Expression of Stem Cell Markers in High-LET Space Radiation-Induced Intestinal Tumors in Apc1638N/+ Mouse Intestine
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiment and Collection of the Samples
2.2. Immunohistochemistry
2.3. Fluorescence Microscopy
2.4. Imaging, Quantification, and Statistical Analysis
3. Results
3.1. LGR5 Expression in 56Fe-Irradiated Mouse Intestinal Tumors and Normal Mucosa
3.2. CD133 and MSI1 Expression in Tumors
3.3. Detection of DCLK1 Expression in Irradiated Intestinal Tumors
3.4. Assessment of ALDH1 and BMI1 Expression in Low- and High-LET Induced Tumors
3.5. Evaluation of β-Catenin Expression and Nuclear Localization in HZE-Induced Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahmanifard, F.; de Wet, W.C.; Schwadron, N.A.; Owens, M.J.; Jordan, A.P.; Wilson, J.K.; Joyce, C.J.; Spence, H.E.; Smith, C.W.; Townsend, L.W. Galactic Cosmic Radiation in the Interplanetary Space Through a Modern Secular Minimum. Space Weather 2020, 18, e2019SW002428. [Google Scholar] [CrossRef]
- Asaithamby, A.; Uematsu, N.; Chatterjee, A.; Story, M.D.; Burma, S.; Chen, D.J. Repair of HZE-particle-induced DNA double-strand breaks in normal human fibroblasts. Radiat. Res. 2008, 169, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Lawson, G.W.; Ripperdan, R.; Ortiz, L.; Luderer, U. Charged-Iron-Particles Found in Galactic Cosmic Rays are Potent Inducers of Epithelial Ovarian Tumors. Radiat. Res. 2018, 190, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.; Suman, S.; Kallakury, B.V.; Fornace, A.J. Heavy ion radiation exposure triggered higher intestinal tumor frequency and greater β-catenin activation than γ radiation in APC(Min/+) mice. PLoS ONE 2013, 8, e59295. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Suman, S.; Fornace, A.J., Jr.; Datta, K. Space radiation triggers persistent stress response, increases senescent signaling, and decreases cell migration in mouse intestine. Proc. Natl. Acad. Sci. USA 2018, 115, E9832–E9841. [Google Scholar] [CrossRef]
- Shuryak, I.; Fornace, A.J.; Datta, K.; Suman, S.; Kumar, S.; Sachs, R.K.; Brenner, D.J. Scaling Human Cancer Risks from Low LET to High LET when Dose-Effect Relationships are Complex. Radiat. Res. 2017, 187, 476–482. [Google Scholar] [CrossRef]
- Suman, S.; Kumar, S.; Moon, B.H.; Strawn, S.J.; Thakor, H.; Fan, Z.; Shay, J.W.; Fornace, A.J.; Datta, K. Relative Biological Effectiveness of Energetic Heavy Ions for Intestinal Tumorigenesis Shows Male Preponderance and Radiation Type and Energy Dependence in APC(1638N/+) Mice. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 131–138. [Google Scholar] [CrossRef]
- Kumar, S. Intestinal stem cells acquire premature senescence and senescence associated secretory phenotype concurrent with persistent DNA damage after heavy ion radiation in mice. Aging 2019, 11, 1445–1458. [Google Scholar] [CrossRef]
- Suman, S.; Kumar, S.; Fornace, A.J.; Datta, K. Decreased RXRα is Associated with Increased β-Catenin/TCF4 in (56)Fe-Induced Intestinal Tumors. Front. Oncol. 2015, 5, 218. [Google Scholar] [CrossRef]
- Li, X.B.; Yang, G.; Zhu, L.; Tang, Y.L.; Zhang, C.; Ju, Z.; Yang, X.; Teng, Y. Gastric Lgr5(+) stem cells are the cellular origin of invasive intestinal-type gastric cancer in mice. Cell Res. 2016, 26, 838–849. [Google Scholar] [CrossRef]
- Yanai, H.; Atsumi, N.; Tanaka, T.; Nakamura, N.; Komai, Y.; Omachi, T.; Tanaka, K.; Ishigaki, K.; Saiga, K.; Ohsugi, H.; et al. Intestinal cancer stem cells marked by Bmi1 or Lgr5 expression contribute to tumor propagation via clonal expansion. Sci. Rep. 2017, 7, 41838. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W. Determining if telomeres matter in colon cancer initiation or progression. J. Natl. Cancer Inst. 2013, 105, 1166–1168. [Google Scholar] [CrossRef]
- Hirata, A.; Hatano, Y.; Niwa, M.; Hara, A.; Tomita, H. Heterogeneity in Colorectal Cancer Stem Cells. Cancer Prev. Res. (Phila) 2019, 12, 413–420. [Google Scholar] [CrossRef]
- Collins, T.J. ImageJ for microscopy. Biotechniques 2007, 43, 25–30. [Google Scholar] [CrossRef]
- Crowe, A.R.; Yue, W. Semi-quantitative Determination of Protein Expression using Immunohistochemistry Staining and Analysis: An Integrated Protocol. Bio Protoc. 2019, 9, e3465. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.H.; Hynes, M.J.; Zhang, T.; Ginestier, C.; Dontu, G.; Appelman, H.; Fields, J.Z.; Wicha, M.S.; Boman, B.M. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009, 69, 3382–3389. [Google Scholar] [CrossRef]
- Boman, B.M.; Walters, R.; Fields, J.Z.; Kovatich, A.J.; Zhang, T.; Isenberg, G.A.; Goldstein, S.D.; Palazzo, J.P. Colonic crypt changes during adenoma development in familial adenomatous polyposis: Immunohistochemical evidence for expansion of the crypt base cell population. Am. J. Pathol. 2004, 165, 1489–1498. [Google Scholar] [CrossRef]
- Xu, L.; Lin, W.; Wen, L.; Li, G. Lgr5 in cancer biology: Functional identification of Lgr5 in cancer progression and potential opportunities for novel therapy. Stem Cell Res. Ther. 2019, 10, 219. [Google Scholar] [CrossRef]
- Jang, B.G.; Kim, H.S.; Chang, W.Y.; Bae, J.M.; Kim, W.H.; Kang, G.H. Expression Profile of LGR5 and Its Prognostic Significance in Colorectal Cancer Progression. Am. J. Pathol. 2018, 188, 2236–2250. [Google Scholar] [CrossRef]
- Wu, C.; Qiu, S.; Lu, L.; Zou, J.; Li, W.F.; Wang, O.; Zhao, H.; Wang, H.; Tang, J.; Chen, L.; et al. RSPO2-LGR5 signaling has tumour-suppressive activity in colorectal cancer. Nat. Commun. 2014, 5, 3149. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Geng, L.; Wang, D.; Yi, H.; Talmon, G.; Wang, J. R-Spondin1/LGR5 Activates TGFβ Signaling and Suppresses Colon Cancer Metastasis. Cancer Res. 2017, 77, 6589–6602. [Google Scholar] [CrossRef] [PubMed]
- Tirado, F.R.; Bhanja, P.; Castro-Nallar, E.; Olea, X.D.; Salamanca, C.; Saha, S. Radiation-induced toxicity in rectal epithelial stem cell contributes to acute radiation injury in rectum. Stem Cell Res. Ther. 2021, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.I.; Ghiani, M.; Lefort, A.; Libert, F.; Strollo, S.; Vassart, G. LGR5 deficiency deregulates Wnt signaling and leads to precocious Paneth cell differentiation in the fetal intestine. Dev. Biol. 2009, 331, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.; Kljavin, N.M.; Ybarra, R.; de Sauvage, F.J. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 2014, 14, 149–159. [Google Scholar] [CrossRef]
- Kawamoto, A.; Tanaka, K.; Saigusa, S.; Toiyama, Y.; Morimoto, Y.; Fujikawa, H.; Iwata, T.; Matsushita, K.; Yokoe, T.; Yasuda, H.; et al. Clinical significance of radiation-induced CD133 expression in residual rectal cancer cells after chemoradiotherapy. Exp. Ther. Med. 2012, 3, 403–409. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saigusa, S.; Tanaka, K.; Toiyama, Y.; Yokoe, T.; Okugawa, Y.; Kawamoto, A.; Yasuda, H.; Morimoto, Y.; Fujikawa, H.; Inoue, Y.; et al. Immunohistochemical features of CD133 expression: Association with resistance to chemoradiotherapy in rectal cancer. Oncol. Rep. 2010, 24, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Sheng, W.Q.; Du, X. CD133: A cancer stem cells marker, is used in colorectal cancers. World J. Gastroenterol. 2013, 19, 2603–2611. [Google Scholar] [CrossRef]
- Moon, B.S.; Jeong, W.J.; Park, J.; Kim, T.I.; Min, D.S.; Choi, K.Y. Role of oncogenic K-Ras in cancer stem cell activation by aberrant Wnt/β-catenin signaling. J. Natl. Cancer Inst. 2014, 106, djt373. [Google Scholar] [CrossRef]
- Khalek, F.J.A.; Gallicano, G.I.; Mishra, L. Colon cancer stem cells. Gastrointest. Cancer Res. GCR 2010, 1, S16–S23. [Google Scholar]
- Forouzanfar, M.; Lachinani, L.; Dormiani, K.; Nasr-Esfahani, M.H.; Gure, A.O.; Ghaedi, K. Intracellular functions of RNA-binding protein, Musashi1, in stem and cancer cells. Stem Cell Res. Ther. 2020, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Chandrakesan, P.; May, R.; Weygant, N.; Qu, D.; Berry, W.L.; Sureban, S.M.; Ali, N.; Rao, C.; Huycke, M.; Bronze, M.S.; et al. Intestinal tuft cells regulate the ATM mediated DNA Damage response via Dclk1 dependent mechanism for crypt restitution following radiation injury. Sci. Rep. 2016, 6, 37667. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Popov, V.L.; O’Connell, M.R.; Stevenson, H.L.; Lee, B.S.; Obeid, R.A.; Luthra, G.K.; Singh, P. A novel antibody against cancer stem cell biomarker, DCLK1-S, is potentially useful for assessing colon cancer risk after screening colonoscopy. Lab. Investig. 2017, 97, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, C.; Mahdavinezhad, A.; Saidijam, M.; Bahreini, F.; Sedighi Pashaki, A.; Gholami, M.H.; Najafi, R. DCLK1 Inhibition Sensitizes Colorectal Cancer Cells to Radiation Treatment. Int. J. Mol. Cell Med. 2021, 10, 23–33. [Google Scholar]
- Westphalen, C.B.; Asfaha, S.; Hayakawa, Y.; Takemoto, Y.; Lukin, D.J.; Nuber, A.H.; Brandtner, A.; Setlik, W.; Remotti, H.; Muley, A.; et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J. Clin. Investig. 2014, 124, 1283–1295. [Google Scholar] [CrossRef]
- Facchino, S.; Abdouh, M.; Chatoo, W.; Bernier, G. BMI1 confers radioresistance to normal and cancerous neural stem cells through recruitment of the DNA damage response machinery. J. Neurosci. 2010, 30, 10096–10111. [Google Scholar] [CrossRef]
- Zhang, T.; Ahn, K.; Emerick, B.; Modarai, S.R.; Opdenaker, L.M.; Palazzo, J.; Schleiniger, G.; Fields, J.Z.; Boman, B.M. APC mutations in human colon lead to decreased neuroendocrine maturation of ALDH+ stem cells that alters GLP-2 and SST feedback signaling: Clue to a link between WNT and retinoic acid signalling in colon cancer development. PLoS ONE 2020, 15, e0239601. [Google Scholar] [CrossRef]
- Yu, T.; Chen, X.; Zhang, W.; Colon, D.; Shi, J.; Napier, D.; Rychahou, P.; Lu, W.; Lee, E.Y.; Weiss, H.L.; et al. Regulation of the potential marker for intestinal cells, Bmi1, by β-catenin and the zinc finger protein KLF4: Implications for colon cancer. J. Biol. Chem. 2012, 287, 3760–3768. [Google Scholar] [CrossRef]
- Ascenção, K.; Dilek, N.; Zuhra, K.; Módis, K.; Sato, T.; Szabo, C. Sequential Accumulation of ‘Driver’ Pathway Mutations Induces the Upregulation of Hydrogen-Sulfide-Producing Enzymes in Human Colonic Epithelial Cell Organoids. Antioxidants 2022, 11, 1823. [Google Scholar]
- Katoh, M. Canonical and non-canonical WNT signaling in cancer stem cells and their niches: Cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review). Int. J. Oncol. 2017, 51, 1357–1369. [Google Scholar]
- Sangiorgi, E.; Capecchi, M.R. Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 2008, 40, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bu, X.; Chen, H.; Wang, Q.; Sha, W. Bmi-1 promotes the invasion and migration of colon cancer stem cells through the downregulation of E-cadherin. Int. J. Mol. Med. 2016, 38, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Karim, B.O.; Rhee, K.J.; Liu, G.; Yun, K.; Brant, S.R. Prom1 function in development, intestinal inflammation, and intestinal tumorigenesis. Front. Oncol. 2014, 4, 323. [Google Scholar]
- Zhu, L.; Gibson, P.; Currle, D.S.; Tong, Y.; Richardson, R.J.; Bayazitov, I.T.; Poppleton, H.; Zakharenko, S.; Ellison, D.W.; Gilbertson, R.J. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature 2009, 457, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Chandrakesan, P.; Yao, J.; Qu, D.; May, R.; Weygant, N.; Ge, Y.; Ali, N.; Sureban, S.M.; Gude, M.; Vega, K.; et al. Dclk1, a tumor stem cell marker, regulates pro-survival signaling and self-renewal of intestinal tumor cells. Mol. Cancer 2017, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, S.Y.; Jeon, S.E.; Choi, J.H.; Lee, C.J.; Jang, T.Y.; Yun, H.J.; Lee, Y.; Kim, P.; Cho, S.H.; et al. DCLK1 promotes colorectal cancer stemness and aggressiveness via the XRCC5/COX2 axis. Theranostics 2022, 12, 5258–5271. [Google Scholar] [CrossRef]
- Liu, W.; Wang, S.; Sun, Q.; Yang, Z.; Liu, M.; Tang, H. DCLK1 promotes epithelial-mesenchymal transition via the PI3K/Akt/NF-κB pathway in colorectal cancer. Int. J. Cancer 2018, 142, 2068–2079. [Google Scholar] [CrossRef]
- Chiremba, T.T.; Neufeld, K.L. Constitutive Musashi1 expression impairs mouse postnatal development and intestinal homeostasis. Mol. Biol. Cell 2021, 32, 28–44. [Google Scholar] [CrossRef]
- Plateroti, M.; de Araujo, P.R.; da Silva, A.E.; Penalva, L.O. The RNA-Binding Protein Musashi1: A Major Player in Intestinal Epithelium Renewal and Colon Cancer Development. Curr. Colorectal Cancer Rep. 2012, 8, 290–297. [Google Scholar] [CrossRef][Green Version]
- Barker, N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014, 15, 19–33. [Google Scholar] [CrossRef]



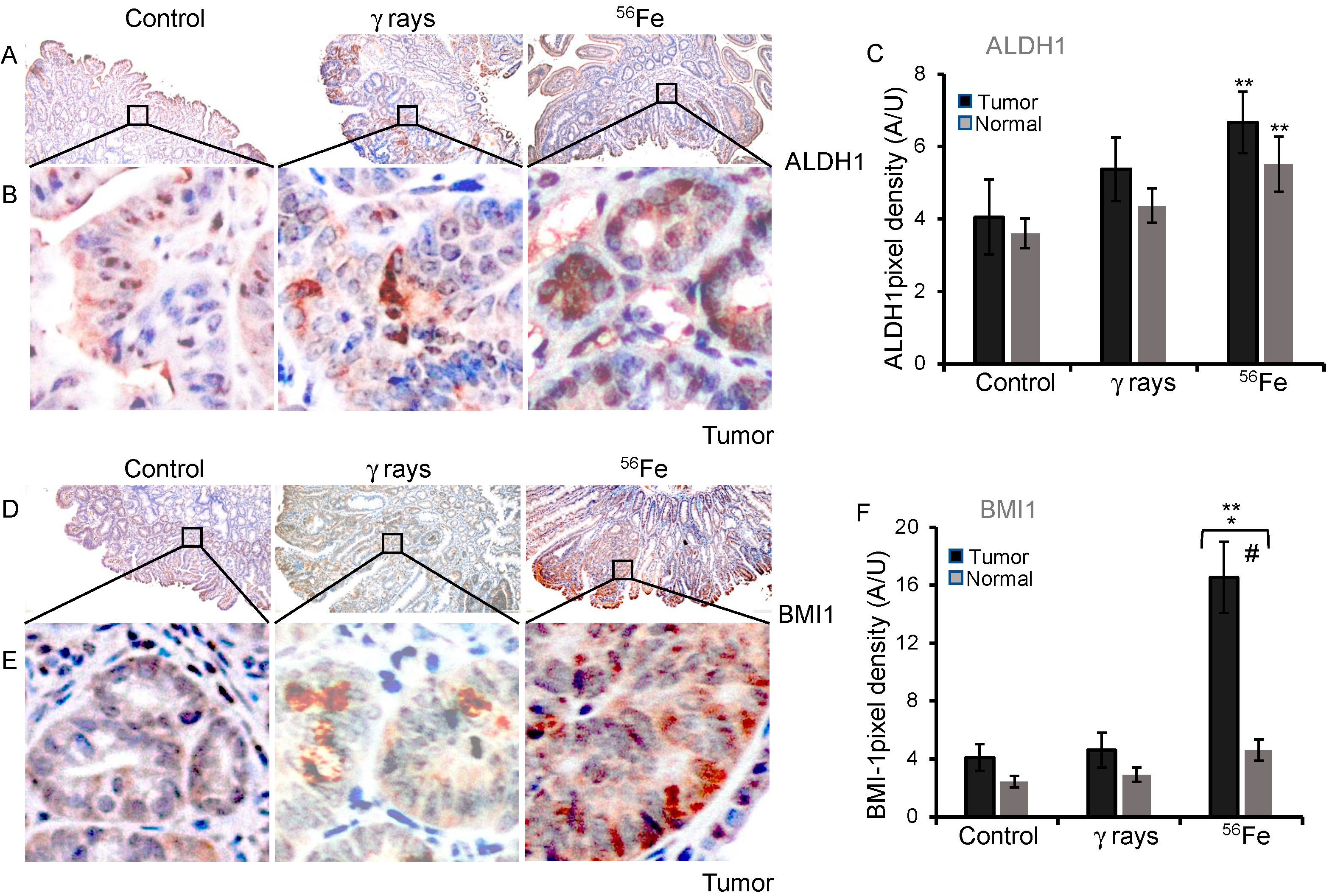
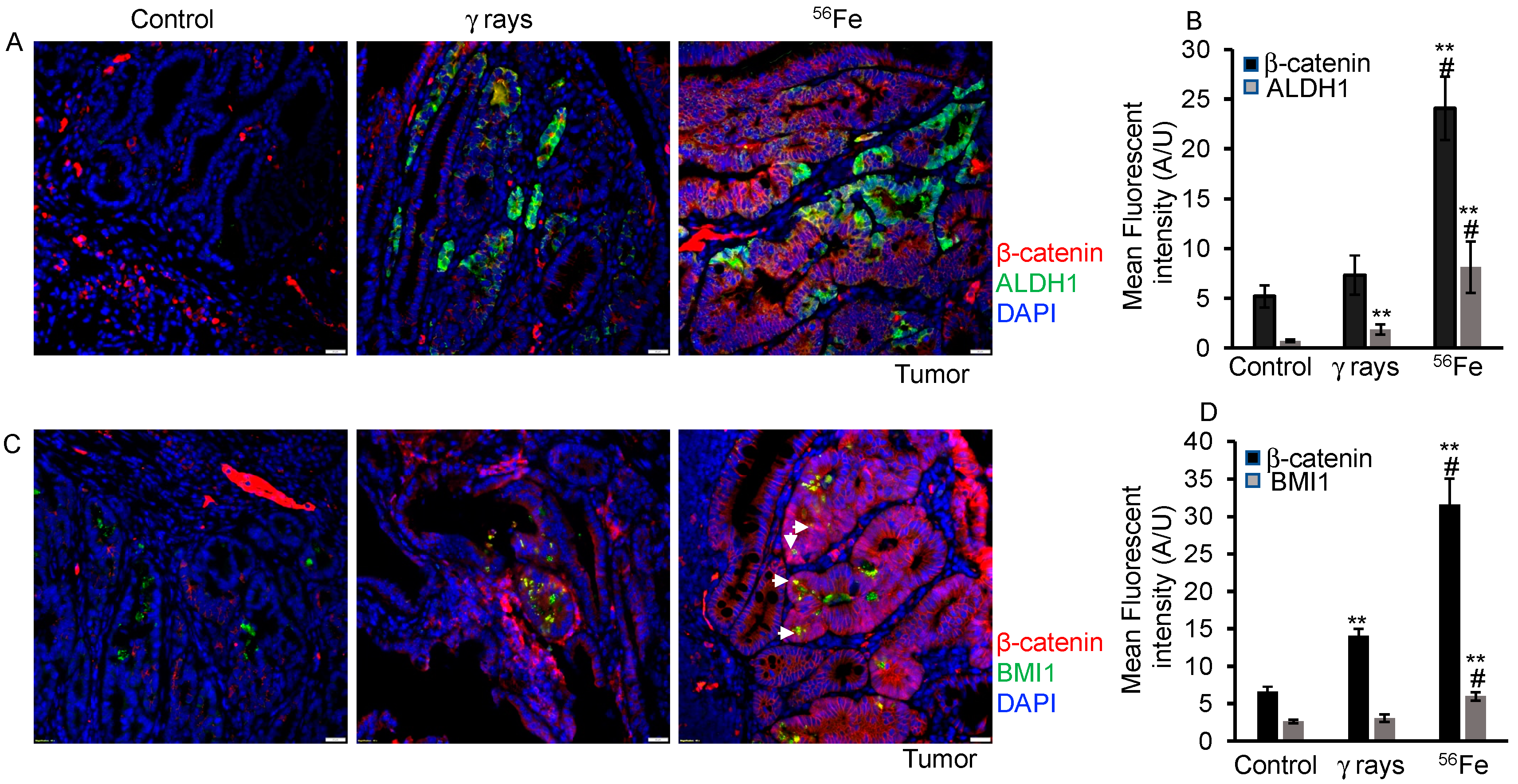
| ISC Marker | Tissue | Sham | γ-rays | 56Fe |
|---|---|---|---|---|
| ALDH1 | Tumor | 4.05 ± 1.04 | 5.37 ± 0.88 | 6.66 ± 0.85 |
| Normal | 3.61 ± 0.41 | 4.37 ± 0.48 | 5.51 ± 0.76 | |
| BMI1 | Tumor | 4.1 ± 0.93 | 4.62 ± 1.19 | 16.53 ± 2.46 |
| Normal | 2.43 ± 0.41 | 2.91 ± 0.51 | 4.60 ± 0.73 |
| ISC Marker | Spontaneous | γ-rays | 56Fe |
|---|---|---|---|
| ALDH1 | 1 | 1.33 | 1.65 * |
| CD133 | 1 | 0.71 | 1.23 |
| MSI1 | 1 | 1.5 | 1.1 |
| BMI1 | 1 | 1.1 | 4.0 * |
| DCLK1 | 1 | 1.4 | 2.6 * |
| LGR5 | 1 | 0.64 | 0.48 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowski, E.; Suman, S.; Kallakury, B.V.S.; Datta, K.; Fornace, A.J., Jr.; Kumar, S. Expression of Stem Cell Markers in High-LET Space Radiation-Induced Intestinal Tumors in Apc1638N/+ Mouse Intestine. Cancers 2023, 15, 4240. https://doi.org/10.3390/cancers15174240
Kwiatkowski E, Suman S, Kallakury BVS, Datta K, Fornace AJ Jr., Kumar S. Expression of Stem Cell Markers in High-LET Space Radiation-Induced Intestinal Tumors in Apc1638N/+ Mouse Intestine. Cancers. 2023; 15(17):4240. https://doi.org/10.3390/cancers15174240
Chicago/Turabian StyleKwiatkowski, Elaina, Shubhankar Suman, Bhaskar V. S. Kallakury, Kamal Datta, Albert J. Fornace, Jr., and Santosh Kumar. 2023. "Expression of Stem Cell Markers in High-LET Space Radiation-Induced Intestinal Tumors in Apc1638N/+ Mouse Intestine" Cancers 15, no. 17: 4240. https://doi.org/10.3390/cancers15174240
APA StyleKwiatkowski, E., Suman, S., Kallakury, B. V. S., Datta, K., Fornace, A. J., Jr., & Kumar, S. (2023). Expression of Stem Cell Markers in High-LET Space Radiation-Induced Intestinal Tumors in Apc1638N/+ Mouse Intestine. Cancers, 15(17), 4240. https://doi.org/10.3390/cancers15174240





