Predictive Factors of Response to Immunotherapy in Lymphomas: A Multicentre Clinical Data Warehouse Study (PRONOSTIM)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Endpoints
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of Patients
3.2. Efficacy Outcomes
3.3. Response Prediction
3.4. Adverse Events
3.5. Toxicity Prediction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Fowler, N.H.; Dickinson, M.; Dreyling, M.; Martinez-Lopez, J.; Kolstad, A.; Butler, J.; Ghosh, M.; Popplewell, L.; Chavez, J.C.; Bachy, E.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Follicular Lymphoma: The Phase 2 ELARA Trial. Nat. Med. 2022, 28, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, C.A.; Chavez, J.C.; Sehgal, A.R.; William, B.M.; Munoz, J.; Salles, G.; Munshi, P.N.; Casulo, C.; Maloney, D.G.; de Vos, S.; et al. Axicabtagene Ciloleucel in Relapsed or Refractory Indolent Non-Hodgkin Lymphoma (ZUMA-5): A Single-Arm, Multicentre, Phase 2 Trial. Lancet Oncol. 2022, 23, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zinzani, P.L.; Fanale, M.A.; Armand, P.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J. Clin. Oncol. 2017, 35, 2125–2132. [Google Scholar] [CrossRef]
- Armand, P.; Engert, A.; Younes, A.; Fanale, M.; Santoro, A.; Zinzani, P.L.; Timmerman, J.M.; Collins, G.P.; Ramchandren, R.; Cohen, J.B.; et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J. Clin. Oncol. 2018, 36, 1428–1439. [Google Scholar] [CrossRef]
- Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma|NEJM. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa2116133 (accessed on 16 January 2023).
- Kamdar, M.; Solomon, S.R.; Arnason, J.; Johnston, P.B.; Glass, B.; Bachanova, V.; Ibrahimi, S.; Mielke, S.; Mutsaers, P.; Hernandez-Ilizaliturri, F.; et al. Lisocabtagene Maraleucel versus Standard of Care with Salvage Chemotherapy Followed by Autologous Stem Cell Transplantation as Second-Line Treatment in Patients with Relapsed or Refractory Large B-Cell Lymphoma (TRANSFORM): Results from an Interim Analysis of an Open-Label, Randomised, Phase 3 Trial. Lancet 2022, 399, 2294–2308. [Google Scholar] [CrossRef]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Achufusi, A.; Armand, P.; Berkenstock, M.K.; Bhatia, S.; Budde, L.E.; Chokshi, S.; Davies, M.; et al. Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 387–405. [Google Scholar] [CrossRef]
- Santomasso, B.D.; Nastoupil, L.J.; Adkins, S.; Lacchetti, C.; Schneider, B.J.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Chimeric Antigen Receptor T-Cell Therapy: ASCO Guideline. J. Clin. Oncol. 2021, 39, 3978–3992. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-Term Safety and Activity of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma (ZUMA-1): A Single-Arm, Multicentre, Phase 1-2 Trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Tam, C.S.; Borchmann, P.; Worel, N.; McGuirk, J.P.; Holte, H.; Waller, E.K.; Jaglowski, S.; Bishop, M.R.; Damon, L.E.; et al. Long-Term Clinical Outcomes of Tisagenlecleucel in Patients with Relapsed or Refractory Aggressive B-Cell Lymphomas (JULIET): A Multicentre, Open-Label, Single-Arm, Phase 2 Study. Lancet Oncol. 2021, 22, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zinzani, P.L.; Lee, H.J.; Armand, P.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; et al. Pembrolizumab in Relapsed or Refractory Hodgkin Lymphoma: 2-Year Follow-up of KEYNOTE-087. Blood 2019, 134, 1144–1153. [Google Scholar] [CrossRef]
- De Filippi, R.; Morabito, F.; Santoro, A.; Tripepi, G.; D’Alò, F.; Rigacci, L.; Ricci, F.; Morelli, E.; Zinzani, P.L.; Pinto, A. Body Mass Index Is Not Associated with Survival Outcomes and Immune-Related Adverse Events in Patients with Hodgkin Lymphoma Treated with the Immune Checkpoint Inhibitor Nivolumab. J. Transl. Med. 2021, 19, 489. [Google Scholar] [CrossRef]
- Fattizzo, B.; Cavallaro, F.; Folino, F.; Barcellini, W. Recent Insights into the Role of the Microbiome in Malignant and Benign Hematologic Diseases. Crit. Rev. Oncol. Hematol. 2021, 160, 103289. [Google Scholar] [CrossRef]
- Swaidani, S.; Roopkumar, J.; Jun-Shim, Y.; Charles, C.; Paul, S.; Kundu, S.; Funchain, P.; Rayman, P.; Pavicic, P.G.; Diaz-Montero, C.M.; et al. Biomarker Assessment of Venous Thromboembolism in Cancer Patients Receiving Checkpoint Blockade. Blood 2019, 134, 3648. [Google Scholar] [CrossRef]
- Colombo, A.; Hav, M.; Singh, M.; Xu, A.; Gamboa, A.; Lemos, T.; Gerdtsson, E.; Chen, D.; Houldsworth, J.; Shaknovich, R.; et al. Single-Cell Spatial Analysis of Tumor Immune Architecture in Diffuse Large B-Cell Lymphoma. Blood Adv. 2022, 6, 4675–4690. [Google Scholar] [CrossRef]
- Hatic, H.; Sampat, D.; Goyal, G. Immune Checkpoint Inhibitors in Lymphoma: Challenges and Opportunities. Ann. Transl. Med. 2021, 9, 1037. [Google Scholar] [CrossRef]
- Roemer, M.G.M.; Advani, R.H.; Ligon, A.H.; Natkunam, Y.; Redd, R.A.; Homer, H.; Connelly, C.F.; Sun, H.H.; Daadi, S.E.; Freeman, G.J.; et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. J. Clin. Oncol. 2016, 34, 2690–2697. [Google Scholar] [CrossRef]
- Jeong, A.-R.; Ball, E.D.; Goodman, A.M. Predicting Responses to Checkpoint Inhibitors in Lymphoma: Are We Up to the Standards of Solid Tumors? Clin. Med. Insights Oncol. 2020, 14, 1179554920976366. [Google Scholar] [CrossRef]
- Chen, C.; Liu, S.; Jiang, X.; Huang, L.; Chen, F.; Wei, X.; Guo, H.; Shao, Y.; Li, Y.; Li, W. Tumor Mutation Burden Estimated by a 69-Gene-Panel Is Associated with Overall Survival in Patients with Diffuse Large B-Cell Lymphoma. Exp. Hematol. Oncol. 2021, 10, 20. [Google Scholar] [CrossRef]
- Jacobson, C.A.; Hunter, B.; Armand, P.; Kamihara, Y.; Ritz, J.; Rodig, S.J.; Wright, K.; Lipschitz, M.; Redd, R.A.; Maus, M.V.; et al. Axicabtagene Ciloleucel in the Real World: Outcomes and Predictors of Response, Resistance and Toxicity. Blood 2018, 132, 92. [Google Scholar] [CrossRef]
- Vercellino, L.; Di Blasi, R.; Kanoun, S.; Tessoulin, B.; Rossi, C.; D’Aveni-Piney, M.; Obéric, L.; Bodet-Milin, C.; Bories, P.; Olivier, P.; et al. Predictive Factors of Early Progression after CAR T-Cell Therapy in Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Blood Adv. 2020, 4, 5607–5615. [Google Scholar] [CrossRef] [PubMed]
- Iacoboni, G.; Villacampa, G.; Martinez-Cibrian, N.; Bailén, R.; Lopez Corral, L.; Sanchez, J.M.; Guerreiro, M.; Caballero, A.C.; Mussetti, A.; Sancho, J.; et al. Real-world Evidence of Tisagenlecleucel for the Treatment of Relapsed or Refractory Large B-cell Lymphoma. Cancer Med. 2021, 10, 3214–3223. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, E.; Pradhan, K.; Sica, R.A.; Bachier-Rodriguez, L.; Mantzaris, I.; Kornblum, N.; Shastri, A.; Gritsman, K.; Goldfinger, M.; Verma, A.; et al. Elevated LDH Greater than 400 U/L Portends Poorer Overall Survival in Diffuse Large B-Cell Lymphoma Patients Treated with CD19 CAR-T Cell Therapy in a Real World Multi-Ethnic Cohort. Exp. Hematol. Oncol. 2021, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Locke, F.L.; Rossi, J.M.; Neelapu, S.S.; Jacobson, C.A.; Miklos, D.B.; Ghobadi, A.; Oluwole, O.O.; Reagan, P.M.; Lekakis, L.J.; Lin, Y.; et al. Tumor Burden, Inflammation, and Product Attributes Determine Outcomes of Axicabtagene Ciloleucel in Large B-Cell Lymphoma. Blood Adv. 2020, 4, 4898–4911. [Google Scholar] [CrossRef]
- Bachy, E.; Le Gouill, S.; Di Blasi, R.; Sesques, P.; Manson, G.; Cartron, G.; Beauvais, D.; Roulin, L.; Gros, F.X.; Rubio, M.T.; et al. A Real-World Comparison of Tisagenlecleucel and Axicabtagene Ciloleucel CAR T Cells in Relapsed or Refractory Diffuse Large B Cell Lymphoma. Nat. Med. 2022, 28, 2145–2154. [Google Scholar] [CrossRef]
- Pennisi, M.; Sanchez-Escamilla, M.; Flynn, J.R.; Shouval, R.; Alarcon Tomas, A.; Silverberg, M.L.; Batlevi, C.; Brentjens, R.J.; Dahi, P.B.; Devlin, S.M.; et al. Modified EASIX Predicts Severe Cytokine Release Syndrome and Neurotoxicity after Chimeric Antigen Receptor T Cells. Blood Adv. 2021, 5, 3397–3406. [Google Scholar] [CrossRef]
- Greenbaum, U.; Strati, P.; Saliba, R.M.; Torres, J.; Rondon, G.; Nieto, Y.; Hosing, C.; Srour, S.A.; Westin, J.; Fayad, L.E.; et al. CRP and Ferritin in Addition to the EASIX Score Predict CAR-T-Related Toxicity. Blood Adv. 2021, 5, 2799–2806. [Google Scholar] [CrossRef]
- Rejeski, K.; Perez, A.; Sesques, P.; Hoster, E.; Berger, C.; Jentzsch, L.; Mougiakakos, D.; Frölich, L.; Ackermann, J.; Bücklein, V.; et al. CAR-HEMATOTOX: A Model for CAR T-Cell-Related Hematologic Toxicity in Relapsed/Refractory Large B-Cell Lymphoma. Blood 2021, 138, 2499–2513. [Google Scholar] [CrossRef] [PubMed]
- Rejeski, K.; Perez, A.; Iacoboni, G.; Penack, O.; Bücklein, V.; Jentzsch, L.; Mougiakakos, D.; Johnson, G.; Arciola, B.; Carpio, C.; et al. The CAR-HEMATOTOX Risk-Stratifies Patients for Severe Infections and Disease Progression after CD19 CAR-T in R/R LBCL. J. Immunother. Cancer 2022, 10, e004475. [Google Scholar] [CrossRef] [PubMed]
- Boardman, A.P.; Gutgarts, V.; Flynn, J.R.; Devlin, S.M.; Tomas, A.A.; Fein, J.A.; Slingerland, J.; Parascondola, A.; Lin, R.J.; Scordo, M.; et al. Predictors and Implications of Renal Injury in Large Cell Lymphoma Patients Receiving CD19 CAR T Cell Therapy; ASH. Blood 2022, 140, 4671–4672. [Google Scholar] [CrossRef]
- Shah, H.; O’Conke, D.; Zhao, R.; Jalota, A.; Patel, M.S.; Winter, A.; Williams, L.S.; Khouri, J.; Jagadeesh, D.; Anwer, F.; et al. Assessment of Major Adverse Cardiac Events (MACE) and Arrhythmias in Patients with Large B-Cell Lymphoma Undergoing Anti-CD19 Chimeric Antigen Receptor T-Cell (CAR-T) Therapy: Impact of Baseline Cardiac Biomarkers; ASH. Blood 2022, 140, 4673–4675. [Google Scholar] [CrossRef]
- Smith, M.; Dai, A.; Ghilardi, G.; Amelsberg, K.V.; Devlin, S.M.; Pajarillo, R.; Slingerland, J.B.; Beghi, S.; Herrera, P.S.; Giardina, P.; et al. Gut Microbiome Correlates of Response and Toxicity Following Anti-CD19 CAR T Cell Therapy. Nat. Med. 2022, 28, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, Z.; Geng, Y.; Gao, L.; Xu, L.; Tang, G.; Ni, X.; Chen, L.; Chen, J.; Wang, T.; et al. The Risk Factors and Early Predictive Model of Hematotoxicity after CD19 Chimeric Antigen Receptor T Cell Therapy. Front. Oncol. 2022, 12, 987965. [Google Scholar] [CrossRef]
- Hasenclever, D.; Diehl, V. A Prognostic Score for Advanced Hodgkin’s Disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N. Engl. J. Med. 1998, 339, 1506–1514. [Google Scholar] [CrossRef]
- A Predictive Model for Aggressive Non-Hodgkin’s Lymphoma|NEJM. Available online: https://www.nejm.org/doi/full/10.1056/nejm199309303291402 (accessed on 5 January 2023).
- Common Terminology Criteria for Adverse Events (CTCAE). 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (accessed on 5 January 2023).
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal Microbiota Transplant Promotes Response in Immunotherapy-Refractory Melanoma Patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef]
- Bigot, F.; Castanon, E.; Baldini, C.; Hollebecque, A.; Carmona, A.; Postel-Vinay, S.; Angevin, E.; Armand, J.-P.; Ribrag, V.; Aspeslagh, S.; et al. Prospective Validation of a Prognostic Score for Patients in Immunotherapy Phase I Trials: The Gustave Roussy Immune Score (GRIm-Score). Eur. J. Cancer 2017, 84, 212–218. [Google Scholar] [CrossRef]
- Aggen, D.H.; Ager, C.R.; Obradovic, A.Z.; Chowdhury, N.; Ghasemzadeh, A.; Mao, W.; Chaimowitz, M.G.; Lopez-Bujanda, Z.A.; Spina, C.S.; Hawley, J.E.; et al. Blocking IL1 Beta Promotes Tumor Regression and Remodeling of the Myeloid Compartment in a Renal Cell Carcinoma Model: Multidimensional Analyses. Clin. Cancer Res. 2021, 27, 608–621. [Google Scholar] [CrossRef]
- Angell, H.K.; Bruni, D.; Barrett, J.C.; Herbst, R.; Galon, J. The Immunoscore: Colon Cancer and Beyond. Clin. Cancer Res. 2020, 26, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex Differences in Immune Responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer Immunotherapy Efficacy and Patients’ Sex: A Systematic Review and Meta-Analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef]
- Duma, N.; Abdel-Ghani, A.; Yadav, S.; Hoversten, K.P.; Reed, C.T.; Sitek, A.N.; Enninga, E.A.L.; Paludo, J.; Aguilera, J.V.; Leventakos, K.; et al. Sex Differences in Tolerability to Anti-Programmed Cell Death Protein 1 Therapy in Patients with Metastatic Melanoma and Non-Small Cell Lung Cancer: Are We All Equal? Oncologist 2019, 24, e1148–e1155. [Google Scholar] [CrossRef]
- van der Kooij, M.K.; Dekkers, O.M.; Aarts, M.J.B.; van den Berkmortel, F.W.P.J.; Boers-Sonderen, M.J.; de Groot, J.W.B.; Hospers, G.A.P.; Piersma, D.; van Rijn, R.S.; Suijkerbuijk, K.P.M.; et al. Sex-Based Differences in Treatment with Immune Checkpoint Inhibition and Targeted Therapy for Advanced Melanoma: A Nationwide Cohort Study. Cancers 2021, 13, 4639. [Google Scholar] [CrossRef]
- Nishijima, T.F.; Muss, H.B.; Shachar, S.S.; Moschos, S.J. Comparison of Efficacy of Immune Checkpoint Inhibitors (ICIs) between Younger and Older Patients: A Systematic Review and Meta-Analysis. Cancer Treat. Rev. 2016, 45, 30–37. [Google Scholar] [CrossRef]
- Baldini, C.; Martin Romano, P.; Voisin, A.-L.; Danlos, F.-X.; Champiat, S.; Laghouati, S.; Kfoury, M.; Vincent, H.; Postel-Vinay, S.; Varga, A.; et al. Impact of Aging on Immune-Related Adverse Events Generated by Anti-Programmed Death (Ligand)PD-(L)1 Therapies. Eur. J. Cancer 2020, 129, 71–79. [Google Scholar] [CrossRef]
- Inc, M.G. Car T-Cells Associated Acute Toxicity in B-Cell Non-Hodgkin Lymphoma: Real-World Study from the Descar-T Registry by Dr. Pierre Sesques. Available online: https://library.ehaweb.org/eha/2022/eha2022-congress/357074/pierre.sesques.car.t-cells.associated.acute.toxicity.in.b-cell.non-hodgkin.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D2%2Asearch%3Dcar-t (accessed on 30 September 2022).
- Neelapu, S.S.; Jacobson, C.A.; Oluwole, O.O.; Munoz, J.; Deol, A.; Miklos, D.B.; Bartlett, N.L.; Braunschweig, I.; Jiang, Y.; Kim, J.J.; et al. Outcomes of Older Patients in ZUMA-1, a Pivotal Study of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma. Blood 2020, 135, 2106–2109. [Google Scholar] [CrossRef]
- Ram, R.; Grisariu, S.; Shargian-Alon, L.; Amit, O.; Bar-On, Y.; Stepensky, P.; Yeshurun, M.; Avni, B.; Hagin, D.; Perry, C.; et al. Toxicity and Efficacy of Chimeric Antigen Receptor T-Cell Therapy in Patients with Diffuse Large B-Cell Lymphoma above the Age of 70 Years Compared to Younger Patients—A Matched Control Multicenter Cohort Study. Haematologica 2022, 107, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Wudhikarn, K.; Bansal, R.; Khurana, A.; Hathcock, M.A.; Bennani, N.N.; Paludo, J.; Villasboas, J.C.; Wang, Y.; Johnston, P.B.; Ansell, S.M.; et al. The Impact of Obesity and Body Weight on the Outcome of Patients with Relapsed/Refractory Large B-Cell Lymphoma Treated with Axicabtagene Ciloleucel. Blood Cancer J. 2021, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Westin, J.R.; Tam, C.S.; Borchmann, P.; Jaeger, U.; McGuirk, J.P.; Holte, H.; Waller, E.K.; Jaglowski, S.; Bishop, M.R.; Andreadis, C.; et al. Correlative Analyses of Patient and Clinical Characteristics Associated with Efficacy in Tisagenlecleucel-Treated Relapsed/Refractory Diffuse Large B-Cell Lymphoma Patients in the Juliet Trial. Blood 2019, 134, 4103. [Google Scholar] [CrossRef]
- Karschnia, P.; Jordan, J.T.; Forst, D.A.; Arrillaga-Romany, I.C.; Batchelor, T.T.; Baehring, J.M.; Clement, N.F.; Gonzalez Castro, L.N.; Herlopian, A.; Maus, M.V.; et al. Clinical Presentation, Management, and Biomarkers of Neurotoxicity after Adoptive Immunotherapy with CAR T Cells. Blood 2019, 133, 2212–2221. [Google Scholar] [CrossRef]
- Jacobson, C.A.; Hunter, B.D.; Redd, R.; Rodig, S.J.; Chen, P.-H.; Wright, K.; Lipschitz, M.; Ritz, J.; Kamihara, Y.; Armand, P.; et al. Axicabtagene Ciloleucel in the Non-Trial Setting: Outcomes and Correlates of Response, Resistance, and Toxicity. J. Clin. Oncol. 2020, 38, 3095–3106. [Google Scholar] [CrossRef] [PubMed]
- Brammer, J.E.; Braunstein, Z.; Katapadi, A.; Porter, K.; Biersmith, M.; Guha, A.; Vasu, S.; Yildiz, V.O.; Smith, S.A.; Buck, B.; et al. Early Toxicity and Clinical Outcomes after Chimeric Antigen Receptor T-Cell (CAR-T) Therapy for Lymphoma. J. Immunother. Cancer 2021, 9, e002303. [Google Scholar] [CrossRef]

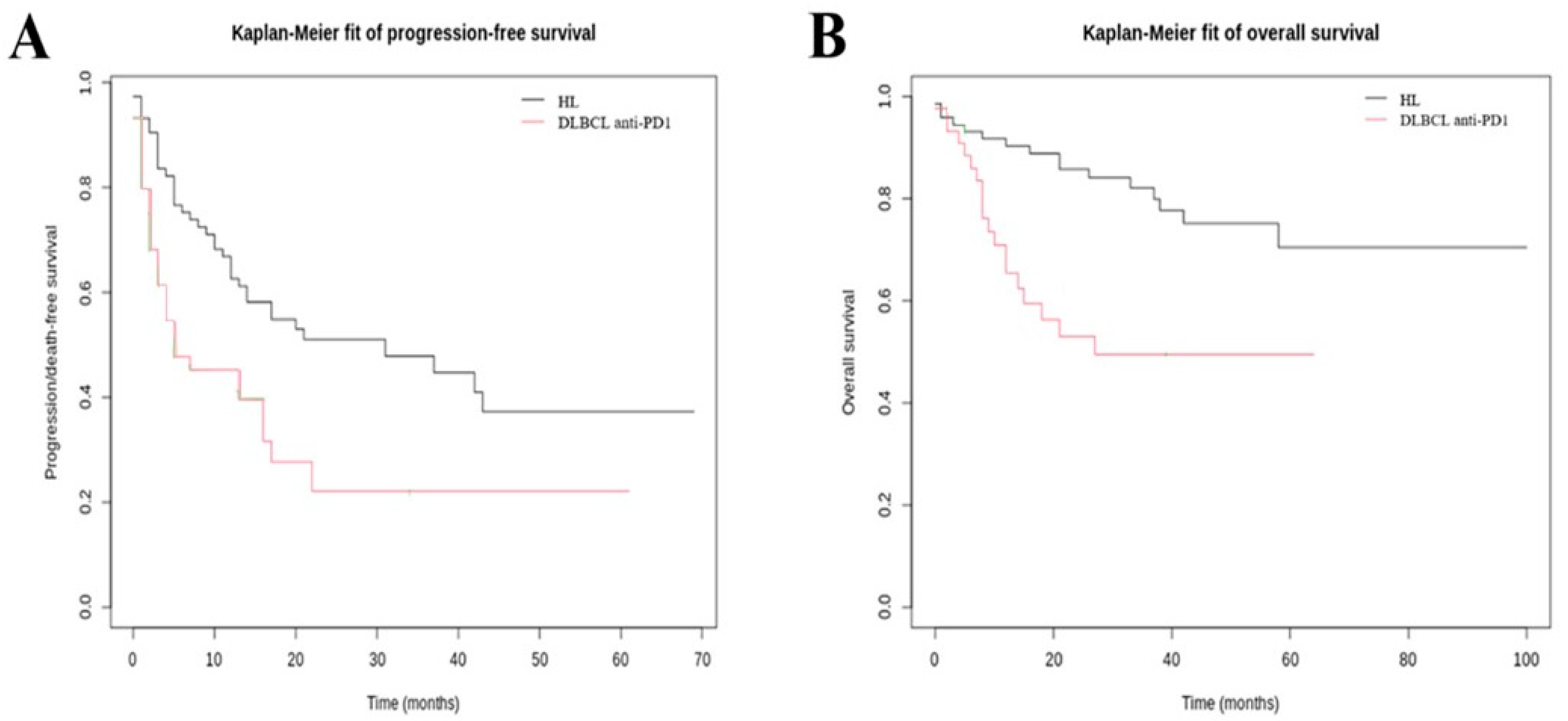

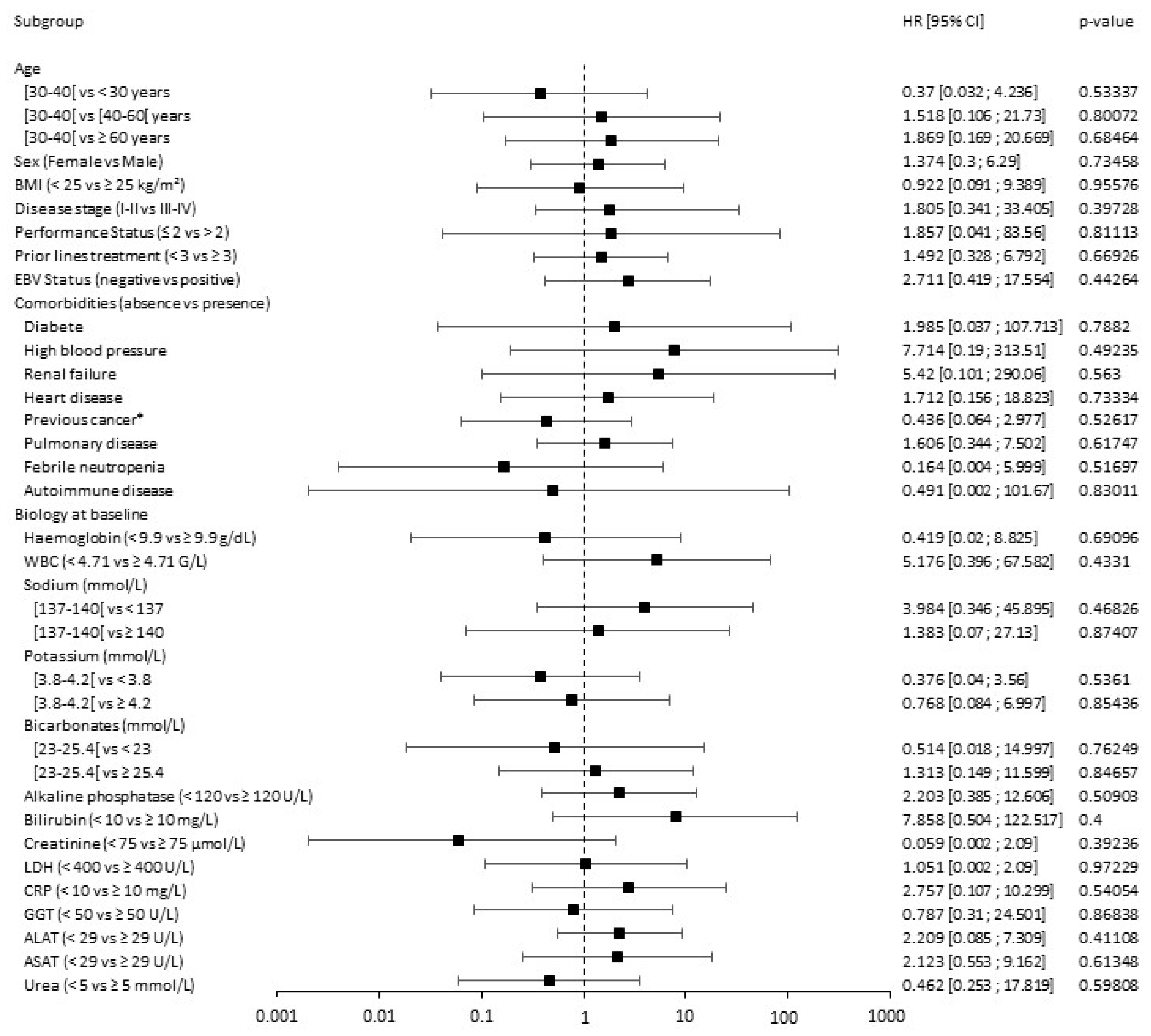
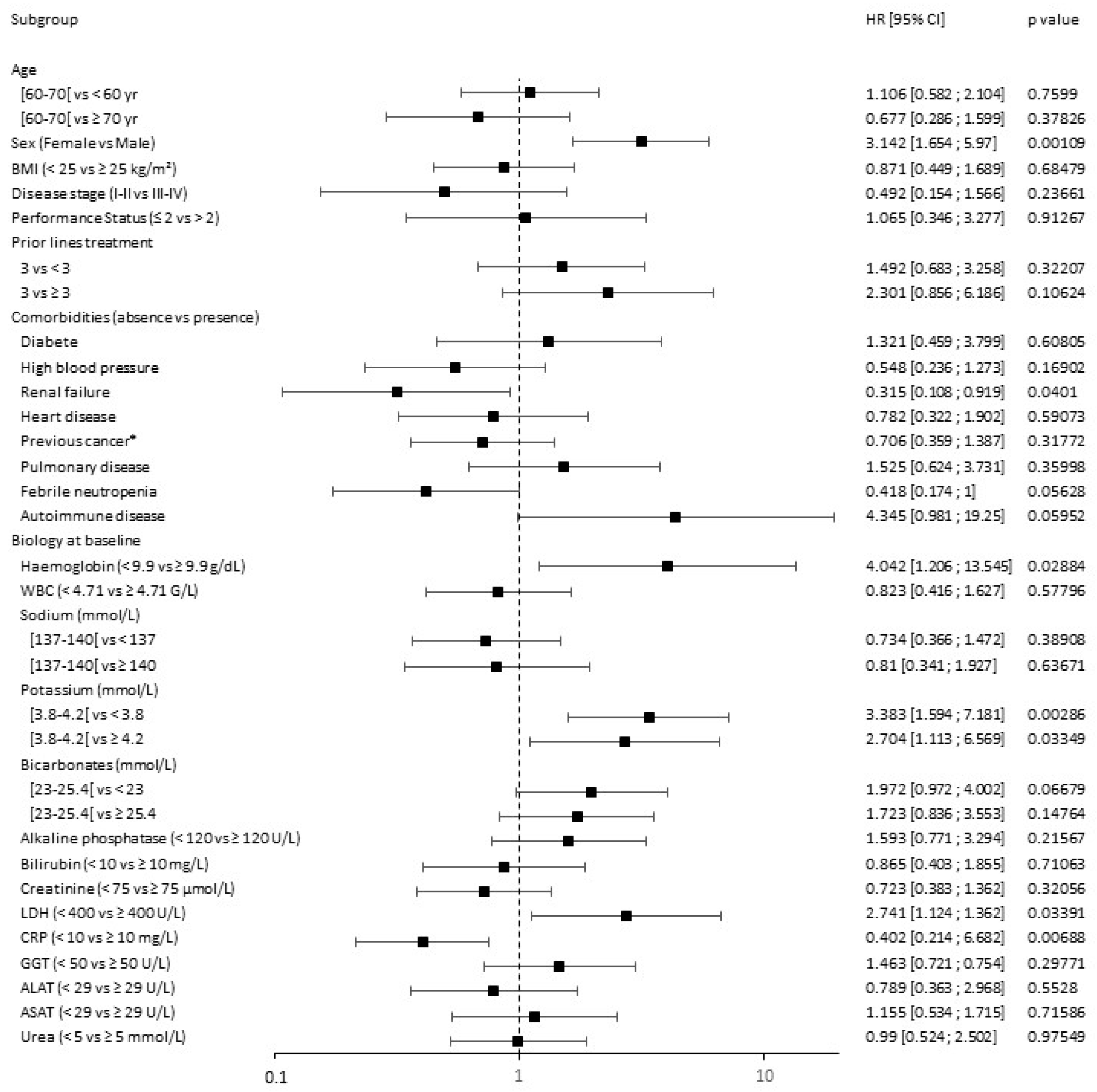
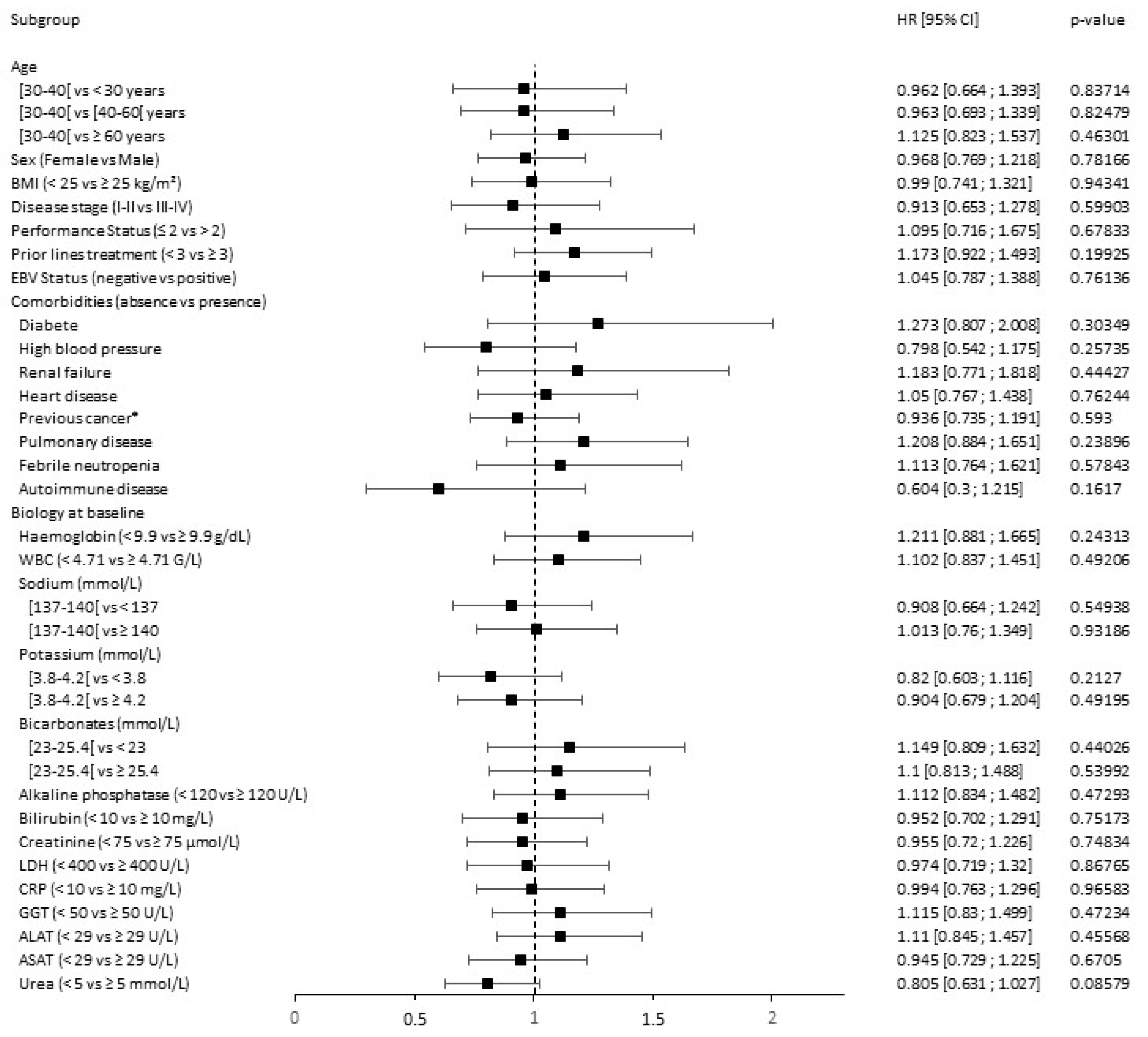

| Hodgkin Lymphoma Cohort | DLBCL Anti-PD-1 Treated Cohort | DLBCL CAR T Cells Treated Cohort | ||
|---|---|---|---|---|
| Characteristics | n = 73 | n = 44 | n = 132 | |
| Sex | ||||
| Male | n (%) | 47 (64.4) | 22 (50.0) | 82 (62.1) |
| Female | n (%) | 26 (35.6) | 22 (50.0) | 50 (37.9) |
| Age at time of (1st) treatment infusion (years) | Med [IQR] | 54.0 [34.0–71.0] | 57.0 [38.5–74.2] | 63.0 [55.0–69.2] |
| min; max | 18.0; 86.0 | 18.0; 84.0 | 25.0; 80.0 | |
| BMI (kg/m2) | Med [IQR] | 23.5 [19.7–25.8] | 23.6 [20.3–26.4] | 24.5 [21.7–27.1] |
| min; max | 14.9; 34.7 | 13.3; 31.6 | 14.6; 39.5 | |
| Including obesity background | n (%) | 6 (8.2) | 1 (2.3) | 6 (4.5) |
| Comorbidities | ||||
| Diabete | n (%) | 3 (4.1) | 5 (11.4) | 11 (8.3) |
| High blood pressure | n (%) | 13 (17.8) | 6 (13.6) | 31 (23.5) |
| Renal failure | n (%) | 9 (12.3) | 4 (9.1) | 15 (11.4) |
| Heart disease | n (%) | 18 (24.7) | 7 (15.9) | 29 (22.0) |
| Previous cancer * | n (%) | 18 (24.7) | 15 (34.1) | 36 (27.3) |
| Pulmonary disease | n (%) | 11 (15.1) | 7 (15.9) | 16 (12.1) |
| Febrile neutropenia | n (%) | 6 (8.2) | 6 (13.6) | 17 (12.9) |
| Autoimmune disease | n (%) | 2 (2.7) | 1 (2.3) | 5 (3.8) |
| EBV positive status | n (%) | 18 (24.7) | 6 (13.6) | 12 (9.1) |
| Stage at diagnosis | ||||
| Local disease (I/II stages) | n (%) | 9 (12.3) | 5 (11.4) | 9 (6.8) |
| Dissiminated disease (III/IV stages) | n (%) | 64 (87.7) | 39 (88.6) | 123 (93.2) |
| PS at diagnosis | ||||
| 0–2 | n (%) | 53 (89.8) | 27 (87.1) | 118 (93.6) |
| 3–4 | n (%) | 6 (10.2) | 4 (12.9) | 8 (6.4) |
| Missing | n (%) | 14 (19.2) | 13 (29.5) | 6 (4.5) |
| Pronostic score at diagnosis | ||||
| IPI | ||||
| 0–3 | n (%) | - | 15 (83.3%) | 69 (71.1%) |
| ≥4 | n (%) | - | 3.0 (16.7%) | 28 (28.8%) |
| Missing | n (%) | - | 26 (59.1) | 35 (26.5) |
| IPS | ||||
| 0–3 | n (%) | 9 (34.6%) | - | - |
| ≥4 | n (%) | 17 (65.4%) | - | - |
| Missing | n (%) | 47 (64.4) | - | - |
| Prior lines treatment | Med [IQR] | 2.0 [1.0–3.0] | 1.5 [1.0–3.0] | 2.0 [2.0–3.0] |
| min; max | 0.0; 7.0 | 1.0; 9.0 | 0.0; 6.0 | |
| Including autograft | n (%) | 16 (21.9) | 6 (13.6) | 27 (20.5) |
| Last PFS (months) | Med [IQR] | 5.0 [2.5–10.0] | 5.0 [2.2–10.8] | 3.0 [2.0–6.0] |
| min; max | 1.0; 79.0 | 1.0; 72.0 | 1.0; 53.0 | |
| Missing | n (%) | 10 (13.7) | 2 (4.5) | 1 (0.8) |
| Biology at baseline | ||||
| Potassium (mmol/L) | Med [IQR] | 4.0 [3.8–4.3] | 4.1 [3.8–4.3] | 4.1 [3.7–4.3] |
| min; max | 3.0; 6.3 | 3.0; 5.1 | 2.4; 6.4 | |
| Missing | n (%) | 1 (1.4) | 7 (15.9) | 1 (0.8) |
| Hemoglobin (g/dL) | Med [IQR] | 11.4 [9.8–13.0] | 11.4 [9.7–12.8] | 10.5 [9.2–12.3] |
| min; max | 5.0; 16.9 | 7.7; 15.7 | 3.3; 15.5 | |
| Missing | n (%) | 0 (0.0) | 3 (6.8) | 3 (2.3) |
| LDH (U/L) | Med [IQR] | 266.0 [197.0–327.5] | 250.5 [202.5–337.5] | 334.5 [229.5–544.2] |
| min; max | 107.0; 2224.0 | 156.0; 1109.0 | 134.0; 4428.0 | |
| Missing | n (%) | 2 (2.7) | 10 (22.7) | 4 (3.0) |
| Hodgkin Lymphoma Cohort | DLBCL Anti-PD-1 Treated Cohort | DLBCL CAR T Cells Treated Cohort | ||
|---|---|---|---|---|
| Adverse events | n = 73 | n = 44 | n = 132 | |
| CRS | n (%) | - | - | 103 (78.0) |
| Including grade ≥3 | n (%) | - | - | 15 (11.4) |
| ICANS | n (%) | - | - | 47 (35.6) |
| Including grade ≥3 | n (%) | - | - | 16 (12.1) |
| Hematological disorders grade ≥3 | n (%) | 35 (47.9) | 16 (36.4) | 40 (30.3) |
| Hepatic disorders grade ≥3 | n (%) | 11 (15.1) | 4 (9.1) | 34 (25.8) |
| Kidney disorders grade ≥3 | n (%) | 5 (6.8) | 2 (4.5) | 30 (22.7) |
| Endocrine disorders grade ≥3 | n (%) | 7 (9.6) | 2 (4.5) | 1 (0.8) |
| Infections grade ≥3 | n (%) | 6.0 (8.2) | 0.0 (0.0) | 35.0 (26.5) |
| Cardiac disorders grade ≥3 | n (%) | 6.0 (8.2) | 0.0 (0.0) | 9.0 (6.8) |
| Skin disorders grade ≥3 | n (%) | 5 (6.8) | 1 (2.3) | 3 (2.3) |
| Neurological disorders grade ≥3 | n (%) | 4 (5.5) | 1 (2.3) | 1 (0.8) |
| Digestive disorders grade ≥3 | n (%) | 3 (4.1) | 3 (6.8) | 12 (9.1) |
| Respiratory and lung disorders grade ≥3 | n (%) | 3.0 (4.1) | 0.0 (0.0) | 1.0 (0.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Detroit, M.; Collier, M.; Beeker, N.; Willems, L.; Decroocq, J.; Deau-Fischer, B.; Vignon, M.; Birsen, R.; Moufle, F.; Leclaire, C.; et al. Predictive Factors of Response to Immunotherapy in Lymphomas: A Multicentre Clinical Data Warehouse Study (PRONOSTIM). Cancers 2023, 15, 4028. https://doi.org/10.3390/cancers15164028
Detroit M, Collier M, Beeker N, Willems L, Decroocq J, Deau-Fischer B, Vignon M, Birsen R, Moufle F, Leclaire C, et al. Predictive Factors of Response to Immunotherapy in Lymphomas: A Multicentre Clinical Data Warehouse Study (PRONOSTIM). Cancers. 2023; 15(16):4028. https://doi.org/10.3390/cancers15164028
Chicago/Turabian StyleDetroit, Marion, Mathis Collier, Nathanaël Beeker, Lise Willems, Justine Decroocq, Bénédicte Deau-Fischer, Marguerite Vignon, Rudy Birsen, Frederique Moufle, Clément Leclaire, and et al. 2023. "Predictive Factors of Response to Immunotherapy in Lymphomas: A Multicentre Clinical Data Warehouse Study (PRONOSTIM)" Cancers 15, no. 16: 4028. https://doi.org/10.3390/cancers15164028
APA StyleDetroit, M., Collier, M., Beeker, N., Willems, L., Decroocq, J., Deau-Fischer, B., Vignon, M., Birsen, R., Moufle, F., Leclaire, C., Balladur, E., Deschamps, P., Chauchet, A., Batista, R., Limat, S., Treluyer, J.-M., Ricard, L., Stocker, N., Hermine, O., ... Zerbit, J. (2023). Predictive Factors of Response to Immunotherapy in Lymphomas: A Multicentre Clinical Data Warehouse Study (PRONOSTIM). Cancers, 15(16), 4028. https://doi.org/10.3390/cancers15164028







