bernia, E.; Diago, A.; Llombart, B.; Sanmartin, O. Cemiplimab in Advanced Cutaneous Squamous Cell Carcinoma: Real-World Experience in a Monographic Oncology Center. Actas Dermosifiliogr. 2022, 113, T610–T615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhong, A.; Chen, J. Immune checkpoint inhibitors in advanced cutaneous squamous cell carcinoma: A systemic review and meta-analysis. Ski. Res. Technol. 2023, 29, e13229. [Google Scholar] [CrossRef]
- Fine, J.D.; Johnson, L.B.; Weiner, M.; Li, K.P.; Suchindran, C. Epidermolysis bullosa and the risk of life-threatening cancers: The National EB Registry experience, 1986–2006. J. Am. Acad. Dermatol. 2009, 60, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Mellerio, J.E.; Robertson, S.J.; Bernardis, C.; Diem, A.; Fine, J.D.; George, R.; Goldberg, D.; Halmos, G.B.; Harries, M.; Jonkman, M.F.; et al. Management of cutaneous squamous cell carcinoma in patients with epidermolysis bullosa: Best clinical practice guidelines. Br. J. Dermatol. 2016, 174, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Khaddour, K.; Gorell, E.S.; Dehdashti, F.; Tang, J.Y.; Ansstas, G. Induced Remission of Metastatic Squamous Cell Carcinoma with an Immune Checkpoint Inhibitor in a Patient with Recessive Dystrophic Epidermolysis Bullosa. Case Rep. Oncol. 2020, 13, 911–915. [Google Scholar] [CrossRef]
- Piccerillo, A.; El Hachem, M.; De Vito, R.; De Luca, E.V.; Peris, K. Pembrolizumab for Treatment of a Patient With Multiple Cutaneous Squamous Cell Carcinomas and Recessive Dystrophic Epidermolysis Bullosa. JAMA Dermatol. 2020, 156, 708. [Google Scholar] [CrossRef]
- Duong, T.; Wong, D.; Barrett, A.; Price, H. Successful use of immunotherapy to treat advanced cutaneous squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. BMJ Case Rep. 2021, 14, e238966. [Google Scholar] [CrossRef]
- Rodriguez, J.E.; Naigeon, M.; Goldschmidt, V.; Roulleaux Dugage, M.; Seknazi, L.; Danlos, F.X.; Champiat, S.; Marabelle, A.; Michot, J.-M.; Massard, C.; et al. Immunosenescence, inflammaging, and cancer immunotherapy efficacy. Expert Rev. Anticancer. Ther. 2022, 22, 915–926. [Google Scholar] [CrossRef]
- Poropatich, K.; Fontanarosa, J.; Samant, S.; Sosman, J.A.; Zhang, B. Cancer Immunotherapies: Are They as Effective in the Elderly? Drugs Aging 2017, 34, 567–581. [Google Scholar] [CrossRef]
- Daste, A.; Domblides, C.; Gross-Goupil, M.; Chakiba, C.; Quivy, A.; Cochin, V.; de Mones, E.; Larmonier, N.; Soubeyran, P.; Ravaud, A. Immune checkpoint inhibitors and elderly people: A review. Eur. J. Cancer 2017, 82, 155–166. [Google Scholar] [CrossRef]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- De Wolf, K.; Kruse, V.; Sundahl, N.; Van Gele, M.; Chevolet, I.; Speeckaert, R.; Brochez, L.; Ost, P. A phase II trial of stereotactic body radiotherapy with concurrent anti-PD1 treatment in metastatic melanoma: Evaluation of clinical and immunologic response. J. Transl. Med. 2017, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, A.B.; Nirschl, C.J.; Kochel, C.M.; Nirschl, T.R.; Francica, B.J.; Velarde, E.; Deweese, T.L.; Drake, C.G. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol. Res. 2015, 3, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, X.; Chen, D.; Yu, J. Radiotherapy combined with immunotherapy: The dawn of cancer treatment. Signal Transduct. Target. Ther. 2022, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Reynders, K.; Illidge, T.; Siva, S.; Chang, J.Y.; De Ruysscher, D. The abscopal effect of local radiotherapy: Using immunotherapy to make a rare event clinically relevant. Cancer Treat. Rev. 2015, 41, 503–510. [Google Scholar] [CrossRef]
- Twyman-Saint Victor, C.; Rech, A.J.; Maity, A.; Rengan, R.; Pauken, K.E.; Stelekati, E.; Benci, J.L.; Xu, B.; Dada, H.; Odorizzi, P.M.; et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015, 520, 373–377. [Google Scholar] [CrossRef]
- Lavaud, J.; Blom, A.; Longvert, C.; Fort, M.; Funck-Brentano, E.; Saiag, P. Pembrolizumab and concurrent hypo-fractionated radiotherapy for advanced non-resectable cutaneous squamous cell carcinoma. Eur. J. Dermatol. 2019, 29, 636–640. [Google Scholar] [CrossRef]
- Johnson, D.B.; Sullivan, R.J.; Ott, P.A.; Carlino, M.S.; Khushalani, N.I.; Ye, F.; Guminski, A.; Puzanov, I.; Lawrence, D.P.; Buchbinder, E.I.; et al. Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol. 2016, 2, 234–240. [Google Scholar] [CrossRef]
- Menzies, A.M.; Johnson, D.B.; Ramanujam, S.; Atkinson, V.G.; Wong, A.N.M.; Park, J.J.; McQuade, J.L.; Shoushtari, A.N.; Tsai, K.K.; Eroglu, Z.; et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann. Oncol. 2017, 28, 368–376. [Google Scholar] [CrossRef]
- Abdel-Wahab, N.; Shah, M.; Lopez-Olivo, M.A.; Suarez-Almazor, M.E. Use of Immune Checkpoint Inhibitors in the Treatment of Patients With Cancer and Preexisting Autoimmune Disease. Ann. Intern. Med. 2018, 168, 121–130. [Google Scholar] [CrossRef]
- Kennedy, L.C.; Bhatia, S.; Thompson, J.A.; Grivas, P. Preexisting Autoimmune Disease: Implications for Immune Checkpoint Inhibitor Therapy in Solid Tumors. J. Natl. Compr. Cancer Netw. 2019, 17, 750–757. [Google Scholar] [CrossRef]
- Haanen, J.; Ernstoff, M.S.; Wang, Y.; Menzies, A.M.; Puzanov, I.; Grivas, P.; Larkin, J.; Peters, S.; Thompson, J.A.; Obeid, M. Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: Review of the literature and personalized risk-based prevention strategy. Ann. Oncol. 2020, 31, 724–744. [Google Scholar] [CrossRef] [PubMed]
- Meserve, J.; Facciorusso, A.; Holmer, A.K.; Annese, V.; Sandborn, W.J.; Singh, S. Systematic review with meta-analysis: Safety and tolerability of immune checkpoint inhibitors in patients with pre-existing inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2021, 53, 374–382. [Google Scholar] [CrossRef]
- Lusa, A.; Alvarez, C.; Saxena Beem, S.; Schwartz, T.A.; Ishizawar, R. Immune-related adverse events in patients with pre-existing autoimmune rheumatologic disease on immune checkpoint inhibitor therapy. BMC Rheumatol. 2022, 6, 64. [Google Scholar] [CrossRef]
- McCarter, K.R.; Wolfgang, T.; Arabelovic, S.; Wang, X.; Yoshida, K.; Banasiak, E.P.; Qian, G.; Kowalski, E.N.; Vanni, K.M.M.; LeBoeuf, N.R.; et al. Mortality and immune-related adverse events after immune checkpoint inhibitor initiation for cancer among patients with pre-existing rheumatoid arthritis: A retrospective, comparative, cohort study. Lancet Rheumatol. 2023, 5, e274–e283. [Google Scholar] [CrossRef]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Maher, V.E.; Fernandes, L.L.; Weinstock, C.; Tang, S.; Agarwal, S.; Brave, M.; Ning, Y.-M.; Singh, H.; Suzman, D.; Xu, J.; et al. Analysis of the Association Between Adverse Events and Outcome in Patients Receiving a Programmed Death Protein 1 or Programmed Death Ligand 1 Antibody. J. Clin. Oncol. 2019, 37, 2730–2737. [Google Scholar] [CrossRef]
- Rogado, J.; Sánchez-Torres, J.M.; Romero-Laorden, N.; Ballesteros, A.I.; Pacheco-Barcia, V.; Ramos-Leví, A.; Arranz, R.; Lorenzo, A.; Gullón, P.; Donnay, O.; et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur. J. Cancer 2019, 109, 21–27. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Kicinski, M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Khattak, A.; Carlino, M.S.; et al. Association Between Immune-Related Adverse Events and Recurrence-Free Survival Among Patients With Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo. JAMA Oncol. 2020, 6, 519. [Google Scholar] [CrossRef]
- Bastacky, M.L.; Wang, H.; Fortman, D.; Rahman, Z.; Mascara, G.P.; Brenner, T.; Najjar, Y.G.; Luke, J.J.; Kirkwood, J.M.; Zarour, H.M.; et al. Immune-Related Adverse Events in PD-1 Treated Melanoma and Impact Upon Anti-Tumor Efficacy: A Real World Analysis. Front. Oncol. 2021, 11, 749064. [Google Scholar] [CrossRef]
- Serna-Higuita, L.M.; Amaral, T.; Forschner, A.; Leiter, U.; Flatz, L.; Seeber, O.; Thomas, I.; Garbe, C.; Eigentler, T.K.; Martus, P. Association between Immune-Related Adverse Events and Survival in 319 Stage IV Melanoma Patients Treated with PD-1-Based Immunotherapy: An Approach Based on Clinical Chemistry. Cancers 2021, 13, 6141. [Google Scholar] [CrossRef] [PubMed]
- Conroy, M.; Naidoo, J. Immune-related adverse events and the balancing act of immunotherapy. Nat. Commun. 2022, 13, 392. [Google Scholar] [CrossRef]
- Watson, A.S.; Goutam, S.; Stukalin, I.; Ewanchuk, B.W.; Sander, M.; Meyers, D.E.; Pabani, A.; Cheung, W.Y.; Heng, D.Y.C.; Cheng, T.; et al. Association of Immune-Related Adverse Events, Hospitalization, and Therapy Resumption With Survival Among Patients With Metastatic Melanoma Receiving Single-Agent or Combination Immunotherapy. JAMA Netw. Open 2022, 5, e2245596. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Nishio, M.; Mok, T.S.K.; Reck, M.; Finley, G.G.; Kaul, M.D.; Yu, W.; Paranthaman, N.; et al. Association of Immune-Related Adverse Events With Efficacy of Atezolizumab in Patients With Non–Small Cell Lung Cancer. JAMA Oncol. 2023, 9, 527. [Google Scholar] [CrossRef] [PubMed]
- Gross, N.D.; Miller, D.M.; Khushalani, N.I.; Divi, V.; Ruiz, E.S.; Lipson, E.J.; Meier, F.; Su, Y.B.; Swiecicki, P.L.; Atlas, J.; et al. Neoadjuvant Cemiplimab for Stage II to IV Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 387, 1557–1568. [Google Scholar] [CrossRef]
- Zuur, C.L.; Breukers, S.; Machuca-Ostos, M.; Boere, T.; Smit, L.; De Boer, J.P.; Cornelissen, S.; Navran, A.; Van Houdt, W.J.; Westerink, B.; et al. Towards organ preservation and cure via 2 infusions of immunotherapy only, in patients normally undergoing extensive and mutilating curative surgery for cutaneous squamous cell carcinoma: An investigator-initiated randomized phase II trial—The MATISSE trial. J. Clin. Oncol. 2023, 41, 9507. [Google Scholar] [CrossRef]
- Uprety, D.; Mandrekar, S.J.; Wigle, D.; Roden, A.C.; Adjei, A.A. Neoadjuvant Immunotherapy for NSCLC: Current Concepts and Future Approaches. J. Thorac. Oncol. 2020, 15, 1281–1297. [Google Scholar] [CrossRef]
- Menzies, A.M.; Amaria, R.N.; Rozeman, E.A.; Huang, A.C.; Tetzlaff, M.T.; van de Wiel, B.A.; Lo, S.; Tarhini, A.A.; Burton, E.M.; Pennington, T.E.; et al. Pathological response and survival with neoadjuvant therapy in melanoma: A pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat. Med. 2021, 27, 301–309. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Bossi, P.; Alberti, A.; Bergamini, C.; Resteghini, C.; Locati, L.D.; Alfieri, S.; Cavalieri, S.; Colombo, E.; Gurizzan, C.; Lorini, L.; et al. Immunotherapy followed by cetuximab in locally advanced/metastatic (LA/M) cutaneous squamous cell carcinomas (cSCC): The I-TACKLE trial. J. Clin. Oncol. 2022, 40, 9520. [Google Scholar] [CrossRef]

| Characteristics—n (%) | SCC (n = 36) |
|---|---|
| Sex | |
| Female | 9 (25) |
| Male | 27 (75) |
| Age | |
| Median (min-max)/IQR | 75.4 (27.9 to 100.1)/(72.4 to 84.4) |
| 27.9 to 69 years of age | 6 (16.7) |
| 70 to 79 years of age | 17 (47.2) |
| 80 to 89 years of age | 8 (22.2) |
| 90 to 100.1 years of age | 5 (13.9) |
| ECOG performance status | |
| 0 | 10 (27.8) |
| 1 | 16 (44.4) |
| 2 | 8 (22.2) |
| 3 | 2 (5.6) |
| Comorbidity | |
| Rheumatological disease on IS drug | 2 (5.6) |
| Solid organ transplant recipient | 2 (5.6) |
| Hematological malignancy | 10 (27.8) |
| EB | 2 (5.6) |
| None | 20 (55.6) |
| Primary site | |
| Head and neck | 25 (69.4) |
| Limbs | 6 (16.7) |
| Torso | 2 (5.6) |
| Unknown | 3 (8.3) |
| Primary treatment | |
| Surgery | 21 (58.3) |
| Surgery + adjuvant RT | 9 (25) |
| RT alone | 3 (8.3) |
| ICI | 2 (5.5) |
| Other systemic therapy | 1 (2.8) |
| Extent of disease | |
| Locally advanced/Unresectable | 26 (72.2) |
| Distant metastasis | 10 (27.8) |
| AJCC clinical stage at ICI start | |
| Recurrent Stage I | 1 (2.8) |
| Recurrent Stage II | 3 (8.3) |
| Recurrent Stage III | 7 (19.4) |
| Stage IV at presentation | 4 (11.1) |
| Recurrent stage IV | 21 (58.3) |
| ICI line of therapy | |
| First-line | 35 (97.2) |
| Second line | 1 (2.8) |
| Concomitant radiation therapy | |
| No | 28 (77.8) |
| Concurrent to ICI at ICI start | 3 (8.3) |
| Completed in the 2 weeks pre-start of ICI | 1 (2.8) |
| Concurrent to ICI for oligoprogression of disease | 4 (11.1) |
| Disease progression | 8 (30.8%) |
| Adverse reactions | 5 (19.2%) |
| Achieved maximum benefit | 5 (19.2%) |
| Maximum number of doses | 3 (11.5%) |
| Other | 3 (11.5%) |
| Death | 2 (7.7%) |
| Variable | HR | 95% CI | p Value | |
|---|---|---|---|---|
| Sex | Female (ref.) | |||
| Male | 0.796 | 0.28–2.65 | 0.669 | |
| Age | <75 years (ref.) | |||
| ≥75 years | 1.073 | 0.39–2.91 | 0.890 | |
| ECOG | 0–1 (ref.) | |||
| ≥2 | 1.043 | 0.37–2.96 | 0.937 | |
| Grade 1–2 toxicity | No (ref.) | |||
| Yes | 0.357 | 0.14–0.94 | 0.037 | |
| Grade ≥ 3 toxicity | Yes (ref.) | |||
| No | 0.210 | 0.06–0.65 | 0.006 | |
| Scenario | Localized (ref.) | |||
| Metastatic | 1.645 | 0.61–4.46 | 0.328 | |
| Comorbidities | No (ref.) | |||
| Yes | 0.930 | 0.36–2.41 | 0.881 | |
| BOR | Responders (ref.) | 2.996 | ||
| Non-responders | 1.82–4.93 | 0.000002 | ||
| Variable | HR | 95% CI | p Value | |
|---|---|---|---|---|
| Sex | Female (ref.) | |||
| Male | 0.835 | 0.933 | 0.35–4.95 | |
| Age | <75 years (ref.) | |||
| ≥75 years | 0.22–3.15 | 1.185 | 0.695 | |
| ECOG | 0–1 (ref.) | |||
| ≥2 | 0.790 | 0.35–3.98 | 0.291 | |
| Grade 1–2 toxicity | Yes (ref.) | |||
| No | 1.054 | 0.784 | 0.07–1.18 | |
| Grade ≥ 3 toxicity | Yes (ref.) | |||
| No | 0.31–3.62 | 1.306 | 0.085 | |
| Scenario | Localized (ref.) | |||
| Metastatic | 3.896 | 1.10–13.87 | 0.036 | |
| Comorbidities | No (ref.) | |||
| Yes | 0.487 | 0.15–1.63 | 0.244 | |
| BOR | Responders (ref.) | |||
| Non-responders | 12.85 | 2.67–61.84 | 0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch Hein, E.C.; Vilbert, M.; Hirsch, I.; Fernando Ribeiro, M.; Muniz, T.P.; Fournier, C.; Abdulalem, K.; Saldanha, E.F.; Martinez, E.; Spreafico, A.; et al. Immune Checkpoint Inhibitors in Advanced Cutaneous Squamous Cell Carcinoma: Real-World Experience from a Canadian Comprehensive Cancer Centre. Cancers 2023, 15, 4312. https://doi.org/10.3390/cancers15174312
Koch Hein EC, Vilbert M, Hirsch I, Fernando Ribeiro M, Muniz TP, Fournier C, Abdulalem K, Saldanha EF, Martinez E, Spreafico A, et al. Immune Checkpoint Inhibitors in Advanced Cutaneous Squamous Cell Carcinoma: Real-World Experience from a Canadian Comprehensive Cancer Centre. Cancers. 2023; 15(17):4312. https://doi.org/10.3390/cancers15174312
Chicago/Turabian StyleKoch Hein, Erica C., Maysa Vilbert, Ian Hirsch, Mauricio Fernando Ribeiro, Thiago P. Muniz, Cynthia Fournier, Khaled Abdulalem, Erick F. Saldanha, Erika Martinez, Anna Spreafico, and et al. 2023. "Immune Checkpoint Inhibitors in Advanced Cutaneous Squamous Cell Carcinoma: Real-World Experience from a Canadian Comprehensive Cancer Centre" Cancers 15, no. 17: 4312. https://doi.org/10.3390/cancers15174312
APA StyleKoch Hein, E. C., Vilbert, M., Hirsch, I., Fernando Ribeiro, M., Muniz, T. P., Fournier, C., Abdulalem, K., Saldanha, E. F., Martinez, E., Spreafico, A., Hogg, D. H., Butler, M. O., & Saibil, S. D. (2023). Immune Checkpoint Inhibitors in Advanced Cutaneous Squamous Cell Carcinoma: Real-World Experience from a Canadian Comprehensive Cancer Centre. Cancers, 15(17), 4312. https://doi.org/10.3390/cancers15174312






