Conditional Survival in Prostate Cancer in the Nordic Countries Elucidates the Timing of Improvements
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
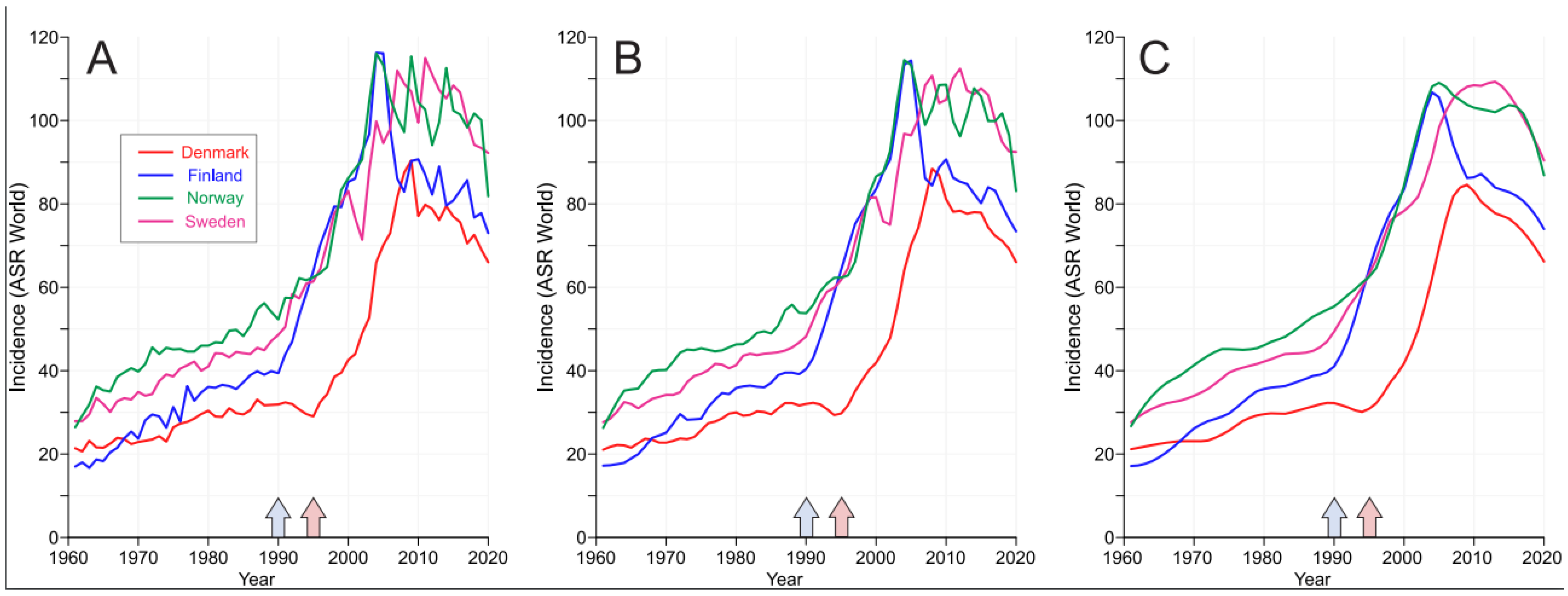
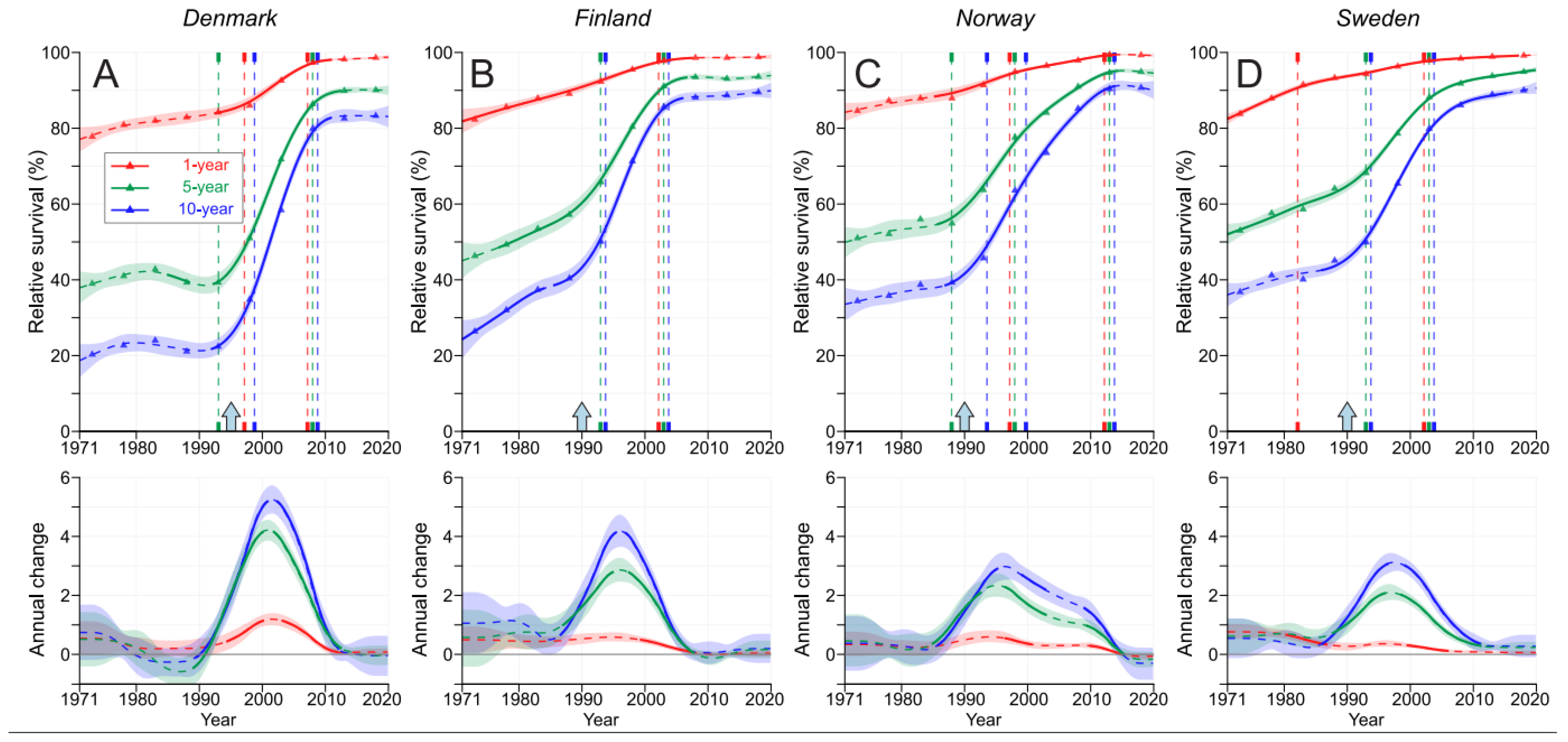
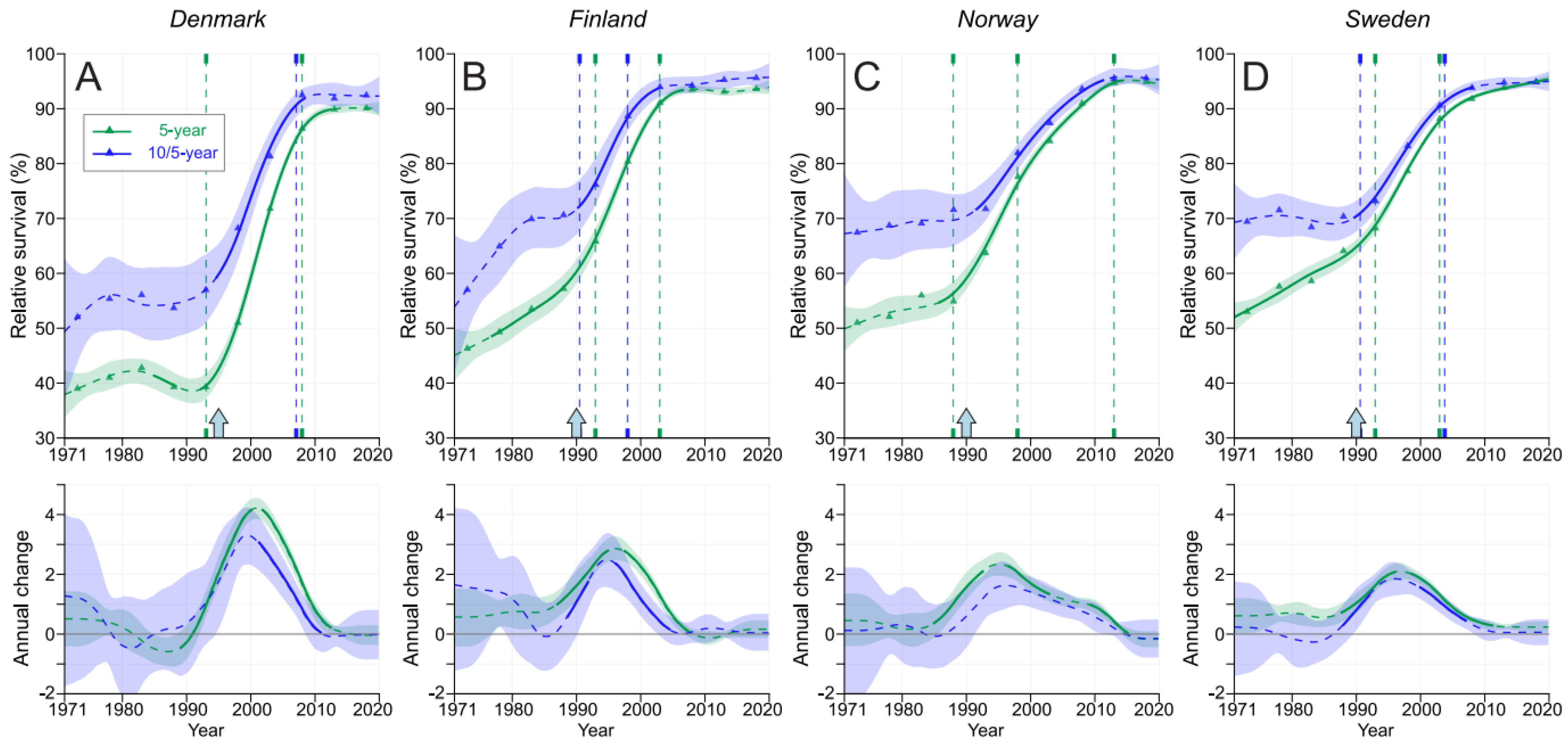
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brasso, K.; Ingimarsdóttir, I.J.; Rusch, E.; Engholm, G.; Adolfsson, J.; Tryggvadóttir, L.; Jónsson, E.; Bill-Axelson, A.; Holmberg, E.; Storm, H.H. Differences in survival from prostate cancer in Denmark, Iceland and Sweden. Eur. J. Cancer 2013, 49, 1984–1992. [Google Scholar] [CrossRef]
- Møller, M.H.; Kristiansen, I.S.; Beisland, C.; Rørvik, J.; Støvring, H. Trends in stage-specific incidence of prostate cancer in Norway, 1980–2010: A population-based study. BJU Int. 2016, 118, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Kavasmaa, O.T.; Tyomkin, D.B.; Mehik, A.; Parpala, T.M.; Tonttila, P.; Paananen, I.; Kunelius, P.; Vaarala, M.H.; Ohtonen, P.; Hellström, P.A. Changing trends in symptomatology, diagnostics, stage and survival of prostate cancer in Northern Finland during a period of 20 years. World J. Surg. Oncol. 2013, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.D.; Hu, J.; Talala, K.; Tammela, T.; Taari, K.; Auvinen, A. Estimating the rate of overdiagnosis with prostate cancer screening: Evidence from the Finnish component of the European Randomized Study of Screening for Prostate Cancer. Cancer Causes Control 2021, 32, 1299–1313. [Google Scholar] [CrossRef]
- Vickers, A.J.; Sjoberg, D.D.; Ulmert, D.; Vertosick, E.; Roobol, M.J.; Thompson, I.; Heijnsdijk, E.A.; De Koning, H.; Atoria-Swartz, C.; Scardino, P.T.; et al. Empirical estimates of prostate cancer overdiagnosis by age and prostate-specific antigen. BMC Med. 2014, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Enikeev, D.; Misrai, V.; Rijo, E.; Sukhanov, R.; Chinenov, D.; Gazimiev, M.; Taratkin, M.; Azilgareeva, C.; Morozov, A.; Herrmann, T.R.W.; et al. EAU, AUA and NICE Guidelines on Surgical and Minimally Invasive Treatment of Benign Prostate Hyperplasia: A Critical Appraisal of the Guidelines Using the AGREE-II Tool. Urol. Int. 2022, 106, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Y.; Yang, D.; Yang, C.; Mao, L. Current state of biomarkers for the diagnosis and assessment of treatment efficacy of prostate cancer. Discov. Med. 2019, 27, 235–243. [Google Scholar] [PubMed]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef]
- Westerberg, M.; Franck Lissbrant, I.; Damber, J.E.; Robinson, D.; Garmo, H.; Stattin, P. Temporal changes in survival in men with de novo metastatic prostate cancer: Nationwide population-based study. Acta Oncol. 2020, 59, 106–111. [Google Scholar] [CrossRef]
- Storås, A.H.; Fosså, S.D.; Ursin, G.; Andreassen, B.K. Survival trends for patients with primary metastatic prostate cancer before and after the introduction of new antitumor drugs. Prostate Cancer Prostatic Dis. 2023, 26, 53–58. [Google Scholar] [CrossRef]
- Devasia, T.P.; Mariotto, A.B.; Nyame, Y.A.; Etzioni, R. Estimating the Number of Men Living with Metastatic Prostate Cancer in the United States. Cancer Epidemiol. Biomarkers Prev. 2023, 32, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Borno, H.T.; Cowan, J.E.; Zhao, S.; Broering, J.M.; Carroll, P.R.; Ryan, C.J. Examining initial treatment and survival among men with metastatic prostate cancer: An analysis from the CaPSURE registry. Urol. Oncol. 2020, 38, e791–e793. [Google Scholar] [CrossRef] [PubMed]
- Riihimaki, M.; Thomsen, H.; Brandt, A.; Sundquist, J.; Hemminki, K. What do prostate cancer patients die of? Oncologist 2011, 16, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.F.; Lambert, P.C.; Mozumder, S.I.; Broggio, J.; Rutherford, M.J. Conditional crude probabilities of death for English cancer patients. Br. J. Cancer 2019, 121, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Riihimäki, M.; Thomsen, H.; Sundquist, K.; Sundquist, J.; Hemminki, K. Clinical landscape of cancer metastases. Cancer Med. 2018, 7, 5534–5542. [Google Scholar] [CrossRef] [PubMed]
- Helgstrand, J.T.; Røder, M.A.; Klemann, N.; Toft, B.G.; Brasso, K.; Vainer, B.; Iversen, P. Diagnostic characteristics of lethal prostate cancer. Eur. J. Cancer 2017, 84, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Orrason, A.W.; Westerberg, M.; Garmo, H.; Lissbrant, I.F.; Robinson, D.; Stattin, P. Changes in treatment and mortality in men with locally advanced prostate cancer between 2000 and 2016: A nationwide, population-based study in Sweden. BJU Int. 2020, 126, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, J.; Försti, A.; Hemminki, A.; Hemminki, K. Survival trends in solid cancers in the Nordic countries through 50 years. Eur. J. Cancer 2022, 175, 77–85. [Google Scholar] [CrossRef]
- Janssen-Heijnen, M.L.; Gondos, A.; Bray, F.; Hakulinen, T.; Brewster, D.H.; Brenner, H.; Coebergh, J.W. Clinical relevance of conditional survival of cancer patients in europe: Age-specific analyses of 13 cancers. J. Clin. Oncol. 2010, 28, 2520–2528. [Google Scholar] [CrossRef]
- Rosiello, G.; Palumbo, C.; Knipper, S.; Pecoraro, A.; Luzzago, S.; Deuker, M.; Mistretta, F.A.; Tian, Z.; Fossati, N.; Gallina, A.; et al. Contemporary conditional cancer-specific survival after radical nephroureterectomy in patients with nonmetastatic urothelial carcinoma of upper urinary tract. J. Surg. Oncol. 2020, 121, 1154–1161. [Google Scholar] [CrossRef]
- Tappero, S.; Cano Garcia, C.; Incesu, R.B.; Piccinelli, M.L.; Barletta, F.; Morra, S.; Scheipner, L.; Tian, Z.; Saad, F.; Shariat, S.F.; et al. Conditional survival for non-metastatic muscle-invasive adenocarcinoma of the urinary bladder after radical cystectomy. Surg. Oncol. 2023, 48, 101947. [Google Scholar] [CrossRef] [PubMed]
- Pukkala, E.; Engholm, G.; Hojsgaard Schmidt, L.K.; Storm, H.; Khan, S.; Lambe, M.; Pettersson, D.; Olafsdottir, E.; Tryggvadottir, L.; Hakanen, T.; et al. Nordic Cancer Registries—An overview of their procedures and data comparability. Acta Oncol. 2018, 57, 440–455. [Google Scholar] [CrossRef] [PubMed]
- Engholm, G.; Ferlay, J.; Christensen, N.; Bray, F.; Gjerstorff, M.L.; Klint, A.; Køtlum, J.E.; Olafsdóttir, E.; Pukkala, E.; Storm, H.H. NORDCAN—A Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010, 49, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Larønningen, S.; Ferlay, J.; Beydogan, H.; Bray, F.; Engholm, G.; Ervik, M.; Gulbrandsen, J.; Hansen, H.; Hansen, H.; Johannesen, T.; et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries; Version 9.2; Association of the Nordic Cancer Registries; Cancer Registry of Norway: Oslo, Norway, 2022. [Google Scholar]
- Storm, H.H.; Klint, A.; Tryggvadóttir, L.; Gislum, M.; Engholm, G.; Bray, F.; Hakulinen, T. Trends in the survival of patients diagnosed with malignant neoplasms of lymphoid, haematopoietic, and related tissue in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol. 2010, 49, 694–712. [Google Scholar] [CrossRef] [PubMed]
- Engholm, G.; Gislum, M.; Bray, F.; Hakulinen, T. Trends in the survival of patients diagnosed with cancer in the Nordic countries 1964–2003 followed up to the end of 2006. Material and methods. Acta Oncol. 2010, 49, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, F.E.; Andersson, T.M.; Lambe, M.; Engholm, G.; Mørch, L.S.; Johannesen, T.B.; Virtanen, A.; Pettersson, D.; Ólafsdóttir, E.J.; Birgisson, H.; et al. Trends in cancer survival in the Nordic countries 1990–2016: The NORDCAN survival studies. Acta Oncol. 2020, 59, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Tichanek, F.; Försti, A.; Liska, V.; Hemminki, A.; Hemminki, K. Survival in Colon, Rectal and Small Intestinal Cancers in the Nordic Countries through a Half Century. Cancers 2023, 15, 991. [Google Scholar] [CrossRef] [PubMed]
- Kvåle, R.; Auvinen, A.; Adami, H.O.; Klint, A.; Hernes, E.; Møller, B.; Pukkala, E.; Storm, H.H.; Tryggvadottir, L.; Tretli, S.; et al. Interpreting trends in prostate cancer incidence and mortality in the five Nordic countries. J. Natl. Cancer Inst. 2007, 99, 1881–1887. [Google Scholar] [CrossRef]
- Seikkula, H.; Kaipia, A.; Boström, P.J.; Malila, N.; Pitkäniemi, J.; Seppä, K. Periodic trends in geographical variation of prostate cancer incidence and mortality in Finland between 1985 and 2019. Acta Oncol. 2022, 61, 1209–1215. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Taitt, H.E. Global Trends and Prostate Cancer: A Review of Incidence, Detection, and Mortality as Influenced by Race, Ethnicity, and Geographic Location. Am. J. Mens Health 2018, 12, 1807–1823. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Han, K.; Shin, D.W.; Park, S.H.; Shin, H.B. Conditional Relative Survival and Competing Mortality of Patients with Prostate Cancer in Korea: A Nationwide Cohort Study. Cancer Epidemiol. Biomarkers Prev. 2021, 30, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Luyendijk, M.; Visser, O.; Blommestein, H.M.; de Hingh, I.; Hoebers, F.J.P.; Jager, A.; Sonke, G.S.; de Vries, E.G.E.; Uyl-de Groot, C.A.; Siesling, S. Changes in survival in de novo metastatic cancer in an era of new medicines. J. Natl. Cancer Inst. 2023, 115, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Francini, E.; Gray, K.P.; Xie, W.; Shaw, G.K.; Valença, L.; Bernard, B.; Albiges, L.; Harshman, L.C.; Kantoff, P.W.; Taplin, M.E.; et al. Time of metastatic disease presentation and volume of disease are prognostic for metastatic hormone sensitive prostate cancer (mHSPC). Prostate 2018, 78, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, L.; Bill-Axelson, A.; Steineck, G.; Garmo, H.; Palmgren, J.; Johansson, E.; Adami, H.O.; Johansson, J.E. Results from the Scandinavian Prostate Cancer Group Trial Number 4: A randomized controlled trial of radical prostatectomy versus watchful waiting. J. Natl. Cancer Institute. Monogr. 2012, 2012, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.; Vellekoop, A.; Ahmed, H.U.; Catto, J.; Emberton, M.; Nam, R.; Rosario, D.J.; Scattoni, V.; Lotan, Y. Systematic review of complications of prostate biopsy. Eur. Urol. 2013, 64, 876–892. [Google Scholar] [CrossRef]
- Lundberg, F.E.; Birgisson, H.; Johannesen, T.B.; Engholm, G.; Virtanen, A.; Pettersson, D.; Ólafsdóttir, E.J.; Lambe, M.; Lambert, P.C.; Mørch, L.S.; et al. Survival trends in patients diagnosed with colon and rectal cancer in the nordic countries 1990–2016: The NORDCAN survival studies. Eur. J. Cancer 2022, 172, 76–84. [Google Scholar] [CrossRef]

| Country | 1971–1975 | 2016–2020 | Increase, Fold |
|---|---|---|---|
| Denmark | 4869 | 22712 | 4.7 |
| Finland | 3411 | 25990 | 7.6 |
| Norway | 6061 | 25412 | 4.2 |
| Sweden | 16561 | 52130 | 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zitricky, F.; Försti, A.; Hemminki, A.; Hemminki, O.; Hemminki, K. Conditional Survival in Prostate Cancer in the Nordic Countries Elucidates the Timing of Improvements. Cancers 2023, 15, 4132. https://doi.org/10.3390/cancers15164132
Zitricky F, Försti A, Hemminki A, Hemminki O, Hemminki K. Conditional Survival in Prostate Cancer in the Nordic Countries Elucidates the Timing of Improvements. Cancers. 2023; 15(16):4132. https://doi.org/10.3390/cancers15164132
Chicago/Turabian StyleZitricky, Frantisek, Asta Försti, Akseli Hemminki, Otto Hemminki, and Kari Hemminki. 2023. "Conditional Survival in Prostate Cancer in the Nordic Countries Elucidates the Timing of Improvements" Cancers 15, no. 16: 4132. https://doi.org/10.3390/cancers15164132
APA StyleZitricky, F., Försti, A., Hemminki, A., Hemminki, O., & Hemminki, K. (2023). Conditional Survival in Prostate Cancer in the Nordic Countries Elucidates the Timing of Improvements. Cancers, 15(16), 4132. https://doi.org/10.3390/cancers15164132









