Long-Term Periodic and Conditional Survival Trends in Prostate, Testicular, and Penile Cancers in the Nordic Countries, Marking Timing of Improvements
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
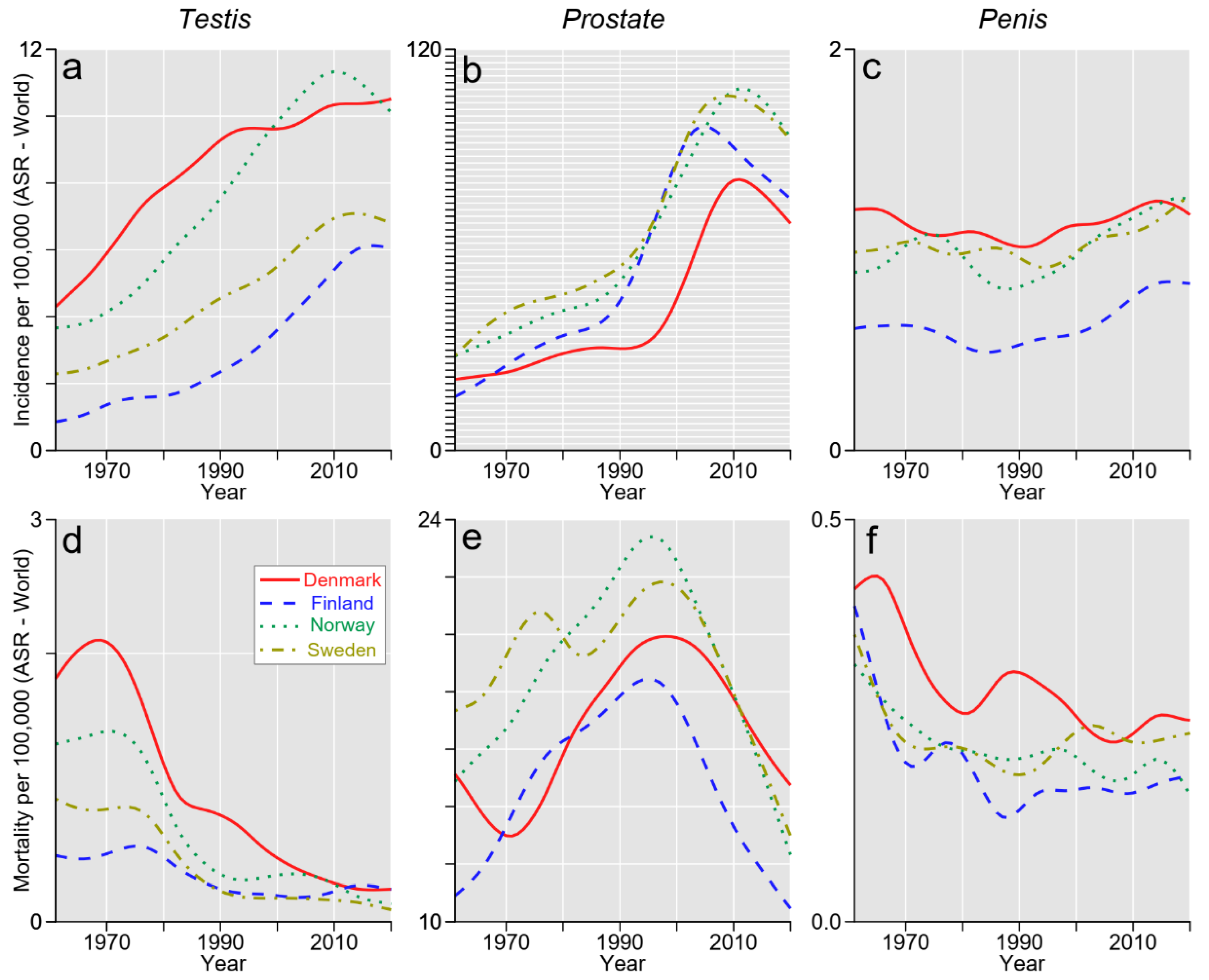
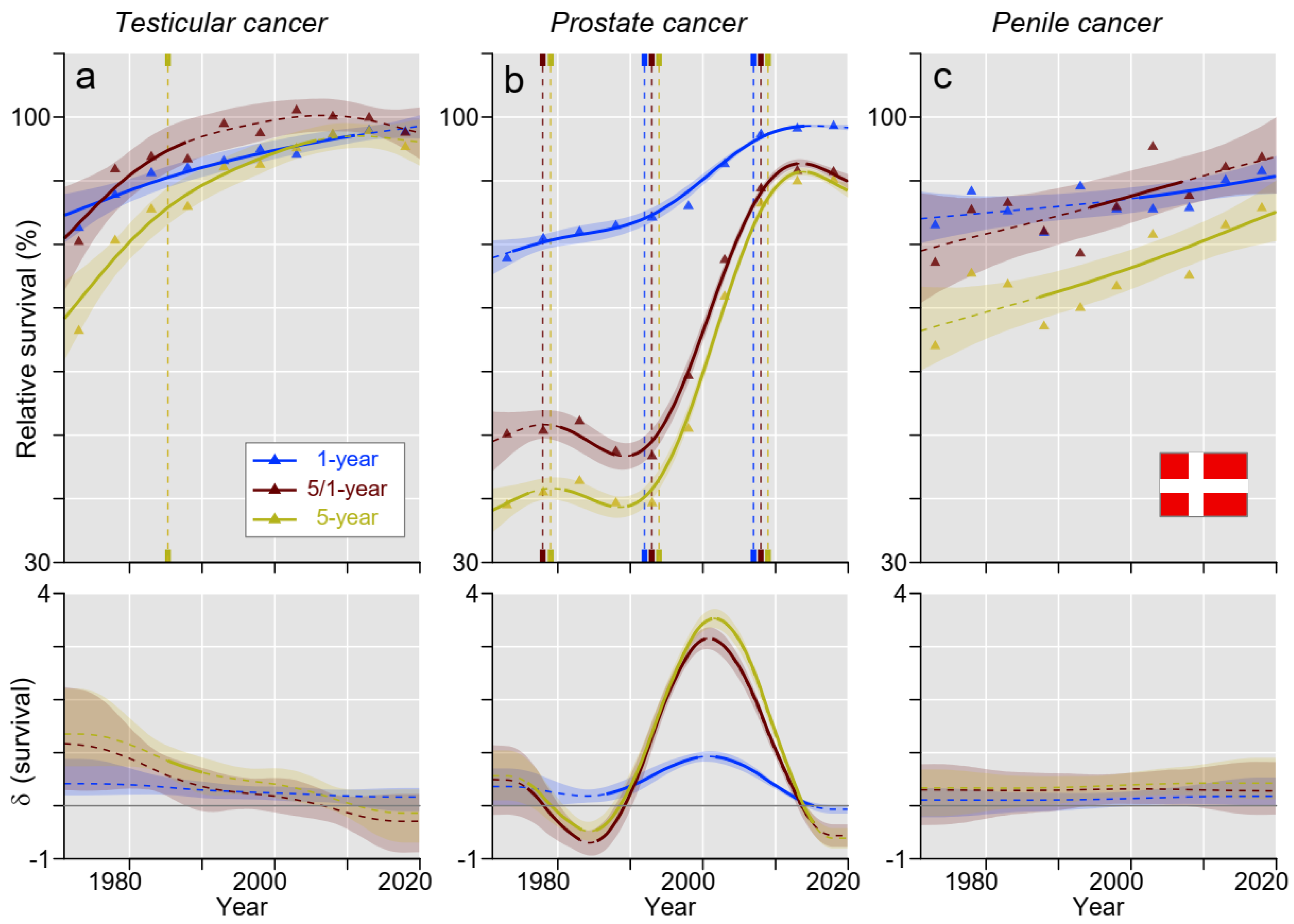
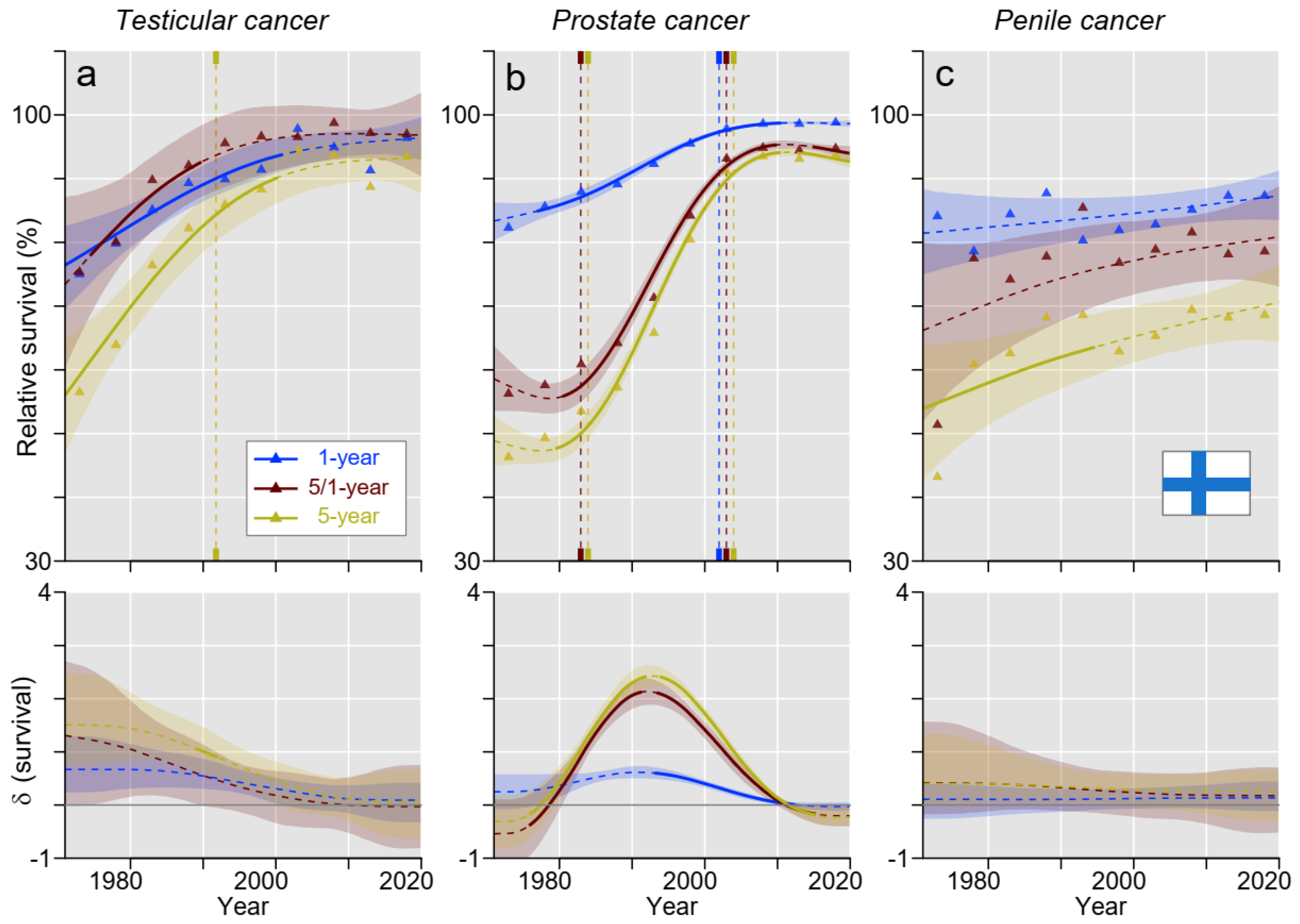
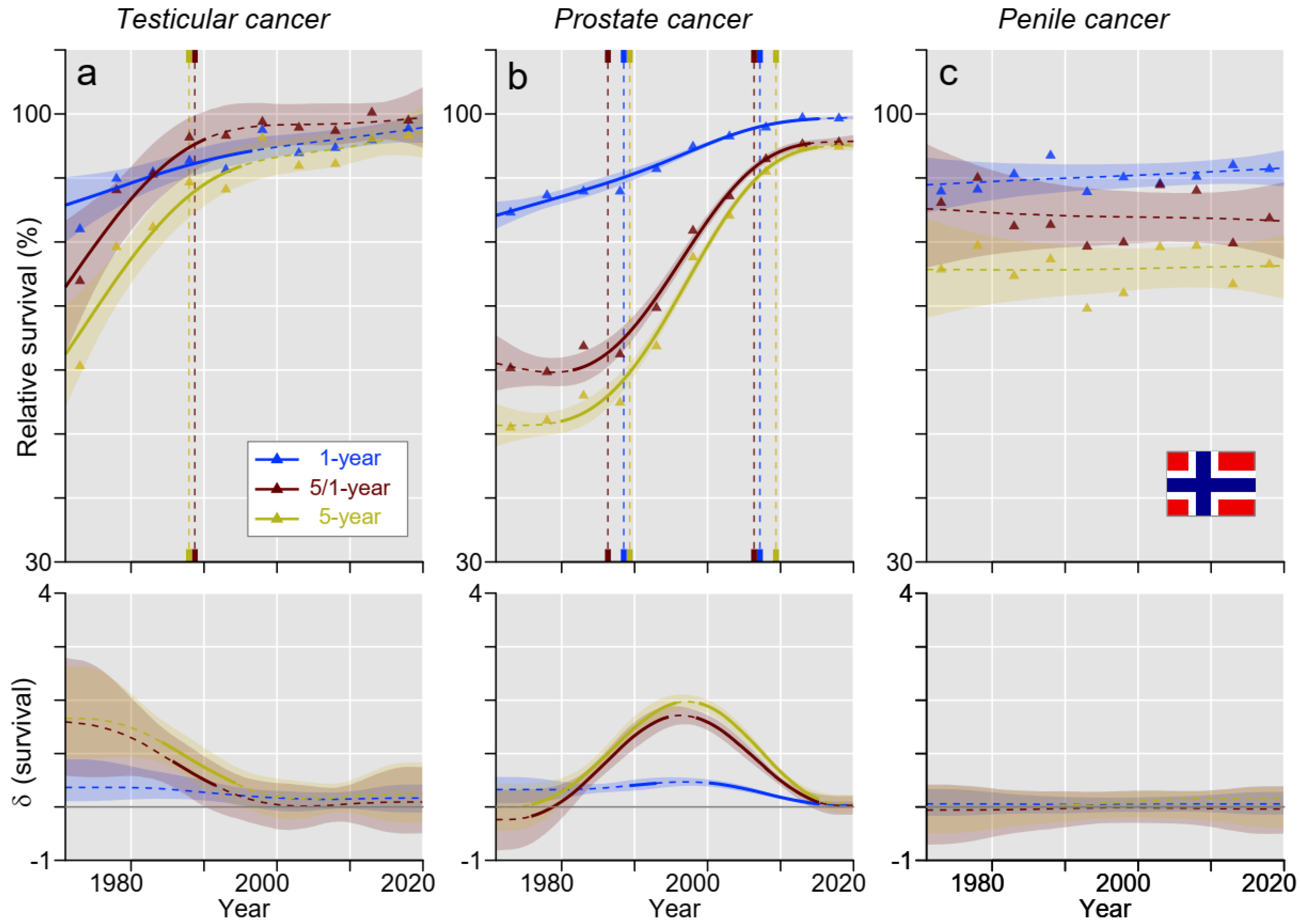
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [PubMed]
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.S.; Bannon, F.; Ahn, J.V.; Johnson, C.J.; Bonaventure, A.; et al. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015, 385, 977–1010. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Försti, A.; Liska, V.; Kanerva, A.; Hemminki, O.; Hemminki, A. Long-term survival trends in solid cancers in the Nordic countries marking timing of improvements. Int. J. Cancer 2023, 152, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, J.; Försti, A.; Hemminki, A.; Hemminki, K. Survival trends in solid cancers in the Nordic countries through 50 years. Eur. J. Cancer 2022, 175, 77–85. [Google Scholar] [CrossRef]
- Wong, K.F.; Lambert, P.C.; Mozumder, S.I.; Broggio, J.; Rutherford, M.J. Conditional crude probabilities of death for English cancer patients. Br. J. Cancer 2019, 121, 883–889. [Google Scholar] [CrossRef]
- Ellis, L.; Woods, L.M.; Estève, J.; Eloranta, S.; Coleman, M.P.; Rachet, B. Cancer incidence, survival and mortality: Explaining the concepts. Int. J. Cancer 2014, 135, 1774–1782. [Google Scholar] [CrossRef]
- Bower, H.; Björkholm, M.; Dickman, P.W.; Höglund, M.; Lambert, P.C.; Andersson, T.M. Life Expectancy of Patients With Chronic Myeloid Leukemia Approaches the Life Expectancy of the General Population. J. Clin. Oncol. 2016, 34, 2851–2857. [Google Scholar] [CrossRef]
- Hanna, N.; Einhorn, L.H. Testicular cancer: A reflection on 50 years of discovery. J. Clin. Oncol. 2014, 32, 3085–3092. [Google Scholar] [CrossRef]
- Luyendijk, M.; Visser, O.; Blommestein, H.M.; de Hingh, I.H.J.T.; Hoebers, F.J.P.; Jager, A.; Sonke, G.S.; de Vries, E.G.E.; Groot, C.A.U.-D.; Siesling, S. Changes in survival in de novo metastatic cancer in an era of new medicines. J. Natl. Cancer Inst. 2023, 115, 628–635. [Google Scholar] [CrossRef]
- Maso, L.D.; Panato, C.; Tavilla, A.; Guzzinati, S.; Serraino, D.; Mallone, S.; Botta, L.; Boussari, O.; Capocaccia, R.; Colonna, M.; et al. Cancer cure for 32 cancer types: Results from the EUROCARE-5 study. Int. J. Epidemiol. 2020, 49, 1517–1525. [Google Scholar] [CrossRef]
- Lundberg, F.E.; Andersson, T.M.-L.; Lambe, M.; Engholm, G.; Mørch, L.S.; Johannesen, T.B.; Virtanen, A.; Pettersson, D.; Ólafsdóttir, E.J.; Birgisson, H.; et al. Trends in cancer survival in the Nordic countries 1990–2016: The NORDCAN survival studies. Acta Oncol. 2020, 59, 1266–1274. [Google Scholar] [CrossRef]
- Hemminki, K.; Försti, A.; Hemminki, A. Survival in colon and rectal cancers in Finland and Sweden through 50 years. BMJ Open Gastroenterol. 2021, 8, e000644. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.F.; Rumgay, H.; Dunlop, C.; Ryan, M.; Quartly, F.; Cox, A.; Deas, A.; Elliss-Brookes, L.; Gavin, A.; Hounsome, L.; et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br. J. Cancer 2018, 118, 1130–1141. [Google Scholar] [CrossRef]
- Bräuner, E.V.; Lim, Y.-H.; Koch, T.; Uldbjerg, C.S.; Gregersen, L.S.; Pedersen, M.K.; Frederiksen, H.; Petersen, J.H.; Coull, B.A.; Andersson, A.-M.; et al. Endocrine Disrupting Chemicals and Risk of Testicular Cancer: A Systematic Review and Meta-analysis. J. Clin. Endocrinol. Metab. 2021, 106, e4834–e4860. [Google Scholar] [CrossRef] [PubMed]
- Brookman-May, S.D.; Campi, R.; Henríquez, J.D.S.; Klatte, T.; Langenhuijsen, J.F.; Brausi, M.; Linares-Espinos, E.; Volpe, A.; Marszalek, M.; Akdogan, B.; et al. Latest Evidence on the Impact of Smoking, Sports, and Sexual Activity as Modifiable Lifestyle Risk Factors for Prostate Cancer Incidence, Recurrence, and Progression: A Systematic Review of the Literature by the European Association of Urology Section of Oncological Urology (ESOU). Eur. Urol. Focus 2019, 5, 756–787. [Google Scholar]
- Engholm, G.; Ferlay, J.; Christensen, N.; Bray, F.; Gjerstorff, M.L.; Klint, A.; Køtlum, J.E. NORDCAN—A Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010, 49, 725–736. [Google Scholar] [CrossRef]
- Pukkala, E.; Engholm, G.; Schmidt, L.K.H.; Storm, H.; Khan, S.; Lambe, M.; Pettersson, D.; Ólafsdóttir, E.; Tryggvadóttir, L.; Hakanen, T.; et al. Nordic Cancer Registries—An overview of their procedures and data comparability. Acta Oncol. 2018, 57, 440–455. [Google Scholar] [CrossRef]
- Larønningen, S.; Ferlay, J.; Beydogan, H.; Bray, F.; Engholm, G.; Ervik, M.; Gulbrandsen, J.; Hansen, H.L.; Hansen, H.M.; Johannsen, T.B.; et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries; Association of the Nordic Cancer Registries; Cancer Registry of Norway: Oslo, Norway, 2022. [Google Scholar]
- Storm, H.H.; Klint, A.; Tryggvadóttir, L.; Gislum, M.; Engholm, G.; Bray, F.; Hakulinen, T. Trends in the survival of patients diagnosed with malignant neoplasms of lymphoid, haematopoietic, and related tissue in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol. 2010, 49, 694–712. [Google Scholar] [CrossRef]
- Engholm, G.; Gislum, M.; Bray, F.; Hakulinen, T. Trends in the survival of patients diagnosed with cancer in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol. 2010, 49, 545–560. [Google Scholar] [CrossRef]
- Tichanek, F.; Försti, A.; Liska, V.; Hemminki, A.; Hemminki, K. Survival in Colon, Rectal and Small Intestinal Cancers in the Nordic Countries through a Half Century. Cancers 2023, 15, 991. [Google Scholar] [CrossRef]
- Bürkner, P. An R package for Bayesian multilevel models using Stan. J. Stat. Softw. 2017, 80, 1–28. [Google Scholar] [CrossRef]
- Bürkner, P. Advanced Bayesian multilevel modeling with the R package (brms). R J. 2018, 10, 395–411. [Google Scholar] [CrossRef]
- Carpenter, B.; Gelman, A.; Hoffman, M.D.; Lee, D.; Goodrich, B.; Betancourt, M.; Brubaker, M.; Guo, J.; Li, P.; Riddell, A. Stan: A probabilistic programming language. J. Stat. Softw. 2017, 76, 1. [Google Scholar] [CrossRef] [PubMed]
- Fosså, S.D.; Aass, N.; Kaalhus, O. Testicular cancer in young Norwegians. J. Surg. Oncol. 1988, 39, 43–63. [Google Scholar] [CrossRef]
- Hellesnes, R.; Myklebust, T.Å.; Fosså, S.D.; Bremnes, R.M.; Karlsdottir, Á.; Kvammen, Ø.; Tandstad, T.; Wilsgaard, T.; Negaard, H.F.S.; Haugnes, H.S. Testicular Cancer in the Cisplatin Era: Causes of Death and Mortality Rates in a Population-Based Cohort. J. Clin. Oncol. 2021, 39, 3561–3573. [Google Scholar] [CrossRef]
- Zhang, L.; Hemminki, O.; Chen, T.; Yu, H.; Zheng, G.; Chattopadhyay, S.; Försti, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. Second cancers and causes of death in patients with testicular cancer in Sweden. PLoS ONE 2019, 14, e0214410. [Google Scholar] [CrossRef]
- Canellos, G.P.; Rosenberg, S.A.; Friedberg, J.W.; Lister, T.A.; Devita, V.T. Treatment of Hodgkin lymphoma: A 50-year perspective. J. Clin. Oncol. 2014, 32, 163–168. [Google Scholar] [CrossRef]
- Sud, A.; Thomsen, H.; Sundquist, K.; Houlston, R.S.; Hemminki, K. Risk of second cancer in Hodgkin lymphoma survivors and the influence of family history. J. Clin. Oncol. 2017, 35, 1584–1590. [Google Scholar] [CrossRef]
- Møller, M.H.; Kristiansen, I.S.; Beisland, C.; Rørvik, J.; Støvring, H. Trends in stage-specific incidence of prostate cancer in Norway, 1980–2010: A population-based study. BJU Int. 2016, 118, 547–555. [Google Scholar] [CrossRef]
- Hemminki, K.; Rawal, R.; Bermejo, J.L. Prostate cancer screening, changing age-specific incidence trends and implications on familial risk. Int. J. Cancer 2005, 113, 312–315. [Google Scholar] [CrossRef]
- Welch, H.G.; Black, W.C. Overdiagnosis in Cancer. J. Natl. Cancer Inst. 2010, 102, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Welch, H.G.; Kramer, B.S.; Black, W.C. Epidemiologic Signatures in Cancer. N. Engl. J. Med. 2019, 381, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Bratt, O.; Carlsson, S.; Fransson, P.; Kindblom, J.; Stranne, J.; Karlsson, C.T. The Swedish national guidelines on prostate cancer, part 2: Recurrent, metastatic and castration resistant disease. Scand. J. Urol. 2022, 56, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Bratt, O.; Carlsson, S.; Fransson, P.; Thellenberg Karlsson, C.; Stranne, J.; Kindblom, J. The Swedish national guidelines on prostate cancer, part 1: Early detection, diagnostics, staging, patient support and primary management of non-metastatic disease. Scand. J. Urol. 2022, 56, 265–273. [Google Scholar] [CrossRef]
- Ulvskog, E.; Drevin, L.; Persson, E.K.; Lambe, M.; Kirrander, P.; Ahlgren, J. Oncological therapy to Swedish men with metastatic penile cancer 2000–2015. Acta Oncol. 2021, 60, 42–49. [Google Scholar] [CrossRef]
- Skeppner, E.; Andersson, S.O.; Johansson, J.E.; Windahl, T. Initial symptoms and delay in patients with penile carcinoma. Scand. J. Urol. Nephrol. 2012, 46, 319–325. [Google Scholar] [CrossRef]
- Baekhøj Kortsen, D.; Predbjørn Krarup, K.; Jakobsen, J.K. DaPeCa-9—Cohabitation and socio-economic conditions predict penile cancer-specific survival in a national clinical study from Denmark. Scand. J. Urol. 2021, 55, 486–490. [Google Scholar] [CrossRef]
- Hemminki, K.; Kanerva, A.; Försti, A.; Hemminki, A. Cervical, vaginal and vulvar cancer incidence and survival trends in Denmark, Finland, Norway and Sweden with implications to treatment. BMC Cancer 2022, 22, 456. [Google Scholar] [CrossRef]
- Van Poppel, H.; Watkin, N.A.; Osanto, S.; Moonen, L.; Horwich, A.; Kataja, V. Penile cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. 6), vi115–vi124. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.A.; Campbell, M.T.; Xie, W.; Farah, S.; Bilen, M.A.; Schmidt, A.L.; Sonpavde, G.P.; Kilbridge, K.L.; Choudhury, A.D.; Mortazavi, A.; et al. Results of a multicenter, phase 2 study of nivolumab and ipilimumab for patients with advanced rare genitourinary malignancies. Cancer 2021, 127, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, J.K.; Krarup, K.P.; Kirrander, P.; Håkansson, U.; Kaipia, A.; Perttila, I.; Axcrona, K.; Torkelsen, T.K.; Hilmarsson, R.; Jensen, J.B. Penile cancer in Scandinavia: Current practice and future perspectives. Scand. J. Urol. 2016, 50, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, O.R.; Albersen, M.; Parnham, A.; Protzel, C.; Pettaway, C.A.; Ayres, B.; Antunes-Lopes, T.; Barreto, L.; Campi, R.; Crook, J.; et al. European Association of Urology-American Society of Clinical Oncology Collaborative Guideline on Penile Cancer: 2023 Update. Eur. Urol. 2023, 83, 548–560. [Google Scholar] [CrossRef] [PubMed]
- You, E.L.; Henry, M.; Zeitouni, A.G. Human papillomavirus-associated oropharyngeal cancer: Review of current evidence and management. Curr. Oncol. 2019, 26, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, F.B.; Røder, M.A.; Jakobsen, H.; Langkilde, N.C.; Borre, M.; Jakobsen, E.B.; Frey, A.; Lund, L.; Lunden, D.; Dahl, C.; et al. Active Surveillance Versus Radical Prostatectomy in Favorable-risk Localized Prostate Cancer. Clin. Genitourin. Cancer 2019, 17, e814–e821. [Google Scholar] [CrossRef]
- Hemminki, K.; Li, X.; Czene, K. Cancer risks in first generation immigrants to Sweden. Int. J. Cancer 2002, 99, 218–228. [Google Scholar] [CrossRef]
- Westerberg, M.; Franck Lissbrant, I.; Damber, J.E.; Robinson, D.; Garmo, H.; Stattin, P. Temporal changes in survival in men with de novo metastatic prostate cancer: Nationwide population-based study. Acta Oncol. 2020, 59, 106–111. [Google Scholar] [CrossRef]
- Storås, A.H.; Fosså, S.D.; Ursin, G.; Andreassen, B.K. Survival trends for patients with primary metastatic prostate cancer before and after the introduction of new antitumor drugs. Prostate Cancer Prostatic Dis. 2023, 26, 53–58. [Google Scholar] [CrossRef]
- Kirrander, P.; Sherif, A.; Friedrich, B.; Lambe, M.; Håkansson, U. Swedish National Penile Cancer Register: Incidence, tumour characteristics, management and survival. BJU Int. 2016, 117, 287–292. [Google Scholar] [CrossRef]
- Kvammen, Ø.; Myklebust, T.; Solberg, A.; Møller, B.; Klepp, O.H.; Fosså, S.D.; Tandstad, T. Long-term Relative Survival after Diagnosis of Testicular Germ Cell Tumor. Cancer Epidemiol. Biomark. Prev. 2016, 25, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Liu, H.; Sundquist, J. Second cancers after testicular cancer diagnosed after 1980 in Sweden. Ann. Oncol. 2010, 21, 1546–1551. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, F.E.; Birgisson, H.; Johannesen, T.B.; Engholm, G.; Virtanen, A.; Pettersson, D.; Ólafsdóttir, E.J.; Lambe, M.; Lambert, P.C.; Mørch, L.S.; et al. Survival trends in patients diagnosed with colon and rectal cancer in the nordic countries 1990–2016: The NORDCAN survival studies. Eur. J. Cancer 2022, 172, 76–84. [Google Scholar] [CrossRef] [PubMed]
| Population | Prostate | Testis | Penis | |||
|---|---|---|---|---|---|---|
| N | Age | N | Age | N | Age | |
| Denmark | 122,150 | 69 | 13,141 | 38 | 2500 | 70 |
| Finland | 140,571 | 71 | 4507 | 34 | 1197 | 69 |
| Norway | 145,648 | 69 | 10,278 | 36 | 1913 | 70 |
| Sweden | 336,314 | 70 | 11,966 | 38 | 4196 | 71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tichanek, F.; Försti, A.; Hemminki, A.; Hemminki, O.; Hemminki, K. Long-Term Periodic and Conditional Survival Trends in Prostate, Testicular, and Penile Cancers in the Nordic Countries, Marking Timing of Improvements. Cancers 2023, 15, 4261. https://doi.org/10.3390/cancers15174261
Tichanek F, Försti A, Hemminki A, Hemminki O, Hemminki K. Long-Term Periodic and Conditional Survival Trends in Prostate, Testicular, and Penile Cancers in the Nordic Countries, Marking Timing of Improvements. Cancers. 2023; 15(17):4261. https://doi.org/10.3390/cancers15174261
Chicago/Turabian StyleTichanek, Filip, Asta Försti, Akseli Hemminki, Otto Hemminki, and Kari Hemminki. 2023. "Long-Term Periodic and Conditional Survival Trends in Prostate, Testicular, and Penile Cancers in the Nordic Countries, Marking Timing of Improvements" Cancers 15, no. 17: 4261. https://doi.org/10.3390/cancers15174261
APA StyleTichanek, F., Försti, A., Hemminki, A., Hemminki, O., & Hemminki, K. (2023). Long-Term Periodic and Conditional Survival Trends in Prostate, Testicular, and Penile Cancers in the Nordic Countries, Marking Timing of Improvements. Cancers, 15(17), 4261. https://doi.org/10.3390/cancers15174261









