Prognostic Implication of YY1 and CP2c Expression in Patients with Primary Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Clinical Specimens
2.2. Tissue Microarray Construction
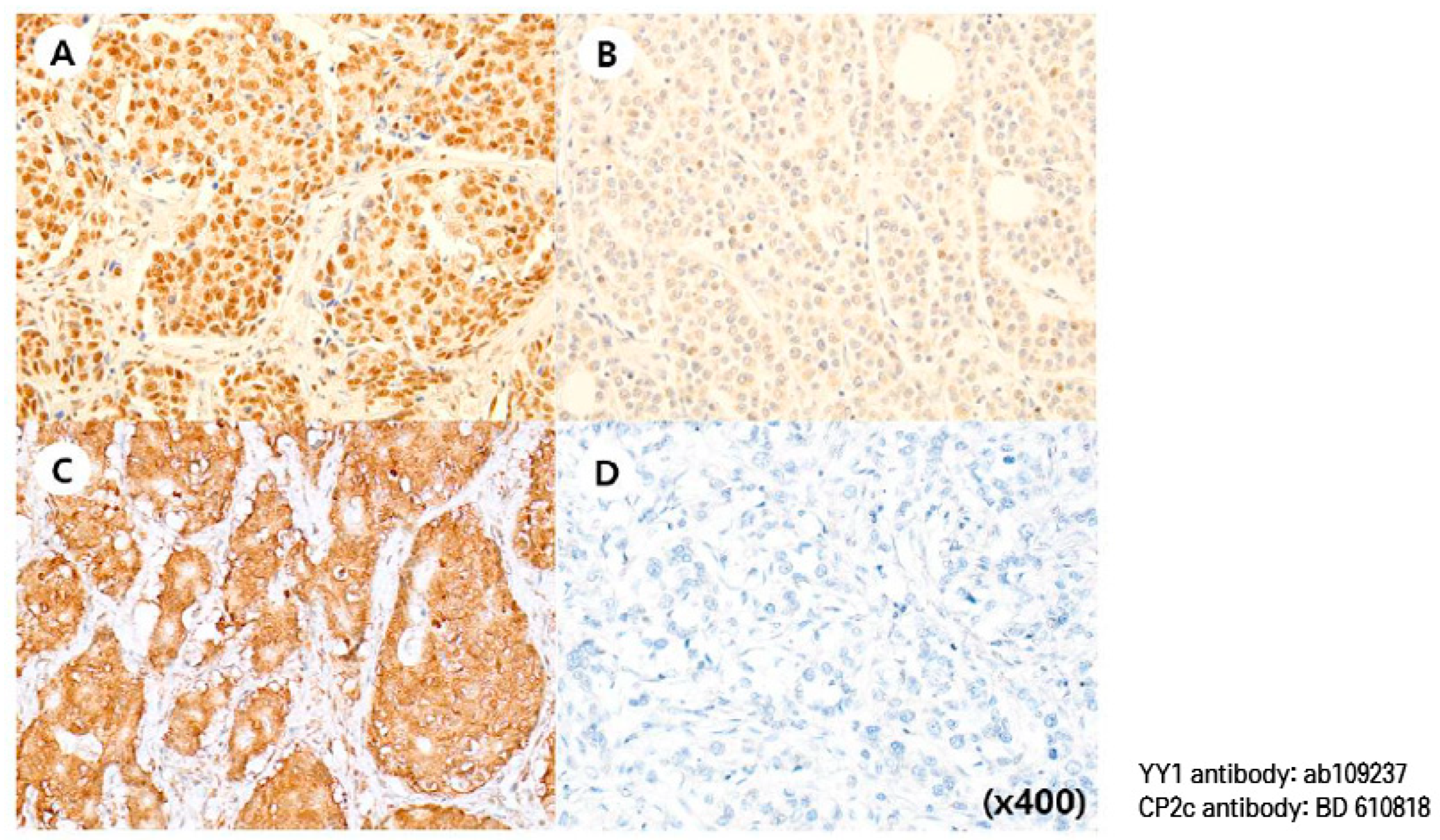
2.3. Immunohistochemical Staining
2.4. Interpretation of IHC Staining
2.5. RT-qPCR from FFPE Tissue
2.6. Western Blot from FFPE Tissue
2.7. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. Clinicopathologic Parameters Versus YY1 Expression
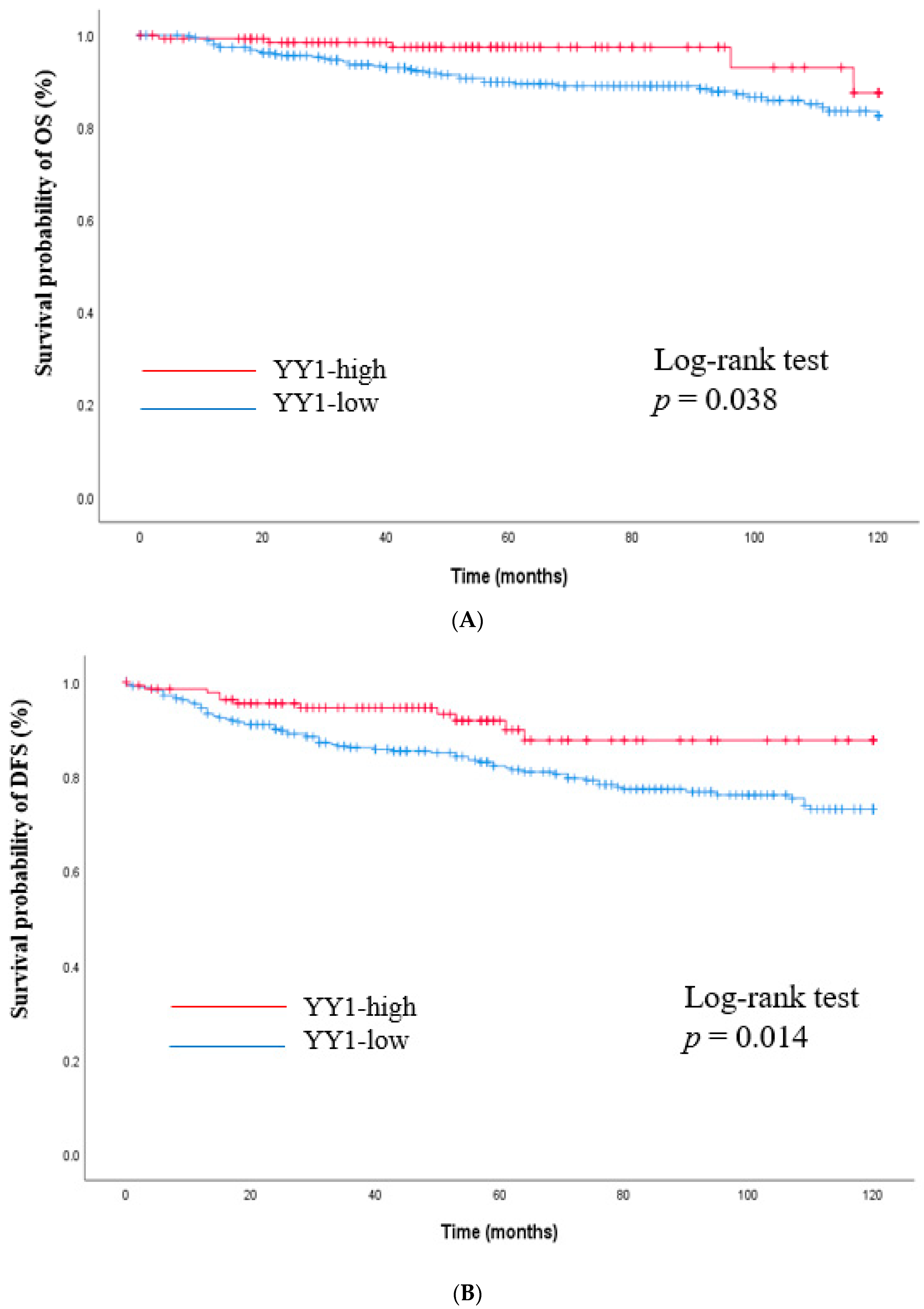
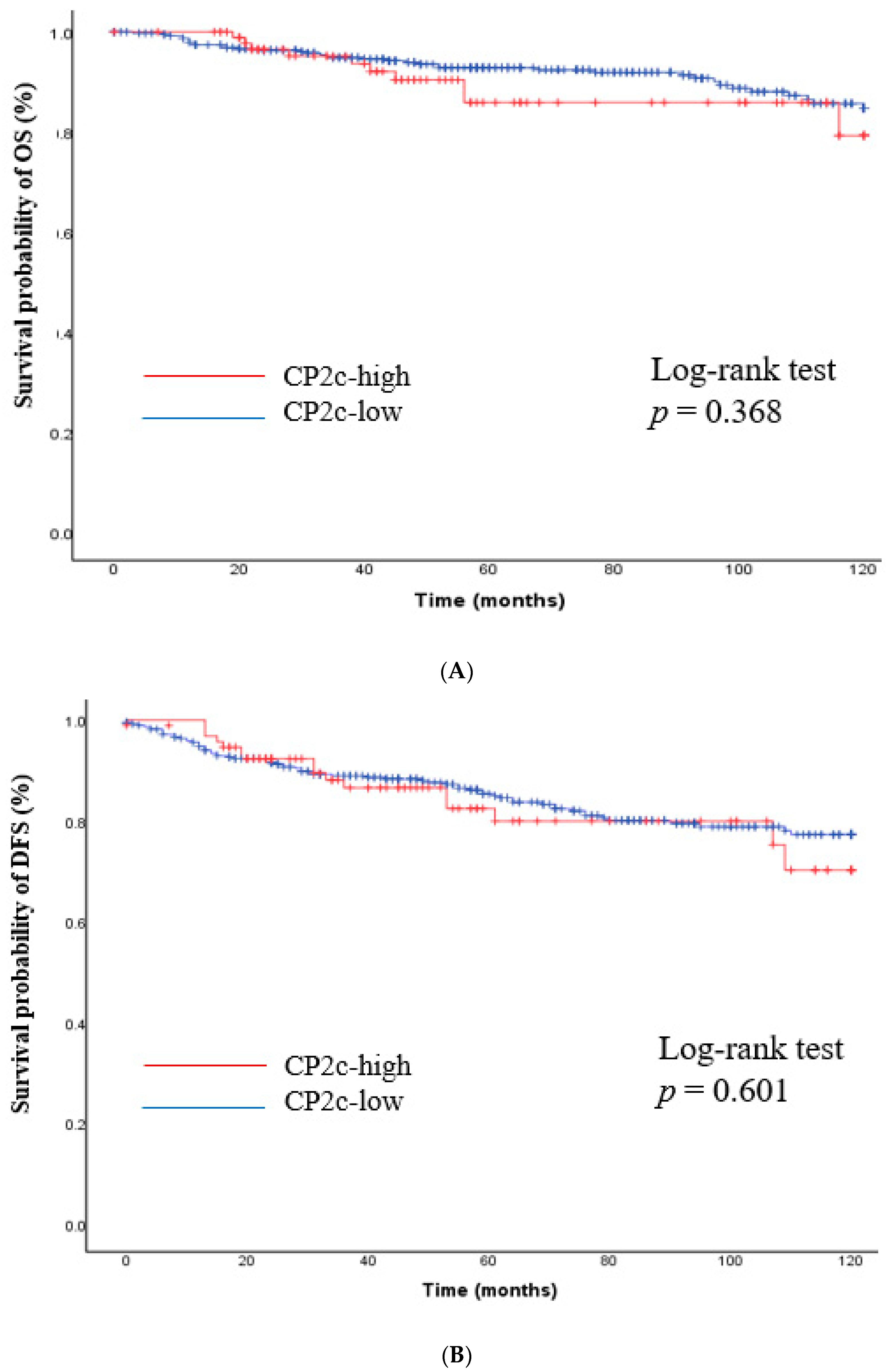
3.3. Survival Outcomes Versus YY1 Expression
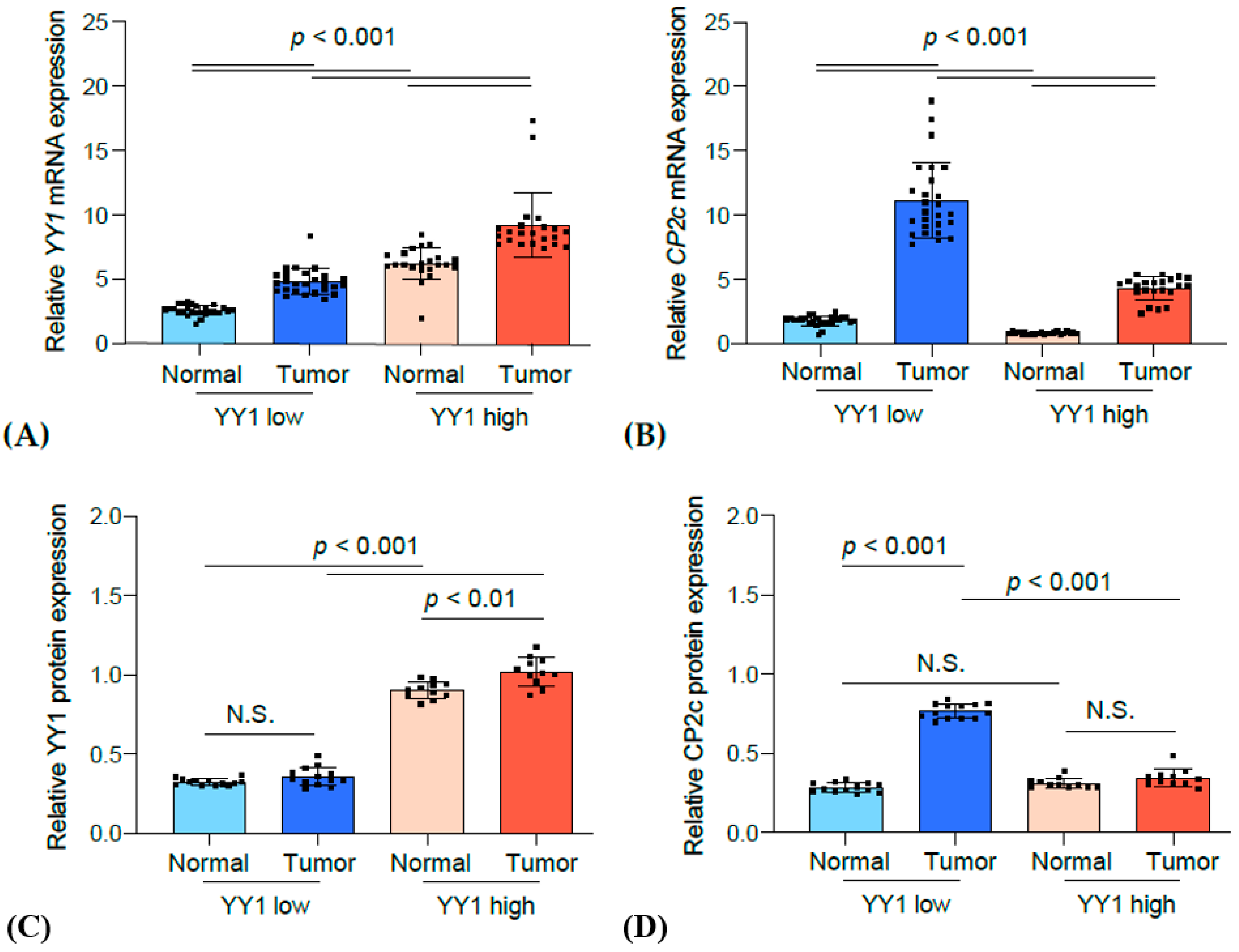
3.4. Correlation Analysis between YY1 and CP2c Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J.; Albain, K.S.; André, F.; Bergh, J.; et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Bonavida, B.; Baritaki, S. The novel role of Yin Yang 1 in the regulation of epithelial to mesenchymal transition in cancer via the dysregulated NF-κB/Snail/YY1/RKIP/PTEN Circuitry. Crit. Rev. Oncog. 2011, 16, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Sarvagalla, S.; Kolapalli, S.P.; Vallabhapurapu, S. The Two Sides of YY1 in Cancer: A Friend and a Foe. Front. Oncol. 2019, 9, 1230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Stovall, D.B.; Inoue, K.; Sui, G. The oncogenic role of Yin Yang 1. Crit. Rev. Oncog. 2011, 16, 163–197. [Google Scholar] [CrossRef] [PubMed]
- Atchison, M.; Basu, A.; Zaprazna, K.; Papasani, M. Mechanisms of Yin Yang 1 in oncogenesis: The importance of indirect effects. Crit. Rev. Oncog. 2011, 16, 143–161. [Google Scholar] [CrossRef]
- Huang, T.; Wang, G.; Yang, L.; Peng, B.; Wen, Y.; Ding, G.; Wang, Z. Transcription Factor YY1 Modulates Lung Cancer Progression by Activating lncRNA-PVT1. DNA Cell Biol. 2017, 36, 947–958. [Google Scholar] [CrossRef]
- Xu, C.; Tsai, Y.H.; Galbo, P.M., Jr.; Gong, W.; Storey, A.J.; Xu, Y.; Byrum, S.D.; Xu, L.; Whang, Y.E.; Parker, J.S.; et al. Cistrome analysis of YY1 uncovers a regulatory axis of YY1:BRD2/4-PFKP during tumorigenesis of advanced prostate cancer. Nucleic Acids Res. 2021, 49, 4971–4988. [Google Scholar] [CrossRef]
- Khachigian, L.M. The Yin and Yang of YY1 in tumor growth and suppression. Int. J. Cancer 2018, 143, 460–465. [Google Scholar] [CrossRef]
- Jeon, Y.; Lee, J.T. YY1 tethers Xist RNA to the inactive X nucleation center. Cell 2011, 146, 119–133. [Google Scholar] [CrossRef]
- Sigova, A.A.; Abraham, B.J.; Ji, X.; Molinie, B.; Hannett, N.M.; Guo, Y.E.; Jangi, M.; Giallourakis, C.C.; Sharp, P.A.; Young, R.A. Transcription factor trapping by RNA in gene regulatory elements. Science 2015, 350, 978–981. [Google Scholar] [CrossRef]
- Weintraub, A.S.; Li, C.H.; Zamudio, A.V.; Sigova, A.A.; Hannett, N.M.; Day, D.S.; Abraham, B.J.; Cohen, M.A.; Nabet, B.; Buckley, D.L.; et al. YY1 Is a Structural Regulator of Enhancer-Promoter Loops. Cell 2017, 171, 1573–1588.e28. [Google Scholar] [CrossRef]
- Gabriele, M.; Vulto-van Silfhout, A.T.; Germain, P.L.; Vitriolo, A.; Kumar, R.; Douglas, E.; Haan, E.; Kosaki, K.; Takenouchi, T.; Rauch, A.; et al. YY1 Haploinsufficiency Causes an Intellectual Disability Syndrome Featuring Transcriptional and Chromatin Dysfunction. Am. J. Hum. Genet. 2017, 100, 907–925. [Google Scholar] [CrossRef]
- Allouche, A.; Nolens, G.; Tancredi, A.; Delacroix, L.; Mardaga, J.; Fridman, V.; Winkler, R.; Boniver, J.; Delvenne, P.; Begon, D.Y. The combined immunodetection of AP-2alpha and YY1 transcription factors is associated with ERBB2 gene overexpression in primary breast tumors. Breast Cancer Res. 2008, 10, R9. [Google Scholar] [CrossRef]
- Yang, W.; Feng, B.; Meng, Y.; Wang, J.; Geng, B.; Cui, Q.; Zhang, H.; Yang, Y.; Yang, J. FAM3C-YY1 axis is essential for TGFβ-promoted proliferation and migration of human breast cancer MDA-MB-231 cells via the activation of HSF1. J. Cell. Mol. Med. 2019, 23, 3464–3475. [Google Scholar] [CrossRef]
- Lee, M.H.; Lahusen, T.; Wang, R.H.; Xiao, C.; Xu, X.; Hwang, Y.S.; He, W.W.; Shi, Y.; Deng, C.X. Yin Yang 1 positively regulates BRCA1 and inhibits mammary cancer formation. Oncogene 2012, 31, 116–127. [Google Scholar] [CrossRef]
- Kotarba, G.; Krzywinska, E.; Grabowska, A.I.; Taracha, A.; Wilanowski, T. TFCP2/TFCP2L1/UBP1 transcription factors in cancer. Cancer Lett. 2018, 420, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Ye, Y.F.; Ruan, L.W.; Bao, L.; Wu, M.W.; Zhou, Y. Inhibition of miR-660-5p expression suppresses tumor development and metastasis in human breast cancer. Genet. Mol. Res. 2017, 16, gmr16019479. [Google Scholar] [CrossRef] [PubMed]
- Broniarczyk, J.K.; Warowicka, A.; Kwaśniewska, A.; Wohuń-Cholewa, M.; Kwaśniewski, W.; Goździcka-Józefiak, A. Expression of TSG101 protein and LSF transcription factor in HPV-positive cervical cancer cells. Oncol. Lett. 2014, 7, 1409–1413. [Google Scholar] [CrossRef]
- Fan, R.; Chen, P.; Zhao, D.; Tong, J.L.; Li, J.; Liu, F. Cooperation of deregulated Notch signaling and Ras pathway in human hepatocarcinogenesis. J. Mol. Histol. 2011, 42, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Du, J.; Jin, J.; Qi, X.; Pu, Y.; Fei, B. LSF expression and its prognostic implication in colorectal cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 6024–6031. [Google Scholar] [PubMed]
- Fan, R.H.; Li, J.; Wu, N.; Chen, P.S. Late SV40 factor: A key mediator of Notch signaling in human hepatocarcinogenesis. World J. Gastroenterol. 2011, 17, 3420–3430. [Google Scholar] [CrossRef] [PubMed]
- Veljkovic, J.; Hansen, U. Lineage-specific and ubiquitous biological roles of the mammalian transcription factor LSF. Gene 2004, 343, 23–40. [Google Scholar] [CrossRef]
- Kokoszynska, K.; Ostrowski, J.; Rychlewski, L.; Wyrwicz, L.S. The fold recognition of CP2 transcription factors gives new insights into the function and evolution of tumor suppressor protein p53. Cell Cycle 2008, 7, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.C.; Chung, B.M.; Chae, J.H.; Yang, S.I.; Kim, C.G.; Kim, C.G. Identification and characterization of four novel peptide motifs that recognize distinct regions of the transcription factor CP2. FEBS J. 2005, 272, 1265–1277. [Google Scholar] [CrossRef]
- Kim, J.S.; Chae, J.H.; Cheon, Y.P.; Kim, C.G. Reciprocal localization of transcription factors YY1 and CP2c in spermatogonial stem cells and their putative roles during spermatogenesis. Acta Histochem. 2016, 118, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Lieberthal, J.G.; Kaminsky, M.; Parkhurst, C.N.; Tanese, N. The role of YY1 in reduced HP1alpha gene expression in invasive human breast cancer cells. Breast Cancer Res. 2009, 11, R42. [Google Scholar] [CrossRef]
- Shen, X.; Zhong, J.; Yu, P.; Zhao, Q.; Huang, T. YY1-regulated LINC00152 promotes triple negative breast cancer progression by affecting on stability of PTEN protein. Biochem. Biophys. Res. Commun. 2019, 509, 448–454. [Google Scholar] [CrossRef]
- Kim, J.S.; Son, S.H.; Kim, M.Y.; Choi, D.; Jang, I.S.; Paik, S.S.; Chae, J.H.; Uversky, V.N.; Kim, C.G. Diagnostic and prognostic relevance of CP2c and YY1 expression in hepatocellular carcinoma. Oncotarget 2017, 8, 24389–24400. [Google Scholar] [CrossRef]
- Schnoell, J.; Jank, B.J.; Kadletz-Wanke, L.; Stoiber, S.; Spielvogel, C.P.; Gurnhofer, E.; Kenner, L.; Heiduschka, G. Transcription factors CP2 and YY1 as prognostic markers in head and neck squamous cell carcinoma: Analysis of The Cancer Genome Atlas and a second independent cohort. J. Cancer Res. Clin. Oncol. 2021, 147, 755–765. [Google Scholar] [CrossRef]
- Wan, M.; Huang, W.; Kute, T.E.; Miller, L.D.; Zhang, Q.; Hatcher, H.; Wang, J.; Stovall, D.B.; Russell, G.B.; Cao, P.D.; et al. Yin Yang 1 plays an essential role in breast cancer and negatively regulates p27. Am. J. Pathol. 2012, 180, 2120–2133. [Google Scholar] [CrossRef]



| Variables | Value (%) |
|---|---|
| Age (years, mean ± SD) | 52.8 ± 11.1 |
| Pathologic tumor size | |
| ≤2 cm | 220 (44.7) |
| >2 cm | 265 (54.0) |
| Unknown | 6 (1.2) |
| Pathologic lymph node metastasis | |
| No | 300 (61.1) |
| Yes | 182 (37.1) |
| Unknown | 9 (1.8) |
| Pathologic nodal stage | |
| N0 | 300 (61.1) |
| N1 | 109 (22.2) |
| N2 | 42 (8.6) |
| N3 | 31 (6.3) |
| Unknown | 9 (1.8) |
| AJCC Stage | |
| I | 174 (35.4) |
| II | 220 (44.8) |
| III | 90 (18.3) |
| Unknown | 7 (1.4) |
| Histological grade | |
| 1, 2 | 262 (53.4) |
| 3 | 151 (30.8) |
| Unknown | 78 (15.9) |
| Molecular subtype | |
| HR+/HER2− | 294 (59.9) |
| HER2+ | 96 (19.6) |
| TNBC | 101 (20.6) |
| Lymphovascular invasion | |
| Absence | 164 (33.4) |
| Presence | 87 (17.7) |
| Unknown | 240 (48.9) |
| Ki-67 labelling index | |
| ≤20% | 357 (72.7) |
| >20% | 133 (27.1) |
| Unknown | 1 (0.2) |
| YY1 expression (H-score, mean ± SD) | 28.4 ± 49.9 |
| Variables | YY1 Expression | p Value | |
|---|---|---|---|
| High (n = 138) No. (%) | Low (n = 353) No. (%) | ||
| Age (years, mean ± SD) | 53.7 ± 10.8 | 52.8 ± 11.1 | 0.269 |
| Pathologic tumor size | 0.002 * | ||
| ≤2 cm | 77 (56.6) | 143 (41.0) | |
| >2 cm | 59 (43.4) | 206 (59.0) | |
| Unknown | 2 | 4 | |
| Pathologic lymph node metastasis | 0.051 | ||
| No | 94 (69.1) | 206 (59.5) | |
| Yes | 42 (30.9) | 140 (40.5) | |
| Unknown | 2 | 7 | |
| Pathologic nodal stage | 0.074 | ||
| N0 | 94 (69.1) | 206 (59.5) | |
| N1 | 23 (16.9) | 86 (24.9) | |
| N2 | 14 (10.3) | 28 (8.1) | |
| N3 | 5 (3.7) | 26 (7.5) | |
| Unknown | 2 | 7 | |
| AJCC Stage | <0.001 * | ||
| I | 67 (49.3) | 107 (30.7) | |
| II | 52 (38.2) | 168 (48.3) | |
| III | 17 (12.5) | 73 (21.0) | |
| Unknown | 2 | 5 | |
| Histological grade | <0.001 * | ||
| 1, 2 | 95 (81.9) | 167 (56.2) | |
| 3 | 21 (18.1) | 130 (43.8) | |
| Unknown | 22 | 56 | |
| Molecular subtype | <0.001 * | ||
| HR+/HER2− | 116 (84.1) | 178 (50.4) | |
| HER2+ | 14 (10.1) | 82 (23.2) | |
| TNBC | 8 (5.8) | 93 (26.3) | |
| Lymphovascular invasion | 0.530 | ||
| Absence | 67 (67.7) | 97 (63.8) | |
| Presence | 32 (32.3) | 55 (36.2) | |
| Unknown | 39 | 201 | |
| Ki-67 labeling index | 0.018 * | ||
| ≤20% | 111 (80.4) | 246 (69.9) | |
| >20% | 27 (19.6) | 106 (30.1) | |
| Unknown | 1 | ||
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p Value | Hazard Ratio | 95% CI | p Value | |
| Tumor size | ||||||
| >2cm (Ref. ≤ 2cm) | 3.65 | 2.12–6.30 | <0.0001 * | 3.4 | 1.95–5.91 | <0.0001 * |
| HR status | ||||||
| Positive (Ref. negative) | 0.7 | 0.45–1.08 | 0.11 | - | ||
| HER2 status | ||||||
| Positive (Ref. negative) | 1.39 | 0.85–2.29 | 0.19 | - | ||
| YY1 expression | ||||||
| High (Ref. low) | 0.46 | 0.24–0.87 | 0.016 * | 0.50 | 0.26–0.98 | 0.042 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, C.D.; Son, S.H.; Kim, C.G.; Park, H.; Chung, M.S. Prognostic Implication of YY1 and CP2c Expression in Patients with Primary Breast Cancer. Cancers 2023, 15, 3495. https://doi.org/10.3390/cancers15133495
Cha CD, Son SH, Kim CG, Park H, Chung MS. Prognostic Implication of YY1 and CP2c Expression in Patients with Primary Breast Cancer. Cancers. 2023; 15(13):3495. https://doi.org/10.3390/cancers15133495
Chicago/Turabian StyleCha, Chihwan David, Seung Han Son, Chul Geun Kim, Hosub Park, and Min Sung Chung. 2023. "Prognostic Implication of YY1 and CP2c Expression in Patients with Primary Breast Cancer" Cancers 15, no. 13: 3495. https://doi.org/10.3390/cancers15133495
APA StyleCha, C. D., Son, S. H., Kim, C. G., Park, H., & Chung, M. S. (2023). Prognostic Implication of YY1 and CP2c Expression in Patients with Primary Breast Cancer. Cancers, 15(13), 3495. https://doi.org/10.3390/cancers15133495







