Lymph Node Metastases from Non-Melanoma Skin Cancer of the Head and Neck †
Abstract
Simple Summary
Abstract
1. Introduction
2. Management of Lymph Node Metastases of Non-Melanoma Skin Cancer (NMSC) of the Head and Neck
2.1. Cutaneous Squamous Cell Carcinoma—Epidemiology and Risk Factors
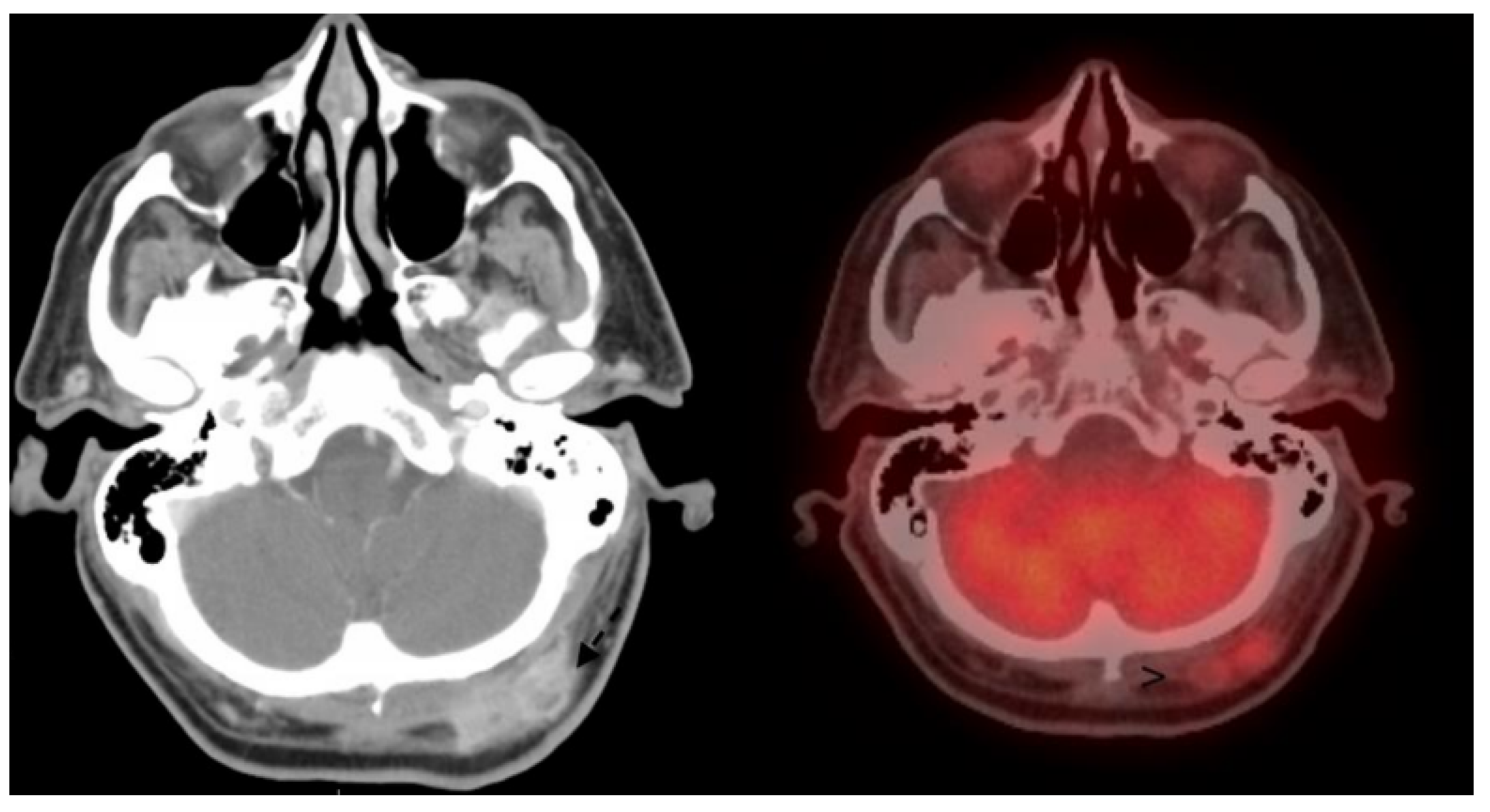
2.2. Cutaneous Squamous Cell Carcinoma—Diagnosis, Evaluation, and Sentinel Lymph Node Biopsy
2.3. Cutaneous Squamous Cell Carcinoma—Treatment and Staging
2.4. Merkel Cell Carcinoma
2.5. Eccrine Cell Carcinoma
2.6. Basal Cell Carcinoma
2.7. Other Non-Melanoma Skin Cancers
3. Clinical Trials and New Treatment Options
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kansara, S.; Bell, D.; Weber, R. Surgical management of non melanoma skin cancer of the head and neck. Oral Oncol. 2020, 100, 104485. [Google Scholar] [CrossRef] [PubMed]
- Newlands, C.; Currie, R.; Memon, A.; Whitaker, S.; Woolford, T. Non-melanoma skin cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130 (Suppl. S2), S125–S132. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, R.; Buralli, L.; De Gaudioc, C. Sentinel lymphonodectomy in non-melanoma skin cancers. Chir. Ital. 2006, 58, 347–351. [Google Scholar] [PubMed]
- Flint, P. Cummings Otolaryngology Head and Neck Surgery, 6th ed.; Elsevier: Philadelphia, PA, USA, 2015. [Google Scholar]
- Rudolph, C.; Schnoor, M.; Eisemann, N.; Katalinic, A. Incidence trends of nonmelanoma skin cancer in Germany from 1998 to 2010. J. Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG 2015, 13, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Schmults, C.D.; Karia, P.S.; Carter, J.B.; Han, J.; Qureshi, A.A. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: A 10-year, single-institution cohort study. JAMA Dermatol. 2013, 149, 541–547. [Google Scholar] [CrossRef]
- Varra, V.; Woody, N.M.; Reddy, C.; Joshi, N.P.; Geiger, J.; Adelstein, D.J.; Burkey, B.B.; Scharpf, J.; Prendes, B.; Lamarre, E.D.; et al. Suboptimal Outcomes in Cutaneous Squamous Cell Cancer of the Head and Neck with Nodal Metastases. Anticancer Res. 2018, 38, 5825–5830. [Google Scholar] [CrossRef]
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef]
- Wehner, M.R.; Shive, M.L.; Chren, M.-M.; Han, J.; Qureshi, A.A.; Linos, E. Indoor tanning and non-melanoma skin cancer: Systematic review and meta-analysis. BMJ 2012, 345, e5909. [Google Scholar] [CrossRef]
- Ramsay, H.M.; Fryer, A.A.; Hawley, C.M.; Smith, A.G.; Nicol, D.L.; Harden, P.N. Factors associated with nonmelanoma skin cancer following renal transplantation in Queensland, Australia. J. Am. Acad. Dermatol. 2003, 49, 397–406. [Google Scholar] [CrossRef]
- Senet, P.; Combemale, P.; Debure, C.; Baudot, N.; Machet, L.; Aout, M.; Vicaut, E.; Lok, C. Angio-Dermatology Group of The French Society of Dermatology Malignancy and chronic leg ulcers: The value of systematic wound biopsies: A prospective, multicenter, cross-sectional study. Arch. Dermatol. 2012, 148, 704–708. [Google Scholar] [CrossRef]
- Kromberg, J.G.; Castle, D.; Zwane, E.M.; Jenkins, T. Albinism and skin cancer in Southern Africa. Clin. Genet. 1989, 36, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Thielmann, H.W.; Popanda, O.; Edler, L.; Jung, E.G. Clinical symptoms and DNA repair characteristics of xeroderma pigmentosum patients from Germany. Cancer Res. 1991, 51, 3456–3470. [Google Scholar] [PubMed]
- Miranda, M.B.; Lauseker, M.; Kraus, M.-P.; Proetel, U.; Hanfstein, B.; Fabarius, A.; Baerlocher, G.M.; Heim, D.; Hossfeld, D.K.; Kolb, H.-J.; et al. Secondary malignancies in chronic myeloid leukemia patients after imatinib-based treatment: Long-term observation in CML Study IV. Leukemia 2016, 30, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J. Am. Acad. Dermatol. 2018, 78, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Kallini, J.R.; Hamed, N.; Khachemoune, A. Squamous cell carcinoma of the skin: Epidemiology, classification, management, and novel trends. Int. J. Dermatol. 2015, 54, 130–140. [Google Scholar] [CrossRef]
- Ciążyńska, M.; Kamińska-Winciorek, G.; Lange, D.; Lewandowski, B.; Reich, A.; Sławińska, M.; Pabianek, M.; Szczepaniak, K.; Hankiewicz, A.; Ułańska, M.; et al. The incidence and clinical analysis of non-melanoma skin cancer. Sci. Rep. 2021, 11, 4337. [Google Scholar] [CrossRef] [PubMed]
- Brougham, N.D.L.; Tan, S.T. The incidence and risk factors of metastasis for cutaneous squamous cell carcinoma--implications on the T-classification system. J. Surg. Oncol. 2014, 110, 876–882. [Google Scholar] [CrossRef]
- Leiter, U.; Keim, U.; Eigentler, T.; Katalinic, A.; Holleczek, B.; Martus, P.; Garbe, C. Incidence, Mortality, and Trends of Nonmelanoma Skin Cancer in Germany. J. Investig. Dermatol. 2017, 137, 1860–1867. [Google Scholar] [CrossRef]
- Miller, D.L.; Weinstock, M.A. Nonmelanoma skin cancer in the United States: Incidence. J. Am. Acad. Dermatol. 1994, 30, 774–778. [Google Scholar] [CrossRef]
- Alam, M.; Ratner, D. Cutaneous squamous-cell carcinoma. N. Engl. J. Med. 2001, 344, 975–983. [Google Scholar] [CrossRef]
- Wilkie, M.D.; Lancaster, J.; Roland, N.J.; Jones, T.M. Elective management of regional nodal basins in cutaneous squamous cell carcinoma of the head and neck: Controversies and contemporary perspectives. Oral Oncol. 2021, 120, 105432. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.K.; Kopf, A.W.; Grin, C.M.; Bart, R.S.; Levenstein, M.J. Recurrence rates of treated basal cell carcinomas. Part 1: Overview. J. Dermatol. Surg. Oncol. 1991, 17, 713–718. [Google Scholar] [CrossRef]
- Silverman, M.K.; Kopf, A.W.; Grin, C.M.; Bart, R.S.; Levenstein, M.J. Recurrence rates of treated basal cell carcinomas. Part 2: Curettage-electrodesiccation. J. Dermatol. Surg. Oncol. 1991, 17, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Schmults, C.D. NCCN Clinical Practice Guidelines in Oncology. Squamous Cell Skin Cancer. J. Natl. Compr. Canc. Netw. 2021, 8, 836–864. [Google Scholar]
- Rowe, D.E.; Carroll, R.J.; Day, C.L. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J. Am. Acad. Dermatol. 1992, 26, 976–990. [Google Scholar] [CrossRef]
- Thompson, A.K.; Kelley, B.F.; Prokop, L.J.; Murad, M.H.; Baum, C.L. Risk Factors for Cutaneous Squamous Cell Carcinoma Recurrence, Metastasis, and Disease-Specific Death: A Systematic Review and Meta-analysis. JAMA Dermatol. 2016, 152, 419–428. [Google Scholar] [CrossRef]
- Kyrgidis, A.; Tzellos, T.G.; Kechagias, N.; Patrikidou, A.; Xirou, P.; Kitikidou, K.; Bourlidou, E.; Vahtsevanos, K.; Antoniades, K. Cutaneous squamous cell carcinoma (SCC) of the head and neck: Risk factors of overall and recurrence-free survival. Eur. J. Cancer 2010, 46, 1563–1572. [Google Scholar] [CrossRef]
- Brantsch, K.D.; Meisner, C.; Schönfisch, B.; Trilling, B.; Wehner-Caroli, J.; Röcken, M.; Breuninger, H. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: A prospective study. Lancet Oncol. 2008, 9, 713–720. [Google Scholar] [CrossRef]
- Gonzalez-Guerrero, M.; Martínez-Camblor, P.; Vivanco, B.; Fernández-Vega, I.; Munguía-Calzada, P.; Gonzalez-Gutierrez, M.P.; Rodrigo, J.P.; Galache, C.; Santos-Juanes, J. The adverse prognostic effect of tumor budding on the evolution of cutaneous head and neck squamous cell carcinoma. J. Am. Acad. Dermatol. 2017, 76, 1139–1145. [Google Scholar] [CrossRef]
- Sahovaler, A.; Krishnan, R.J.; Yeh, D.H.; Zhou, Q.; Palma, D.; Fung, K.; Yoo, J.; Nichols, A.; MacNeil, S.D. Outcomes of Cutaneous Squamous Cell Carcinoma in the Head and Neck Region with Regional Lymph Node Metastasis: A Systematic Review and Meta-analysis. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 352–360. [Google Scholar] [CrossRef]
- Rangwala, S.; Tsai, K.Y. Roles of the immune system in skin cancer. Br. J. Dermatol. 2011, 165, 953–965. [Google Scholar] [CrossRef]
- Yu, S.H.; Bordeaux, J.S.; Baron, E.D. The immune system and skin cancer. Adv. Exp. Med. Biol. 2014, 810, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Santos-Juanes, J.; Fernández-Vega, I.; Lorenzo-Herrero, S.; Sordo-Bahamonde, C.; Martínez-Camblor, P.; García-Pedrero, J.M.; Vivanco, B.; Galache-Osuna, C.; Vazquez-Lopez, F.; Gonzalez, S.; et al. Lectin-like transcript 1 (LLT1) expression is associated with nodal metastasis in patients with head and neck cutaneous squamous cell carcinoma. Arch. Dermatol. Res. 2019, 311, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Munguía-Calzada, P.; Fernández-Vega, I.; Martínez-Camblor, P.; Díaz-Coto, S.; García-Pedrero, J.M.; Vivanco, B.; Osuna, C.G.; Vazquez-Lopez, F.; Rodrigo, J.P.; Santos-Juanes, J. Correlation of focal adhesion kinase expression with nodal metastasis in patients with head and neck cutaneous squamous cell carcinoma. Head Neck 2019, 41, 1290–1296. [Google Scholar] [CrossRef]
- García-Pedrero, J.M.; Martínez-Camblor, P.; Diaz-Coto, S.; Munguia-Calzada, P.; Vallina-Alvarez, A.; Vazquez-Lopez, F.; Rodrigo, J.P.; Santos-Juanes, J. Tumor programmed cell death ligand 1 expression correlates with nodal metastasis in patients with cutaneous squamous cell carcinoma of the head and neck. J. Am. Acad. Dermatol. 2017, 77, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Burton, K.A.; Ashack, K.A.; Khachemoune, A. Cutaneous Squamous Cell Carcinoma: A Review of High-Risk and Metastatic Disease. Am. J. Clin. Dermatol. 2016, 17, 491–508. [Google Scholar] [CrossRef]
- Mooney, C.P.; Martin, R.C.W.; Dirven, R.; Ashford, B.G.; Shannon, K.; Palme, C.E.; Ngo, Q.; Wykes, J.; Davies, S.; Gao, K.; et al. Sentinel Node Biopsy in 105 High-Risk Cutaneous SCCs of the Head and Neck: Results of a Multicenter Prospective Study. Ann. Surg. Oncol. 2019, 26, 4481–4488. [Google Scholar] [CrossRef]
- Weinberg, A.S.; Ogle, C.A.; Shim, E.K. Metastatic Cutaneous Squamous Cell Carcinoma: An Update. Dermatol. Surg. 2007, 33, 885–899. [Google Scholar] [CrossRef]
- Venables, Z.C.; Autier, P.; Nijsten, T.; Wong, K.F.; Langan, S.M.; Rous, B.; Broggio, J.; Harwood, C.; Henson, K.; Proby, C.M.; et al. Nationwide Incidence of Metastatic Cutaneous Squamous Cell Carcinoma in England. JAMA Dermatol. 2019, 155, 298–306. [Google Scholar] [CrossRef]
- Epstein, E.; Epstein, N.N.; Bragg, K.; Linden, G. Metastases from squamous cell carcinomas of the skin. Arch. Dermatol. 1968, 97, 245–251. [Google Scholar] [CrossRef]
- Peat, B.; Insull, P.; Ayers, R. Risk stratification for metastasis from cutaneous squamous cell carcinoma of the head and neck. ANZ J. Surg. 2012, 82, 230–233. [Google Scholar] [CrossRef]
- Wermker, K.; Kluwig, J.; Schipmann, S.; Klein, M.; Schulze, H.J.; Hallermann, C. Prediction score for lymph node metastasis from cutaneous squamous cell carcinoma of the external ear. Eur. J. Surg. Oncol. 2015, 41, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.S.; Mancuso, A.A.; Mendenhall, W.M. Perineural spread of cutaneous squamous and basal cell carcinoma: CT and MR detection and its impact on patient management and prognosis. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 1061–1069. [Google Scholar] [CrossRef]
- de Bondt, R.B.J.; Nelemans, P.J.; Hofman, P.A.M.; Casselman, J.W.; Kremer, B.; van Engelshoven, J.M.A.; Beets-Tan, R.G.H. Detection of lymph node metastases in head and neck cancer: A meta-analysis comparing US, USgFNAC, CT and MR imaging. Eur. J. Radiol. 2007, 64, 266–272. [Google Scholar] [CrossRef]
- Nouri, K.; Rivas, M.P.; Pedroso, F.; Bhatia, R.; Civantos, F. Sentinel lymph node biopsy for high-risk cutaneous squamous cell carcinoma of the head and neck. Arch. Dermatol. 2004, 140, 1284. [Google Scholar] [CrossRef]
- Durham, A.B.; Lowe, L.; Malloy, K.M.; McHugh, J.B.; Bradford, C.R.; Chubb, H.; Johnson, T.M.; McLean, S.A. Sentinel Lymph Node Biopsy for Cutaneous Squamous Cell Carcinoma on the Head and Neck. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 1171–1176. [Google Scholar] [CrossRef]
- Gore, S.M.; Shaw, D.; Martin, R.C.W.; Kelder, W.; Roth, K.; Uren, R.; Gao, K.; Davies, S.; Ashford, B.G.; Ngo, Q.; et al. Prospective study of sentinel node biopsy for high-risk cutaneous squamous cell carcinoma of the head and neck. Head Neck 2016, 38 (Suppl. 1), E884–E889. [Google Scholar] [CrossRef]
- Ross, A.S.; Schmults, C.D. Sentinel lymph node biopsy in cutaneous squamous cell carcinoma: A systematic review of the English literature. Dermatol. Surg. 2006, 32, 1309–1321. [Google Scholar] [CrossRef]
- Kwon, S.; Dong, Z.M.; Wu, P.C. Sentinel lymph node biopsy for high-risk cutaneous squamous cell carcinoma: Clinical experience and review of literature. World J. Surg. Oncol. 2011, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Imafuku, S.; Nakayama, J.; Nakaura, J.; Ito, K.; Shibayama, Y. Sentinel node biopsy for high-risk cutaneous squamous cell carcinoma. Eur. J. Surg. Oncol. 2014, 40, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Lubov, J.; Labbé, M.; Sioufi, K.; Morand, G.B.; Hier, M.P.; Khanna, M.; Sultanem, K.; Mlynarek, A.M. Prognostic factors of head and neck cutaneous squamous cell carcinoma: A systematic review. J. Otolaryngol. Head Neck Surg. 2021, 50, 54. [Google Scholar] [CrossRef] [PubMed]
- Haisma, M.S.; Plaat, B.E.C.; Bijl, H.P.; Roodenburg, J.L.N.; Diercks, G.F.H.; Romeijn, T.R.; Terra, J.B. Multivariate analysis of potential risk factors for lymph node metastasis in patients with cutaneous squamous cell carcinoma of the head and neck. J. Am. Acad. Dermatol. 2016, 75, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, T.; Brodland, D.G.; Arzeno, J.; Sharon, D.J.; Zitelli, J.A. Clinical outcomes of high-risk cutaneous squamous cell carcinomas treated with Mohs surgery alone: An analysis of local recurrence, regional nodal metastases, progression-free survival, and disease-specific death. J. Am. Acad. Dermatol. 2023, 88, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Cancer Staging Form Supplement. American College of Surgeons. Available online: http://www.facs.org/quality-programs/cancer/ajcc/cancer-staging/form-supplement (accessed on 15 February 2022).
- Jambusaria-Pahlajani, A.; Kanetsky, P.A.; Karia, P.S.; Hwang, W.-T.; Gelfand, J.M.; Whalen, F.M.; Elenitsas, R.; Xu, X.; Schmults, C.D. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013, 149, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.S.; Karia, P.S.; Besaw, R.; Schmults, C.D. Performance of the American Joint Committee on Cancer Staging Manual, 8th Edition vs the Brigham and Women’s Hospital Tumor Classification System for Cutaneous Squamous Cell Carcinoma. JAMA Dermatol. 2019, 155, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yuan, S.; Liu, F.; Liu, B.; Zhu, J.; He, W.; Li, W.; Kan, Q. Comparison between wait-and-see policy and elective neck dissection in clinically N0 cutaneous squamous cell carcinoma of head and neck. Medicine 2018, 97, e10782. [Google Scholar] [CrossRef]
- Cannon, R.B.; Dundar, Y.; Thomas, A.; Monroe, M.M.; Buchmann, L.O.; Witt, B.L.; Sowder, A.M.; Hunt, J.P. Elective Neck Dissection for Head and Neck Cutaneous Squamous Cell Carcinoma with Skull Base Invasion. Otolaryngol. Head Neck Surg. 2017, 156, 671–676. [Google Scholar] [CrossRef]
- Amit, M.; Liu, C.; Mansour, J.; Gleber-Netto, F.O.; Tam, S.; Baruch, E.N.; Aashiq, M.; El-Naggar, A.K.; Moreno, A.C.; Rosenthal, D.I.; et al. Elective neck dissection versus observation in patients with head and neck cutaneous squamous cell carcinoma. Cancer 2021, 127, 4413–4420. [Google Scholar] [CrossRef]
- Southwell, K.E.; Chaplin, J.M.; Eisenberg, R.L.; McIvor, N.P.; Morton, R.P. Effect of immunocompromise on metastatic cutaneous squamous cell carcinoma in the parotid and neck. Head Neck 2006, 28, 244–248. [Google Scholar] [CrossRef]
- Palme, C.E.; O’Brien, C.J.; Veness, M.J.; McNeil, E.B.; Bron, L.P.; Morgan, G.J. Extent of parotid disease influences outcome in patients with metastatic cutaneous squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 750–753. [Google Scholar] [CrossRef]
- Veness, M.J.; Palme, C.E.; Smith, M.; Cakir, B.; Morgan, G.J.; Kalnins, I. Cutaneous head and neck squamous cell carcinoma metastatic to cervical lymph nodes (nonparotid): A better outcome with surgery and adjuvant radiotherapy. Laryngoscope 2003, 113, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Gurney, B.; Newlands, C. Management of regional metastatic disease in head and neck cutaneous malignancy. 1. Cutaneous squamous cell carcinoma. Br. J. Oral Maxillofac. Surg. 2014, 52, 294–300. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.J.; McNeil, E.B.; McMahon, J.D.; Pathak, I.; Lauer, C.S.; Jackson, M.A. Significance of clinical stage, extent of surgery, and pathologic findings in metastatic cutaneous squamous carcinoma of the parotid gland. Head Neck 2002, 24, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Gurudutt, V.V.; Genden, E.M. Cutaneous squamous cell carcinoma of the head and neck. J. Skin Cancer 2011, 2011, 502723. [Google Scholar] [CrossRef] [PubMed]
- Agnese, D.M.; Maupin, R.; Tillman, B.; Pozderac, R.D.; Magro, C.; Walker, M.J. Head and neck melanoma in the sentinel lymph node era. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 1121–1124. [Google Scholar] [CrossRef][Green Version]
- Rotman, A.; Kerr, S.J.; Giddings, C.E.B. Elective neck dissection in metastatic cutaneous squamous cell carcinoma to the parotid gland: A systematic review and meta-analysis. Head Neck 2019, 41, 1131–1139. [Google Scholar] [CrossRef]
- Vauterin, T.J.; Veness, M.J.; Morgan, G.J.; Poulsen, M.G.; O’Brien, C.J. Patterns of lymph node spread of cutaneous squamous cell carcinoma of the head and neck. Head Neck 2006, 28, 785–791. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Moncrieff, M.D.; Clark, J.R.; Shannon, K.F.; Gao, K.; Milross, C.G.; O’Brien, C.J. Predicting the pattern of regional metastases from cutaneous squamous cell carcinoma of the head and neck based on location of the primary. Head Neck 2010, 32, 1288–1294. [Google Scholar] [CrossRef]
- D’Souza, J.; Clark, J. Management of the neck in metastatic cutaneous squamous cell carcinoma of the head and neck. Curr. Opin. Otolaryngol. Head Neck Surg. 2011, 19, 99–105. [Google Scholar] [CrossRef]
- Mierzwa, M.L. Radiotherapy for Skin Cancers of the Face, Head, and Neck. Facial Plast. Surg. Clin. 2019, 27, 131–138. [Google Scholar] [CrossRef]
- Veness, M.J.; Morgan, G.J.; Palme, C.E.; Gebski, V. Surgery and adjuvant radiotherapy in patients with cutaneous head and neck squamous cell carcinoma metastatic to lymph nodes: Combined treatment should be considered best practice. Laryngoscope 2005, 115, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, N.; Kutz, J.W.; Myers, L.L.; Isaacson, B.; Sumer, B.D.; Truelson, J.M.; Ahn, C.; Roland, P.S. Patterns of regional metastasis in advanced stage cutaneous squamous cell carcinoma of the auricle. Otolaryngol. Head Neck Surg. 2011, 144, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Palme, C.E.; Morgan, G.J.; Gebski, V.; Wang, A.Y.; Veness, M.J. Predictors of outcome in patients with metastatic cutaneous head and neck squamous cell carcinoma involving cervical lymph nodes: Improved survival with the addition of adjuvant radiotherapy. Head Neck 2012, 34, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.S.; Kus, K.J.B.; Smile, T.D.; Murad, F.; Zhou, G.; Ilori, E.O.; Schoenfeld, J.D.; Margalit, D.N.; Tishler, R.B.; Vidimos, A.T.; et al. Adjuvant radiation following clear margin resection of high T-stage cutaneous squamous cell carcinoma halves the risk of local and locoregional recurrence: A dual-center retrospective study. J. Am. Acad. Dermatol. 2022, 87, 87–94. [Google Scholar] [CrossRef]
- Schmidt, C.; Martin, J.M.; Khoo, E.; Plank, A.; Grigg, R. Outcomes of nodal metastatic cutaneous squamous cell carcinoma of the head and neck treated in a regional center. Head Neck 2015, 37, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- Forest, V.-I.; Clark, J.J.; Veness, M.J.; Milross, C. N1S3: A revised staging system for head and neck cutaneous squamous cell carcinoma with lymph node metastases: Results of 2 Australian Cancer Centers. Cancer 2010, 116, 1298–1304. [Google Scholar] [CrossRef]
- Porceddu, S.V.; Bressel, M.; Poulsen, M.G.; Stoneley, A.; Veness, M.J.; Kenny, L.M.; Wratten, C.; Corry, J.; Cooper, S.; Fogarty, G.B.; et al. Postoperative Concurrent Chemoradiotherapy Versus Postoperative Radiotherapy in High-Risk Cutaneous Squamous Cell Carcinoma of the Head and Neck: The Randomized Phase III TROG 05.01 Trial. J. Clin. Oncol. 2018, 36, 1275–1283. [Google Scholar] [CrossRef]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Amit, M.; Nagarajan, P.; Rubin, M.L.; Yuan, Y.; Bell, D.; El-Naggar, A.K.; Johnson, J.M.; Morrison, W.H.; Rosenthal, D.I.; et al. Pilot Phase II Trial of Neoadjuvant Immunotherapy in Locoregionally Advanced, Resectable Cutaneous Squamous Cell Carcinoma of the Head and Neck. Clin. Cancer Res. 2021, 27, 4557–4565. [Google Scholar] [CrossRef]
- Ibrahim, S.F.; Kasprzak, J.M.; Hall, M.A.; Fitzgerald, A.L.; Siegel, J.J.; Kurley, S.J.; Covington, K.R.; Goldberg, M.S.; Farberg, A.S.; Trotter, S.C.; et al. Enhanced metastatic risk assessment in cutaneous squamous cell carcinoma with the 40-gene expression profile test. Future Oncol. Lond. Engl. 2022, 18, 833–847. [Google Scholar] [CrossRef]
- Arron, S.T.; Blalock, T.W.; Guenther, J.M.; Hyams, D.M.; Ibrahim, S.F.; Koyfman, S.A.; Wysong, A. Clinical Considerations for Integrating Gene Expression Profiling into Cutaneous Squamous Cell Carcinoma Management. J. Drugs Dermatol. JDD 2021, 20, s5–s11. [Google Scholar]
- Walsh, N.M.; Cerroni, L. Merkel cell carcinoma: A review. J. Cutan. Pathol. 2021, 48, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Paulson, K.G.; Park, S.Y.; Vandeven, N.A.; Lachance, K.; Thomas, H.; Chapuis, A.G.; Harms, K.L.; Thompson, J.A.; Bhatia, S.; Stang, A.; et al. Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. J. Am. Acad. Dermatol. 2018, 78, 457–463.e2. [Google Scholar] [CrossRef] [PubMed]
- DeCaprio, J.A. Molecular Pathogenesis of Merkel Cell Carcinoma. Annu. Rev. Pathol. 2021, 16, 69–91. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Moshiri, A.S.; Doumani, R.; Yelistratova, L.; Blom, A.; Lachance, K.; Shinohara, M.M.; Delaney, M.; Chang, O.; McArdle, S.; Thomas, H.; et al. Polyomavirus-Negative Merkel Cell Carcinoma: A More Aggressive Subtype Based on Analysis of 282 Cases Using Multimodal Tumor Virus Detection. J. Investig. Dermatol. 2017, 137, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Harms, P.W.; Collie, A.M.B.; Hovelson, D.H.; Cani, A.K.; Verhaegen, M.E.; Patel, R.M.; Fullen, D.R.; Omata, K.; Dlugosz, A.A.; Tomlins, S.A.; et al. Next generation sequencing of Cytokeratin 20-negative Merkel cell carcinoma reveals ultraviolet-signature mutations and recurrent TP53 and RB1 inactivation. Mod. Pathol. 2016, 29, 240–248. [Google Scholar] [CrossRef]
- Heath, M.; Jaimes, N.; Lemos, B.; Mostaghimi, A.; Wang, L.C.; Peñas, P.F.; Nghiem, P. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: The AEIOU features. J. Am. Acad. Dermatol. 2008, 58, 375–381. [Google Scholar] [CrossRef]
- Tarantola, T.I.; Vallow, L.A.; Halyard, M.Y.; Weenig, R.H.; Warschaw, K.E.; Grotz, T.E.; Jakub, J.W.; Roenigk, R.K.; Brewer, J.D.; Weaver, A.L.; et al. Prognostic factors in Merkel cell carcinoma: Analysis of 240 cases. J. Am. Acad. Dermatol. 2013, 68, 425–432. [Google Scholar] [CrossRef]
- Mazziotta, C.; Cervellera, C.F.; Lanzillotti, C.; Touzé, A.; Gaboriaud, P.; Tognon, M.; Martini, F.; Rotondo, J.C. MicroRNA dysregulations in Merkel cell carcinoma: Molecular mechanisms and clinical applications. J. Med. Virol. 2023, 95, e28375. [Google Scholar] [CrossRef]
- Smith, V.A.; MaDan, O.P.; Lentsch, E.J. Tumor location is an independent prognostic factor in head and neck Merkel cell carcinoma. Otolaryngol. Head Neck Surg. 2012, 146, 403–408. [Google Scholar] [CrossRef]
- Lemos, B.D.; Storer, B.E.; Iyer, J.G.; Phillips, J.L.; Bichakjian, C.K.; Fang, L.C.; Johnson, T.M.; Liegeois-Kwon, N.J.; Otley, C.C.; Paulson, K.G.; et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: Analysis of 5823 cases as the basis of the first consensus staging system. J. Am. Acad. Dermatol. 2010, 63, 751–761. [Google Scholar] [CrossRef] [PubMed]
- van Veenendaal, L.M.; van Akkooi, A.C.J.; Verhoef, C.; Grünhagen, D.J.; Klop, W.M.C.; Valk, G.D.; Tesselaar, M.E.T. Merkel cell carcinoma: Clinical outcome and prognostic factors in 351 patients. J. Surg. Oncol. 2018, 117, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.O.; Yue, B.; Marzban, S.S.; Walls, B.L.; Carr, M.; Jackson, R.S.; Puleo, C.A.; Padhya, T.; Cruse, C.W.; Gonzalez, R.J.; et al. Both tumor depth and diameter are predictive of sentinel lymph node status and survival in Merkel cell carcinoma. Cancer 2015, 121, 3252–3260. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.C.; Busam, K.J.; Chou, J.F.; Panageas, K.S.; Pulitzer, M.P.; Kraus, D.H.; Brady, M.S.; Coit, D.G. Recurrence and survival in patients undergoing sentinel lymph node biopsy for merkel cell carcinoma: Analysis of 153 patients from a single institution. Ann. Surg. Oncol. 2011, 18, 2529–2537. [Google Scholar] [CrossRef]
- Schwartz, J.L.; Griffith, K.A.; Lowe, L.; Wong, S.L.; McLean, S.A.; Fullen, D.R.; Lao, C.D.; Hayman, J.A.; Bradford, C.R.; Rees, R.S.; et al. Features predicting sentinel lymph node positivity in Merkel cell carcinoma. J. Clin. Oncol. 2011, 29, 1036–1041. [Google Scholar] [CrossRef]
- Feldmeyer, L.; Hudgens, C.W.; Ray-Lyons, G.; Nagarajan, P.; Aung, P.P.; Curry, J.L.; Torres-Cabala, C.A.; Mino, B.; Rodriguez-Canales, J.; Reuben, A.; et al. Density, Distribution, and Composition of Immune Infiltrates Correlate with Survival in Merkel Cell Carcinoma. Clin. Cancer Res. 2016, 22, 5553–5563. [Google Scholar] [CrossRef]
- Pellitteri, P.K.; Takes, R.P.; Lewis, J.S.; Devaney, K.O.; Harlor, E.J.; Strojan, P.; Rodrigo, J.P.; Suárez, C.; Rinaldo, A.; Medina, J.E.; et al. Merkel cell carcinoma of the head and neck. Head Neck 2012, 34, 1346–1354. [Google Scholar] [CrossRef]
- Gupta, S.G.; Wang, L.C.; Peñas, P.F.; Gellenthin, M.; Lee, S.J.; Nghiem, P. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: The Dana-Farber experience and meta-analysis of the literature. Arch. Dermatol. 2006, 142, 685–690. [Google Scholar] [CrossRef]
- Colgan, M.B.; Tarantola, T.I.; Weaver, A.L.; Wiseman, G.A.; Roenigk, R.K.; Brewer, J.D.; Otley, C.C. The predictive value of imaging studies in evaluating regional lymph node involvement in Merkel cell carcinoma. J. Am. Acad. Dermatol. 2012, 67, 1250–1256. [Google Scholar] [CrossRef]
- Liu, J.; Larcos, G.; Howle, J.; Veness, M. Lack of clinical impact of 18 F-fluorodeoxyglucose positron emission tomography with simultaneous computed tomography for stage I and II Merkel cell carcinoma with concurrent sentinel lymph node biopsy staging: A single institutional experience from Westmead Hospital, Sydney. Australas. J. Dermatol. 2017, 58, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Hawryluk, E.B.; O’Regan, K.N.; Sheehy, N.; Guo, Y.; Dorosario, A.; Sakellis, C.G.; Jacene, H.A.; Wang, L.C. Positron emission tomography/computed tomography imaging in Merkel cell carcinoma: A study of 270 scans in 97 patients at the Dana-Farber/Brigham and Women’s Cancer Center. J. Am. Acad. Dermatol. 2013, 68, 592–599. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Merkel Cell Carcinoma. Version 1. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf (accessed on 15 February 2022).
- Bichakjian, C.K.; Olencki, T.; Aasi, S.Z.; Alam, M.; Andersen, J.S.; Blitzblau, R.; Bowen, G.M.; Contreras, C.M.; Daniels, G.A.; Decker, R.; et al. Merkel Cell Carcinoma, Version 1.2018, NCCN Clinical Practice Guidelines in On-cology. J. Natl. Compr. Cancer Netw. JNCCN 2018, 16, 742–774. [Google Scholar] [CrossRef]
- Horii, A.; Yoshida, J.; Honjo, Y.; Mitani, K.; Takashima, S.; Kubo, T. Pre-operative assessment of metastatic parotid tumors. Auris. Nasus. Larynx 1998, 25, 277–283. [Google Scholar] [CrossRef]
- Kim, H.J.; Yoon, D.Y.; Hong, J.H.; Yun, E.J.; Baek, S.; Kim, E.S.; Park, M.W.; Kwon, K.H. Intra-parotid lymph node metastasis in patients with non-cutaneous head and neck cancers: Clinical and imaging features for differentiation from simultaneous parotid primary tumor. Acta Radiol. 2020, 61, 1628–1635. [Google Scholar] [CrossRef]
- Mehrany, K.; Otley, C.C.; Weenig, R.H.; Phillips, P.K.; Roenigk, R.K.; Nguyen, T.H. A meta-analysis of the prognostic significance of sentinel lymph node status in Merkel cell carcinoma. Dermatol. Surg. 2002, 28, 113–117; discussion 117. [Google Scholar] [CrossRef] [PubMed]
- Servy, A.; Maubec, E.; Sugier, P.E.; Grange, F.; Mansard, S.; Lesimple, T.; Marinho, E.; Couturaud, B.; Girod, A.; Albert, S.; et al. Merkel cell carcinoma: Value of sentinel lymph-node status and adjuvant radiation therapy. Ann. Oncol. 2016, 27, 914–919. [Google Scholar] [CrossRef]
- Kachare, S.D.; Wong, J.H.; Vohra, N.A.; Zervos, E.E.; Fitzgerald, T.L. Sentinel lymph node biopsy is associated with improved survival in Merkel cell carcinoma. Ann. Surg. Oncol. 2014, 21, 1624–1630. [Google Scholar] [CrossRef]
- Sims, J.R.; Grotz, T.E.; Pockaj, B.A.; Joseph, R.W.; Foote, R.L.; Otley, C.C.; Weaver, A.L.; Jakub, J.W.; Price, D.L. Sentinel lymph node biopsy in Merkel cell carcinoma: The Mayo Clinic experience of 150 patients. Surg. Oncol. 2018, 27, 11–17. [Google Scholar] [CrossRef]
- Fritsch, V.A.; Camp, E.R.; Lentsch, E.J. Sentinel lymph node status in Merkel cell carcinoma of the head and neck: Not a predictor of survival. Head Neck 2014, 36, 571–579. [Google Scholar] [CrossRef]
- Asgari, M.M.; Sokil, M.M.; Warton, E.M.; Iyer, J.; Paulson, K.G.; Nghiem, P. Effect of host, tumor, diagnostic, and treatment variables on outcomes in a large cohort with Merkel cell carcinoma. JAMA Dermatol. 2014, 150, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Liang, E.; Brower, J.V.; Rice, S.R.; Buehler, D.G.; Saha, S.; Kimple, R.J. Merkel Cell Carcinoma Analysis of Outcomes: A 30-Year Experience. PLoS ONE 2015, 10, e0129476. [Google Scholar] [CrossRef] [PubMed]
- Righi, A.; Asioli, S.; Caliendo, V.; Macripò, G.; Picciotto, F.; Risio, M.; Eusebi, V.; Bussolati, G. An ultrasonography-cytology protocol for the diagnostic management of regional nodes in a subset of patients with Merkel cell carcinoma of the skin. Br. J. Dermatol. 2013, 168, 563–570. [Google Scholar] [CrossRef]
- Jabbour, J.; Cumming, R.; Scolyer, R.A.; Hruby, G.; Thompson, J.F.; Lee, S. Merkel cell carcinoma: Assessing the effect of wide local excision, lymph node dissection, and radiotherapy on recurrence and survival in early-stage disease--results from a review of 82 consecutive cases diagnosed between 1992 and 2004. Ann. Surg. Oncol. 2007, 14, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.P.; Holtzman, M.P. Surgical resection improves median overall survival with marginal improvement in long-term survival when compared with definitive radiotherapy in Merkel cell carcinoma: A propensity score matched analysis of the National Cancer Database. Am. J. Surg. 2018, 215, 384–387. [Google Scholar] [CrossRef]
- Adjuvant Radiation Therapy and Chemotherapy in Merkel Cell Carcinoma: Survival Analyses of 6908 Cases From the National Cancer Data Base—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27245173/ (accessed on 5 November 2021).
- Jouary, T.; Leyral, C.; Dreno, B.; Doussau, A.; Sassolas, B.; Beylot-Barry, M.; Renaud-Vilmer, C.; Guillot, B.; Bernard, P.; Lok, C.; et al. Adjuvant prophylactic regional radiotherapy versus observation in stage I Merkel cell carcinoma: A multicentric prospective randomized study. Ann. Oncol. 2012, 23, 1074–1080. [Google Scholar] [CrossRef]
- Bowe, C.M.; Gurney, B.; Whitaker, S.; Newlands, C. Management of regional metastatic disease in cutaneous malignancy of the head and neck. 3. Merkel cell carcinoma. Br. J. Oral Maxillofac. Surg. 2019, 57, 847–856. [Google Scholar] [CrossRef]
- Strom, T.; Carr, M.; Zager, J.S.; Naghavi, A.; Smith, F.O.; Cruse, C.W.; Messina, J.L.; Russell, J.; Rao, N.G.; Fulp, W.; et al. Radiation Therapy is Associated with Improved Outcomes in Merkel Cell Carcinoma. Ann. Surg. Oncol. 2016, 23, 3572–3578. [Google Scholar] [CrossRef]
- Chen, M.M.; Roman, S.A.; Sosa, J.A.; Judson, B.L. The role of adjuvant therapy in the management of head and neck merkel cell carcinoma: An analysis of 4815 patients. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 137–141. [Google Scholar] [CrossRef]
- Shnayder, Y.; Weed, D.T.; Arnold, D.J.; Gomez-Fernandez, C.; Bared, A.; Goodwin, W.J.; Civantos, F.J. Management of the neck in Merkel cell carcinoma of the head and neck: University of Miami experience. Head Neck 2008, 30, 1559–1565. [Google Scholar] [CrossRef]
- Wong, W.G.; Stahl, K.; Olecki, E.J.; Holguin, R.P.; Pameijer, C.; Shen, C. Survival Benefit of Guideline-Concordant Postoperative Radiation for Local Merkel Cell Carcinoma. J. Surg. Res. 2021, 266, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, P.; Bhatia, S.; Lipson, E.J.; Sharfman, W.H.; Kudchadkar, R.R.; Brohl, A.S.; Friedlander, P.A.; Daud, A.; Kluger, H.M.; Reddy, S.A.; et al. Durable Tumor Regression and Overall Survival in Patients with Advanced Merkel Cell Carcinoma Receiving Pembrolizumab as First-Line Therapy. J. Clin. Oncol. 2019, 37, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Bhatia, S.; Amin, A.; Kudchadkar, R.R.; Sharfman, W.H.; Lebbé, C.; Delord, J.-P.; Dunn, L.A.; Shinohara, M.M.; Kulikauskas, R.; et al. Neoadjuvant Nivolumab for Patients with Resectable Merkel Cell Carcinoma in the CheckMate 358 Trial. J. Clin. Oncol. 2020, 38, 2476–2487. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Bhatia, S.; Brohl, A.S.; Hamid, O.; Mehnert, J.M.; Terheyden, P.; Shih, K.C.; Brownell, I.; Lebbé, C.; Lewis, K.D.; et al. Avelumab in patients with previously treated metastatic Merkel cell carcinoma: Long-term data and biomarker analyses from the single-arm phase 2 JAVELIN Merkel 200 trial. J. Immunother. Cancer 2020, 8, e000674. [Google Scholar] [CrossRef]
- Colunga, A.; Pulliam, T.; Nghiem, P. Merkel Cell Carcinoma in the Age of Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2018, 24, 2035–2043. [Google Scholar] [CrossRef]
- Sidiropoulos, M.; Sade, S.; Al-Habeeb, A.; Ghazarian, D. Syringoid eccrine carcinoma: A clinicopathological and immunohistochemical study of four cases. J. Clin. Pathol. 2011, 64, 788–792. [Google Scholar] [CrossRef]
- Larson, K.; Babiker, H.M.; Kovoor, A.; Liau, J.; Eldersveld, J.; Elquza, E. Oral Capecitabine Achieves Response in Metastatic Eccrine Carcinoma. Case Rep. Oncol. Med. 2018, 2018, 7127048. [Google Scholar] [CrossRef]
- Kaseb, H.; Babiker, H.M. Eccrine Carcinoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: http://www.ncbi.nlm.nih.gov/books/NBK541042/ (accessed on 28 October 2021).
- Salih, A.M.; Kakamad, F.H.; Essa, R.A.; Rauf, G.M.; Masrur, S.A.; Shvan, H.M.; Rawezh, Q.S.; Hunar, A.H.; Dahat, A.H.; Othman, S. Porocarcinoma: A systematic review of literature with a single case report. Int. J. Surg. Case Rep. 2016, 30, 13–16. [Google Scholar] [CrossRef]
- van der Horst, M.P.J.; Brenn, T. Update on Malignant Sweat Gland Tumors. Surg. Pathol. Clin. 2017, 10, 383–397. [Google Scholar] [CrossRef]
- Gómez-Zubiaur, A.; Medina-Montalvo, S.; Vélez-Velázquez, M.D.; Polo-Rodríguez, I. Eccrine Porocarcinoma: Patient Characteristics, Clinical and Histopathologic Features, and Treatment in 7 Cases. Actas Dermosifiliogr. 2017, 108, e27–e32. [Google Scholar] [CrossRef]
- Sanchez Petitto, G.; Sarwari, N.M.; Jain, P.; Swaby, M.; Bhattacharjee, M. FDG PET/CT in Malignant Eccrine Spiradenoma. Clin. Nucl. Med. 2017, 42, 125–126. [Google Scholar] [CrossRef]
- Shaw, M.; McKee, P.H.; Lowe, D.; Black, M.M. Malignant eccrine poroma: A study of twenty-seven cases. Br. J. Dermatol. 1982, 107, 675–680. [Google Scholar] [CrossRef]
- Coonley, C.J.; Schauer, P.; Kelsen, D.P.; Sordillo, P.; Huvos, A.G. Chemotherapy of metastatic sweat gland carcinoma. A retrospective review. Am. J. Clin. Oncol. 1985, 8, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Bogner, P.N.; Fullen, D.R.; Lowe, L.; Paulino, A.; Biermann, J.S.; Sondak, V.K.; Su, L.D. Lymphatic mapping and sentinel lymph node biopsy in the detection of early metastasis from sweat gland carcinoma. Cancer 2003, 97, 2285–2289. [Google Scholar] [CrossRef] [PubMed]
- Shiohara, J.; Koga, H.; Uhara, H.; Takata, M.; Saida, T. Eccrine porocarcinoma: Clinical and pathological studies of 12 cases. J. Dermatol. 2007, 34, 516–522. [Google Scholar] [CrossRef]
- Delgado, R.; Kraus, D.; Coit, D.G.; Busam, K.J. Sentinel lymph node analysis in patients with sweat gland carcinoma. Cancer 2003, 97, 2279–2284. [Google Scholar] [CrossRef]
- Scrivener, Y.; Grosshans, E.; Cribier, B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br. J. Dermatol. 2002, 147, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Malone, J.P.; Fedok, F.G.; Belchis, D.A.; Maloney, M.E. Basal cell carcinoma metastatic to the parotid: Report of a new case and review of the literature. Ear. Nose. Throat J. 2000, 79, 511–515, 518–519. [Google Scholar] [CrossRef]
- Jankovic, I.; Kovacevic, P.; Visnjic, M.; Jankovic, D.; Binic, I.; Jankovic, A.; Ilic, I. Application of sentinel lymph node biopsy in cutaneous basosquamous carcinoma. Ann. Dermatol. 2011, 23 (Suppl. 1), S123–S126. [Google Scholar] [CrossRef]
- Yoshida, Y.; Shiomi, T.; Tahira, M.; Yamamoto, O. Metastatic basosquamous carcinoma detected by sentinel lymph node biopsy. J. Dermatol. 2013, 40, 635–637. [Google Scholar] [CrossRef]
- Buffo, T.H.; Stelini, R.F.; Serrano, J.Y.M.; Pontes, L.T.; Magalhães, R.F.; de Moraes, A.M. Mohs micrographic surgery in rare cutaneous tumors: A retrospective study at a Brazilian tertiary university hospital. An. Bras. Dermatol. 2023, 98, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Yesensky, J. Sentinel Lymph Node Biopsy for Cutaneous Squamous Cell Carcinoma of the Head and Neck. 2023. Available online: https://clinicaltrials.gov/study/NCT05108090 (accessed on 7 August 2023).
- Cives, M.; Mannavola, F.; Lospalluti, L.; Sergi, M.C.; Cazzato, G.; Filoni, E.; Cavallo, F.; Giudice, G.; Stucci, L.S.; Porta, C.; et al. Non-Melanoma Skin Cancers: Biological and Clinical Features. Int. J. Mol. Sci. 2020, 21, 5394. [Google Scholar] [CrossRef] [PubMed]
| Tumor Staging System | AJCC 8th Edition | BWH |
|---|---|---|
| T1 | <2 cm in greatest diameter | 0 High-risk factors * |
| T2 Δ | ≥2 cm, but <4 cm in greatest diameter | N/A |
| T2a | N/A | 1 High-risk factor |
| T2b | N/A | 2–3 High-risk factors |
| T3 † | Tumor ≥4 cm in greatest diameter or minor bone invasion or perineural invasion or deep invasion | 4 High-risk factors or bone invasion |
| T4 Δ | ||
| T4a | Tumor with gross cortical bone and/or marrow invasion | N/A |
| T4b | Tumor with skull bone invasion and/or skull base foramen involvement | N/A |
| Durham et al. [47] | Gore et al. [48] | Haisma et al. [53] |
|---|---|---|
| Study Inclusion Criteria for High-Risk Cutaneous Squamous Cell Carcinoma | ||
| Locally recurrent | Local recurrence in the setting of adequate prior resection margins | N/A * |
| Occurrence in a prior radiation or chronic inflammation and/or ulcer site | Carcinoma in a pre-existing scar | |
| Perineural invasion | Perineural invasion | |
| Angiolymphatic invasion | Lymphovascular invasion | |
| Immunosuppression | Immunocompromise | |
| Size of 1 cm or more on cheek, forehead, scalp, neck, or 0.6 cm or more on face mask area | Tumor size > 2 cm | |
| Poorly differentiated | Poorly differentiated | |
| Clark level of IV or V | Invasion into subcutaneous fat or tumor thickness > 5 mm | |
| Breslow depth of 2 mm or more | Location on ear or lip | |
| Rapid growth | ||
| Risk criteria identified after sentinel lymph node biopsy or development of neck metastasis | ||
| Perineural invasion | Perineural invasion | Ear location |
| Angiolymphatic invasion | Lymphovascular invasion | Moderate or poor differentiation |
| Clinical size | Number of high-risk tumor factors | Tumor diameter > 50 mm |
| Tumor thickness > 2 mm | ||
| Incidence of nodal metastasis | ||
| 15% | 14% | 16% |
| Squamous Cell Carcinoma [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83] | |||
| T1-T2N0 | T3N0 | T4N0 | N+ |
| Primary resection, consider SLNB for high risk | Primary resection, consider SLNB vs elective neck dissection (END) +/− parotid vs. RT to nodal basin | T4 lesions may not be amenable to injection that fully encompasses lesion. END vs RT to nodal basin. Consider induction immunotherapy as an alternative to radical surgery. | Therapeutic lymphadenectomy (neck and/or parotid as indicated) in conjunction with primary resection. Adjuvant therapy based on final pathology report |
| Merkel Cell Carcinoma [84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129] | |||
| T1-T2N0 | T3N0 | T4N0 | N+ |
| Resect primary, SLNB recommended | Resect primary, consider SLNB vs END +/− parotid vs. RT to nodal basin | If surgery is performed, consider END. SLNB may be an option if the lesion is not too large and invasive to fully inject. Consider immunotherapy upfront due to high rate of distant disease. | Therapeutic lymphadenectomy neck +/− parotid. If massive resection is necessary, including extensive adenopathy, consider induction immunotherapy (high rate of distant metastases) |
| Eccrine Cell Carcinoma [130,131,132,133,134,135,136,137,138,139,140,141] | |||
| T1-T2N0 | T3N0 | T4N0 | N+ |
| Resect primary tumor. Very limited data available on SLNB which seems logical. | Resect primary tumor. Very limited data available on SLNB vs. END, either of which could be argued for. | Resect primary tumor. Very limited data available on SLNB vs. END, either of which could be argued for. Adjuvant radiation may be necessary. | Therapeutic lymphadenectomy, neck +/− parotid, with primary resection. Radiation probably will be indicated. |
| Basal Cell Carcinoma [142,143,144,145,146] | |||
| T1-T2N0 | T3N0 | T4N0 | N+ |
| Primary resection only | Primary resection only | Primary resection only | Therapeutic Lymphadenectomy |
| Non-epithelial Malignancies | |||
| No surgical management of lymphatics, even if primary is managed surgically. | Management would depend on histology, but may require therapeutic lymphadenectomy | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Civantos, F.; Helmen, Z.M.; Bradley, P.J.; Coca-Pelaz, A.; De Bree, R.; Guntinas-Lichius, O.; Kowalski, L.P.; López, F.; Mäkitie, A.A.; Rinaldo, A.; et al. Lymph Node Metastases from Non-Melanoma Skin Cancer of the Head and Neck. Cancers 2023, 15, 4201. https://doi.org/10.3390/cancers15174201
Civantos F, Helmen ZM, Bradley PJ, Coca-Pelaz A, De Bree R, Guntinas-Lichius O, Kowalski LP, López F, Mäkitie AA, Rinaldo A, et al. Lymph Node Metastases from Non-Melanoma Skin Cancer of the Head and Neck. Cancers. 2023; 15(17):4201. https://doi.org/10.3390/cancers15174201
Chicago/Turabian StyleCivantos, Francisco, Zachary M. Helmen, Patrick J. Bradley, Andrés Coca-Pelaz, Remco De Bree, Orlando Guntinas-Lichius, Luiz P. Kowalski, Fernando López, Antti A. Mäkitie, Alessandra Rinaldo, and et al. 2023. "Lymph Node Metastases from Non-Melanoma Skin Cancer of the Head and Neck" Cancers 15, no. 17: 4201. https://doi.org/10.3390/cancers15174201
APA StyleCivantos, F., Helmen, Z. M., Bradley, P. J., Coca-Pelaz, A., De Bree, R., Guntinas-Lichius, O., Kowalski, L. P., López, F., Mäkitie, A. A., Rinaldo, A., Robbins, K. T., Rodrigo, J. P., Takes, R. P., & Ferlito, A. (2023). Lymph Node Metastases from Non-Melanoma Skin Cancer of the Head and Neck. Cancers, 15(17), 4201. https://doi.org/10.3390/cancers15174201












