Understanding Sleep Disturbances in Prostate Cancer—A Scientometric Analysis of Sleep Assessment, Aetiology, and Its Impact on Quality of Life
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
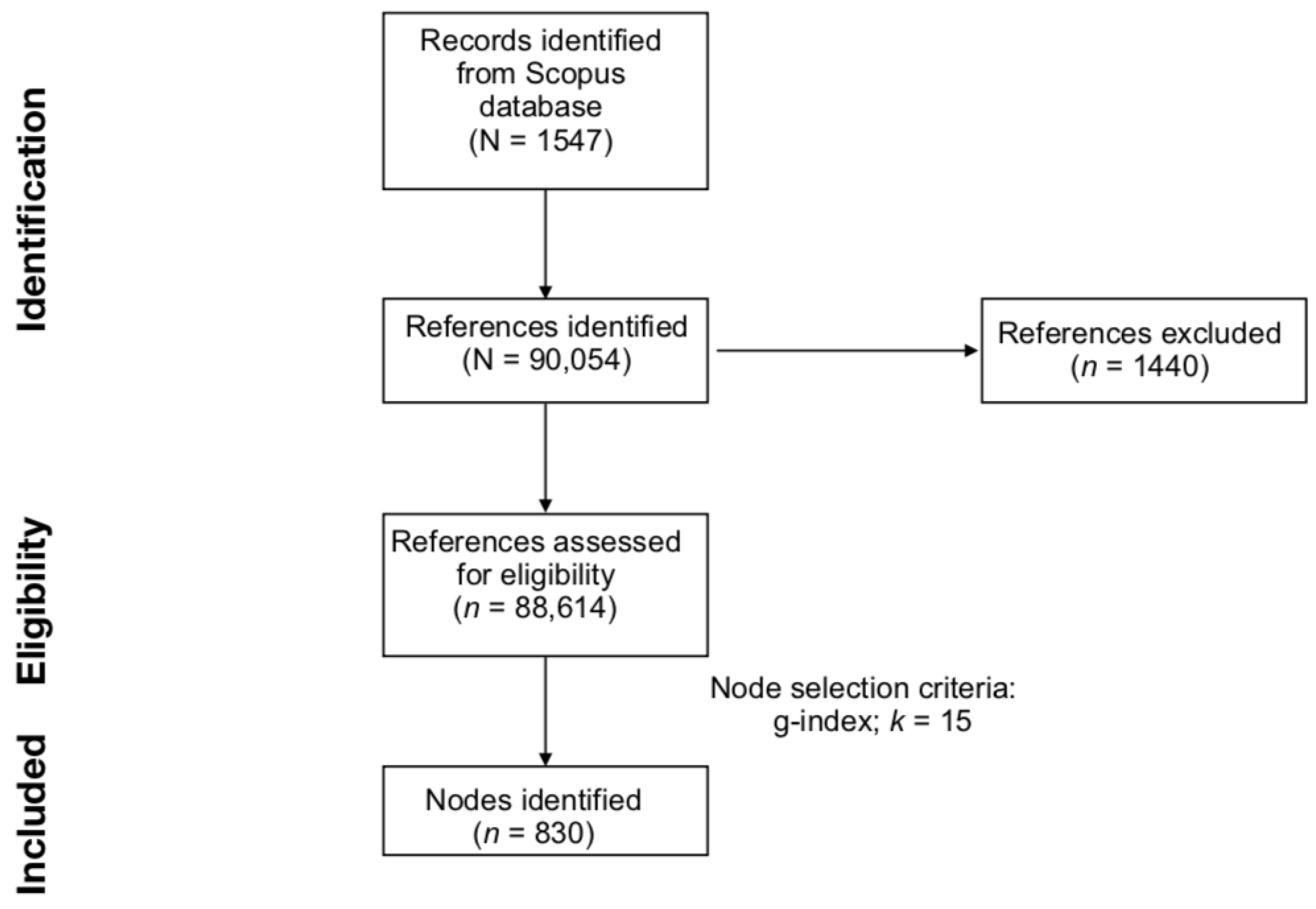
2.1. Data Collection from Scopus
2.2. Data Eligibility
2.3. Document Co-Citation Analysis (DCA)
2.4. DCA Network Evaluation Metrics
3. Results
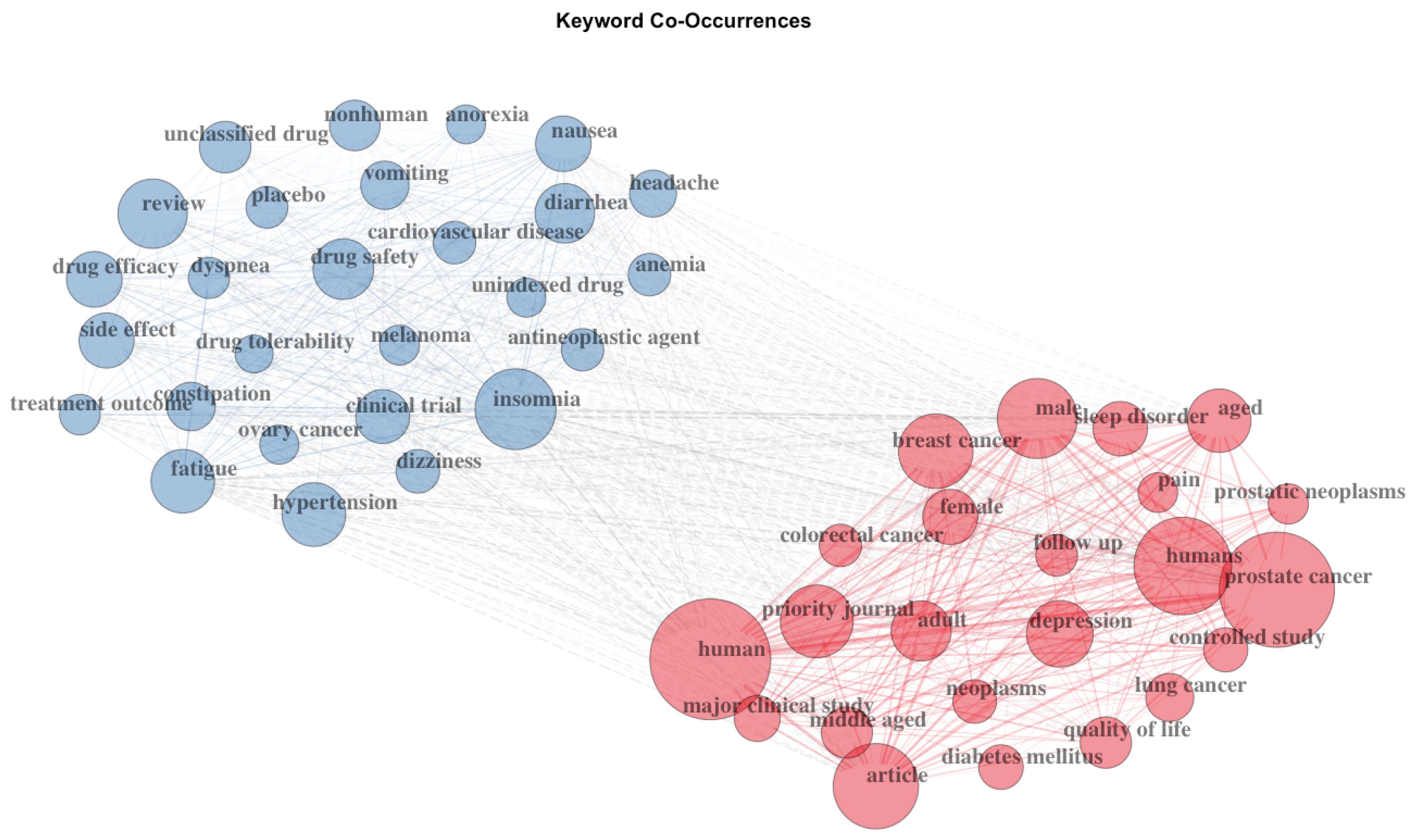
3.1. Bibliometric Analysis on the Citing Documents
3.2. Structural Properties of the Document Co-Citation Analysis Network
3.3. Citation Burstness
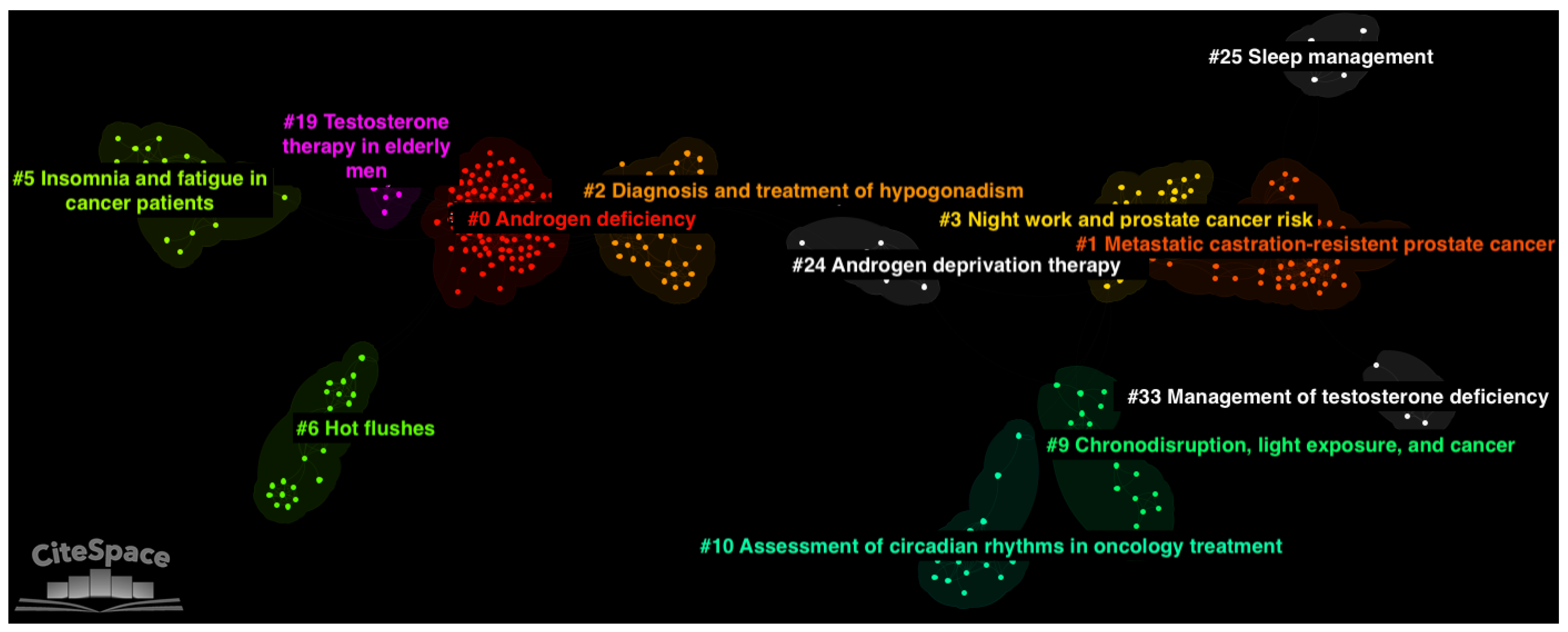
3.4. Thematic Clusters
4. Discussion
4.1. Cluster #5: Insomnia and Fatigue in Cancer Patients
4.2. Cluster #1: Metastatic Castration-Resistant Prostate Cancer
4.3. Cluster #10: Assessment of Circadian Rhythms in Oncology Treatments
4.4. Cluster #25: Sleep Management
4.5. Cluster #3: Night Work and Prostate Cancer Risk
4.6. Cluster #9: Chronodisruption, Light Exposure, and Cancer
4.7. Cluster #19: Testosterone Therapy in Elderly Men
4.8. Cluster #6: Hot Flashes
4.9. Cluster #33: Management of Testosterone Deficiency
4.10. Cluster #24: Androgen Deprivation Therapy
4.11. Cluster #0: Androgen Deficiency and Cluster #2: Diagnosis and Treatment of Hypogonadism
5. Conclusions
- Sleep disturbances and vasomotor symptoms associated with androgen deprivation therapy affect the quality of life for men with prostate cancer.
- Much of the data rely on subjective rather than objective assessment of sleep quality and hot flashes.
- The subjective data do not seem to be able to distinguish between cancer-related fatigue or a sleeping disorder, such as insomnia.
- Actigraphy has been used in clinical settings as a non-invasive method of characterising sleep, but studies thus far have not explored whether sleep quality varies according to the type of androgen-deprivation therapy.
- The limited use of actigraphy is hindered by the lack of use of baseline assessments. This is particularly important given that sleep disturbances are generally considered multifactorial.
Author Contributions
Funding
Conflicts of Interest
References
- Cancer Research UK. Prostate Cancer Incidence Statistics. 2023. Available online: https://www.cancerresearchuk.org/healthprofessional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/incidence (accessed on 15 April 2023).
- Agarwal, P.K.; Sadetsky, N.; Konety, B.R.; Resnick, M.I.; Carroll, P.R. Treatment failure after primary and salvage therapy for prostate cancer: Likelihood, patterns of care, and outcomes. Cancer 2008, 112, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Pound, C.R.; Partin, A.W.; Eisenberger, M.A.; Chan, D.W.; Pearson, J.D.; Walsh, P.C. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999, 281, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.M.; Rezaei, M.M.; Ghoreifi, A.; Kerigh, B.F. Metabolic syndrome in patients with prostate cancer undergoing intermittent androgen-deprivation therapy. Can. Urol. Assoc. J. 2016, 10, E300. [Google Scholar] [CrossRef] [PubMed]
- Saylor, P.J.; Smith, M.R. Metabolic complications of androgen deprivation therapy for prostate cancer. J. Urol. 2009, 181, 1998–2008. [Google Scholar] [CrossRef]
- Savard, M.H.; Savard, J.; Caplette-Gingras, A.; Ivers, H.; Bastien, C. Relationship between objectively recorded hot flashes and sleep disturbances among breast cancer patients: Investigating hot flash characteristics other than frequency. Menopause 2013, 20, 997–1005. [Google Scholar] [CrossRef]
- Habibollahpour, M.; Ranjkesh, F.; Motalebi, S.A.; Mohammadi, F. The Impact of Benson’s relaxation technique on the quality of sleep in the elderly. Top. Geriatr. Rehabil. 2019, 35, 88–94. [Google Scholar] [CrossRef]
- Li, J.; Vitiello, M.V.; Gooneratne, N.S. Sleep in normal aging. Sleep Med. Clin. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Medic, G.; Wille, M.; Hemels, M.E. Short-and long-term health consequences of sleep disruption. Nat. Sci. Sleep 2017, 9, 151–161. [Google Scholar] [CrossRef]
- Okoro, C.A.; Courtney-Long, E.; Cyrus, A.C.; Zhao, G.; Wheaton, A.G. Self-reported short sleep duration among US adults by disability status and functional disability type: Results from the 2016 Behavioral Risk Factor Surveillance System. Disabil. Health J. 2020, 13, 100887. [Google Scholar] [CrossRef]
- Yi, S.J.; Jeong, Y.M.; Kim, J.H. The influence of total sleep time on chronic disease in people with disabilities in South Korea: An analysis of panel data. J. Clin. Sleep Med. 2022, 18, 1307–1318. [Google Scholar] [CrossRef]
- Bonacina, G.; Carollo, A.; Esposito, G. The Genetic Side of the Mood: A Scientometric Review of the Genetic Basis of Mood Disorders. Genes 2023, 14, 352. [Google Scholar] [CrossRef]
- Carollo, A.; Balagtas, J.P.M.; Neoh, M.J.Y.; Esposito, G. A scientometric approach to review the role of the medial preoptic area (MPOA) in parental behavior. Brain Sci. 2021, 11, 393. [Google Scholar] [CrossRef]
- Carollo, A.; Bonassi, A.; Lim, M.; Gabrieli, G.; Setoh, P.; Dimitriou, D.; Aryadoust, V.; Esposito, G. Developmental disabilities across the world: A scientometric review from 1936 to 2020. Res. Dev. Disabil. 2021, 117, 104031. [Google Scholar] [CrossRef]
- Carollo, A.; Lim, M.; Aryadoust, V.; Esposito, G. Interpersonal synchrony in the context of caregiver-child interactions: A document co-citation analysis. Front. Psychol. 2021, 12, 701824. [Google Scholar] [CrossRef]
- Carollo, A.; Fong, S.; Gabrieli, G.; Mulatti, C.; Esposito, G. To wine or not to wine? A scientometric approach to 65+ years of wine preference and selection studies. Br. Food J. 2022, 124, 409–431. [Google Scholar] [CrossRef]
- Cataldo, I.; Lieu, A.A.; Carollo, A.; Bornstein, M.H.; Gabrieli, G.; Lee, A.; Esposito, G. From the cradle to the web: The growth of “sharenting”—A scientometric perspective. Hum. Behav. Emerg. Technol. 2022, 2022, 5607422. [Google Scholar] [CrossRef]
- Lim, M.; Carollo, A.; Chen, S.A.; Esposito, G. Surveying 80 years of psychodrama research: A scientometric review. Front. Psychiatry 2021, 12, 780542. [Google Scholar] [CrossRef]
- Lim, M.; Carollo, A.; Dimitriou, D.; Esposito, G. Recent developments in autism genetic research: A scientometric review from 2018 to 2022. Genes 2022, 13, 1646. [Google Scholar] [CrossRef]
- Lim, M.; Carollo, A.; Neoh, M.J.Y.; Esposito, G. Mapping miRNA Research in Schizophrenia: A Scientometric Review. Int. J. Mol. Sci. 2023, 24, 436. [Google Scholar] [CrossRef]
- Lim, M.; Carollo, A.; Neoh, M.J.Y.; Sacchiero, M.; Azhari, A.; Balboni, G.; Marschik, P.; Nordahl-Hansen, A.; Dimitriou, D.; Esposito, G. Developmental disabilities in Africa: A scientometric review. Res. Dev. Disabil. 2023, 133, 104395. [Google Scholar] [CrossRef]
- Neoh, M.J.Y.; Carollo, A.; Lim, M.; Corazza, O.; Coppola, A.; Esposito, G. The Novel Psychoactive Substances Epidemic: A Scientometric Perspective. Addict. Neurosci. 2022, 5, 100060. [Google Scholar] [CrossRef]
- Neoh, M.J.Y.; Carollo, A.; Lim, M.; Dimitriou, D.; Esposito, G. A Scientometric Review of Obstructive Sleep Apnea and Obesity. Appl. Sci. 2023, 13, 753. [Google Scholar] [CrossRef]
- Carollo, A.; Cataldo, I.; Fong, S.; Corazza, O.; Esposito, G. Unfolding the real-time neural mechanisms in addiction: Functional near-infrared spectroscopy (fNIRS) as a resourceful tool for research and clinical practice. Addict. Neurosci. 2022, 4, 100048. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. bibliometrix: An R-tool for comprehensive science mapping analysis. J. Inf. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Chen, C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 2006, 57, 359–377. [Google Scholar] [CrossRef]
- Chen, C. The citespace manual. Coll. Comput. Inform. 2014, 1, 1–84. [Google Scholar]
- Small, H. Co-citation context analysis and the structure of paradigms. J. Doc. 1980, 36, 183–196. [Google Scholar] [CrossRef]
- Chen, C.; Ibekwe-SanJuan, F.; Hou, J. The structure and dynamics of cocitation clusters: A multiple-perspective cocitation analysis. J. Am. Soc. Inf. Sci. Technol. 2010, 61, 1386–1409. [Google Scholar] [CrossRef]
- Alonso, S.; Cabrerizo, F.J.; Herrera-Viedma, E.; Herrera, F. h-Index: A review focused in its variants, computation and standardization for different scientific fields. J. Inf. 2009, 3, 273–289. [Google Scholar] [CrossRef]
- Egghe, L. Theory and practise of the g-index. Scientometrics 2006, 69, 131–152. [Google Scholar] [CrossRef]
- Chen, C. CiteSpace: A Practical Guide for Mapping Scientific Literature; Nova Science Publishers: Hauppauge, NY, USA, 2016. [Google Scholar]
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- Freeman, L.C. A set of measures of centrality based on betweenness. Sociometry 1977, 40, 35–41. [Google Scholar] [CrossRef]
- Kleinberg, J. Bursty and hierarchical structure in streams. In Proceedings of the Eighth ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, New York, NY, USA, 23–26 July 2002; pp. 91–101. [Google Scholar]
- Chen, C. Science mapping: A systematic review of the literature. J. Data Inf. Sci. 2017, 2, 1–40. [Google Scholar] [CrossRef]
- Haslam, D.W. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef]
- Parker, C.a.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’sullivan, J.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; De Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef]
- Bhasin, S.; Cunningham, G.R.; Hayes, F.J.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Montori, V.M. Testosterone therapy in men with androgen deficiency syndromes: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2010, 95, 2536–2559. [Google Scholar] [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef]
- Basaria, S.; Coviello, A.D.; Travison, T.G.; Storer, T.W.; Farwell, W.R.; Jette, A.M.; Eder, R.; Tennstedt, S.; Ulloor, J.; Zhang, A.; et al. Adverse events associated with testosterone administration. N. Engl. J. Med. 2010, 363, 109–122. [Google Scholar] [CrossRef]
- Sih, R.; Morley, J.E.; Kaiser, F.E.; Perry, H.M., III; Patrick, P.; Ross, C. Testosterone replacement in older hypogonadal men: A 12-month randomized controlled trial. J. Clin. Endocrinol. Metab. 1997, 82, 1661–1667. [Google Scholar] [CrossRef]
- Harman, S.M.; Metter, E.J.; Tobin, J.D.; Pearson, J.; Blackman, M.R. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J. Clin. Endocrinol. Metab. 2001, 86, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.J.; Smith, M.R.; De Bono, J.S.; Molina, A.; Logothetis, C.J.; De Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Amory, J.K.; Watts, N.B.; Easley, K.A.; Sutton, P.R.; Anawalt, B.D.; Matsumoto, A.M.; Bremner, W.J.; Tenover, J.L. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J. Clin. Endocrinol. Metab. 2004, 89, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J.; Peachey, H.; Hannoush, P.; Berlin, J.A.; Loh, L.; Holmes, J.H.; Dlewati, A.; Staley, J.; Santanna, J.; Kapoor, S.C.; et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J. Clin. Endocrinol. Metab. 1999, 84, 1966–1972. [Google Scholar] [CrossRef]
- Bhasin, S.; Cunningham, G.R.; Hayes, F.J.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Montori, V.M. Testosterone therapy in adult men with androgen deficiency syndromes: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2006, 91, 1995–2010. [Google Scholar] [CrossRef]
- Lee, K.; Cho, M.; Miaskowski, C.; Dodd, M. Impaired sleep and rhythms in persons with cancer. Sleep Med. Rev. 2004, 8, 199–212. [Google Scholar] [CrossRef]
- Hervouet, S.; Savard, J.; Simard, S.; Ivers, H.; Laverdière, J.; Vigneault, É.; Fradet, Y.; Lacombe, L. Psychological functioning associated with prostate cancer: Cross-sectional comparison of patients treated with radiotherapy, brachytherapy, or surgery. J. Pain Symptom Manag. 2005, 30, 474–484. [Google Scholar] [CrossRef]
- Simeit, R.; Deck, R.; Conta-Marx, B. Sleep management training for cancer patients with insomnia. Support. Care Cancer 2004, 12, 176–183. [Google Scholar] [CrossRef]
- Savard, J.; Morin, C.M. Insomnia in the context of cancer: A review of a neglected problem. J. Clin. Oncol. 2001, 19, 895–908. [Google Scholar] [CrossRef]
- Davidson, J.R.; MacLean, A.W.; Brundage, M.D.; Schulze, K. Sleep disturbance in cancer patients. Soc. Sci. Med. 2002, 54, 1309–1321. [Google Scholar] [CrossRef]
- Curt, G.A.; Breitbart, W.; Cella, D.; Groopman, J.E.; Horning, S.J.; Itri, L.M.; Johnson, D.H.; Miaskowski, C.; Scherr, S.L.; Portenoy, R.K.; et al. Impact of cancer-related fatigue on the lives of patients: New findings from the Fatigue Coalition. Oncologist 2000, 5, 353–360. [Google Scholar] [CrossRef]
- Eton, D.T.; Lepore, S.J. Prostate cancer and health-related quality of life: A review of the literature. Psycho-Oncology 2002, 11, 307–326. [Google Scholar] [CrossRef]
- Graydon, J.E.; Bubela, N.; Irvine, D.; Vincent, L. Fatigue-reducing strategies used by patients receiving treatment for cancer. Cancer Nurs. 1995, 18, 23–28. [Google Scholar] [CrossRef]
- Fillion, L.; Gélinas, C.; Simard, S.; Savard, J.; Gagnon, P. Validation evidence for the French Canadian adaptation of the Multidimensional Fatigue Inventory as a measure of cancer-related fatigue. Cancer Nurs. 2003, 26, 143–154. [Google Scholar] [CrossRef]
- Davidson, J.R.; Waisberg, J.L.; Brundage, M.D.; MacLean, A.W. Nonpharmacologic group treatment of insomnia: A preliminary study with cancer survivors. Psycho-Oncology 2001, 10, 389–397. [Google Scholar] [CrossRef]
- Dimeo, F.C.; Stieglitz, R.D.; Novelli-Fischer, U.; Fetscher, S.; Keul, J. Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer 1999, 85, 2273–2277. [Google Scholar] [CrossRef]
- Watson, T.; Mock, V. Exercise as an intervention for cancer-related fatigue. Phys. Ther. 2004, 84, 736–743. [Google Scholar] [CrossRef]
- Wagner, L.; Cella, D. Fatigue and cancer: Causes, prevalence and treatment approaches. Br. J. Cancer 2004, 91, 822–828. [Google Scholar] [CrossRef]
- Oudard, S.; Fizazi, K.; Sengeløv, L.; Daugaard, G.; Saad, F.; Hansen, S.; Hjälm-Eriksson, M.; Jassem, J.; Thiery-Vuillemin, A.; Caffo, O.; et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: A randomized phase III trial—FIRSTANA. J. Clin. Oncol. 2017, 35, 3189–3197. [Google Scholar] [CrossRef]
- Beer, T.M.; Hotte, S.J.; Saad, F.; Alekseev, B.; Matveev, V.; Fléchon, A.; Gravis, G.; Joly, F.; Chi, K.N.; Malik, Z.; et al. Custirsen (OGX-011) combined with cabazitaxel and prednisone versus cabazitaxel and prednisone alone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel (AFFINITY): A randomised, open-label, international, phase 3 trial. Lancet Oncol. 2017, 18, 1532–1542. [Google Scholar]
- Sparasci, D.; Napoli, I.; Rossi, L.; Pereira-Mestre, R.; Manconi, M.; Treglia, G.; Marandino, L.; Ottaviano, M.; Turco, F.; Mangan, D.; et al. Prostate cancer and sleep disorders: A systematic review. Cancers 2022, 14, 1784. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Madsen, M.T.; Gögenur, I. Circadian rhythms measured by actigraphy during oncological treatments: A systematic review. Biol. Rhythm. Res. 2015, 46, 329–348. [Google Scholar] [CrossRef]
- Madsen, M.T.; Huang, C.; Gögenur, I. Actigraphy for measurements of sleep in relation to oncological treatment of patients with cancer: A systematic review. Sleep Med. Rev. 2015, 20, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Savard, J.; Ivers, H.; Savard, M.H.; Morin, C.M. Cancer treatments and their side effects are associated with aggravation of insomnia: Results of a longitudinal study. Cancer 2015, 121, 1703–1711. [Google Scholar] [CrossRef]
- Lundh, L.G.; Morin, C.M. Insomnia: Psychological assessment and management. Scand. J. Behav. Ther. 1994, 23, 62. [Google Scholar]
- Berger, A.M.; Kuhn, B.R.; Farr, L.A.; Lynch, J.C.; Agrawal, S.; Chamberlain, J.; Von Essen, S.G. Behavioral therapy intervention trial to improve sleep quality and cancer-related fatigue. Psycho-Oncology 2009, 18, 634–646. [Google Scholar] [CrossRef]
- Epstein, D.R. Randomized trial of a cognitive-behavioral intervention for insomnia in breast cancer survivors. Oncol. Nurs. Forum 2007, 34, E51–E59. [Google Scholar] [CrossRef]
- Delpachitra, S.; Campbell, A.; Wibowo, E. Preference for sleep management strategies among prostate cancer patients: An Aotearoa/New Zealand perspective. Cancer Treat. Res. Commun. 2020, 25, 100219. [Google Scholar] [CrossRef]
- Feng, L.R.; Barb, J.J.; Allen, H.; Regan, J.; Saligan, L. Steroid hormone biosynthesis metabolism is associated with fatigue related to androgen deprivation therapy for prostate cancer. Front. Cell Dev. Biol. 2021, 9, 642307. [Google Scholar] [CrossRef]
- Gonzalez, B.D.; Small, B.J.; Cases, M.G.; Williams, N.L.; Fishman, M.N.; Jacobsen, P.B.; Jim, H.S. Sleep disturbance in men receiving androgen deprivation therapy for prostate cancer: The role of hot flashes and nocturia. Cancer 2018, 124, 499–506. [Google Scholar] [CrossRef]
- Howell, D.; Oliver, T.; Keller-Olaman, S.; Davidson, J.; Garland, S.; Samuels, C.; Savard, J.; Harris, C.; Aubin, M.; Olson, K.; et al. Sleep disturbance in adults with cancer: A systematic review of evidence for best practices in assessment and management for clinical practice. Ann. Oncol. 2014, 25, 791–800. [Google Scholar] [CrossRef]
- Dinh, K.T.; Yang, D.D.; Nead, K.T.; Reznor, G.; Trinh, Q.D.; Nguyen, P.L. Association between androgen deprivation therapy and anxiety among 78 000 patients with localized prostate cancer. Int. J. Urol. 2017, 24, 743–748. [Google Scholar] [CrossRef]
- Choi, T.Y.; Kim, J.I.; Lim, H.J.; Lee, M.S. Acupuncture for managing cancer-related insomnia: A systematic review of randomized clinical trials. Integr. Cancer Ther. 2017, 16, 135–146. [Google Scholar] [CrossRef]
- Deng, N.; Haney, N.M.; Kohn, T.P.; Pastuszak, A.W.; Lipshultz, L.I. The effect of shift work on urogenital disease: A systematic review. Curr. Urol. Rep. 2018, 19, 57. [Google Scholar] [CrossRef]
- Deng, N.; Kohn, T.P.; Lipshultz, L.I.; Pastuszak, A.W. The relationship between shift work and men’s health. Sex. Med. Rev. 2018, 6, 446–456. [Google Scholar] [CrossRef]
- Wendeu-Foyet, M.G.; Bayon, V.; Cénée, S.; Trétarre, B.; Rébillard, X.; Cancel-Tassin, G.; Cussenot, O.; Lamy, P.J.; Faraut, B.; Khedher, S.B.; et al. Night work and prostate cancer risk: Results from the EPICAP Study. Occup. Environ. Med. 2018, 75, 573–581. [Google Scholar] [CrossRef]
- Turner, M.C.; Gracia-Lavedan, E.; Papantoniou, K.; Aragonés, N.; Castaño-Vinyals, G.; Dierssen-Sotos, T.; Amiano, P.; Ardanaz, E.; Marcos-Delgado, A.; Molina-Barceló, A.; et al. Sleep and breast and prostate cancer risk in the MCC-Spain study. Sci. Rep. 2022, 12, 21807. [Google Scholar] [CrossRef]
- Erren, T.; Morfeld, P.; Foster, R.; Reiter, R.; Gross, J.; Westermann, I. Sleep and cancer: Synthesis of experimental data and meta-analyses of cancer incidence among some 1,500,000 study individuals in 13 countries. Chronobiol. Int. 2016, 33, 325–350. [Google Scholar] [CrossRef]
- Porcacchia, A.S.; Câmara, D.A.D.; Andersen, M.L.; Tufik, S. Sleep disorders and prostate cancer prognosis: Biology, epidemiology, and association with cancer development risk. Eur. J. Cancer Prev. 2022, 31, 178–189. [Google Scholar] [CrossRef]
- Fritschi, L.; Glass, D.; Heyworth, J.; Aronson, K.; Girschik, J.; Boyle, T.; Grundy, A.; Erren, T. Hypotheses for mechanisms linking shiftwork and cancer. Med. Hypotheses 2011, 77, 430–436. [Google Scholar] [CrossRef]
- Sigurdardottir, L.G.; Markt, S.C.; Rider, J.R.; Haneuse, S.; Fall, K.; Schernhammer, E.S.; Tamimi, R.M.; Flynn-Evans, E.; Batista, J.L.; Launer, L.; et al. Urinary melatonin levels, sleep disruption, and risk of prostate cancer in elderly men. Eur. Urol. 2015, 67, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Wendeu-Foyet, M.G.; Menegaux, F. Circadian Disruption and Prostate Cancer Risk: An Updated Review of Epidemiological EvidencesCircadian Disruption and Prostate Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2017, 26, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.S.; Lin, C.L. Sleep disorders associated with risk of prostate cancer: A population-based cohort study. BMC Cancer 2019, 19, 146. [Google Scholar] [CrossRef] [PubMed]
- Gapstur, S.M.; Diver, W.R.; Stevens, V.L.; Carter, B.D.; Teras, L.R.; Jacobs, E.J. Work schedule, sleep duration, insomnia, and risk of fatal prostate cancer. Am. J. Prev. Med. 2014, 46, S26–S33. [Google Scholar] [CrossRef] [PubMed]
- Flynn-Evans, E.E.; Mucci, L.; Stevens, R.G.; Lockley, S.W. Shiftwork and prostate-specific antigen in the National Health and Nutrition Examination Survey. J. Natl. Cancer Inst. 2013, 105, 1292–1297. [Google Scholar] [CrossRef]
- Cordina-Duverger, E.; Cénée, S.; Trétarre, B.; Rebillard, X.; Lamy, P.J.; Wendeu-Foyet, G.; Menegaux, F. Sleep patterns and risk of prostate cancer: A population-based case control study in France (EPICAP). Cancer Epidemiol. Biomark. Prev. 2022, 31, 2070–2078. [Google Scholar] [CrossRef]
- Kakizaki, M.; Inoue, K.; Kuriyama, S.; Sone, T.; Matsuda-Ohmori, K.; Nakaya, N.; Fukudo, S.; Tsuji, I. Sleep duration and the risk of prostate cancer: The Ohsaki Cohort Study. Br. J. Cancer 2008, 99, 176–178. [Google Scholar] [CrossRef]
- Kecklund, G.; Axelsson, J. Health consequences of shift work and insufficient sleep. BMJ 2016, 355, i5210. [Google Scholar] [CrossRef]
- Itani, O.; Kaneita, Y. The association between shift work and health: A review. Sleep Biol. Rhythm. 2016, 14, 231–239. [Google Scholar] [CrossRef]
- Erren, T.C.; Morfeld, P.; Stork, J.; Knauth, P.; Von Mülmann, M.J.; Breitstadt, R.; Müller, U.; Emmerich, M.; Piekarski, C. Shift work, chronodisruption and cancer?—The IARC 2007 challenge for research and prevention and 10 theses from the Cologne Colloquium 2008. Scand. J. Work. Environ. Health 2009, 35, 74–79. [Google Scholar] [CrossRef]
- Sigurdardottir, L.G.; Valdimarsdottir, U.A.; Fall, K.; Rider, J.R.; Lockley, S.W.; Schernhammer, E.; Mucci, L.A. Circadian Disruption, Sleep Loss, and Prostate Cancer Risk: A Systematic Review of Epidemiologic StudiesCircadian Disruption, Sleep Loss, and Prostate Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1002–1011. [Google Scholar] [CrossRef]
- Kubo, T.; Ozasa, K.; Mikami, K.; Wakai, K.; Fujino, Y.; Watanabe, Y.; Miki, T.; Nakao, M.; Hayashi, K.; Suzuki, K.; et al. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: Findings from the Japan collaborative cohort study. Am. J. Epidemiol. 2006, 164, 549–555. [Google Scholar] [CrossRef]
- Fuller, P.M.; Lu, J.; Saper, C.B. Differential rescue of light-and food-entrainable circadian rhythms. Science 2008, 320, 1074–1077. [Google Scholar] [CrossRef]
- Kayumov, L.; Casper, R.F.; Hawa, R.J.; Perelman, B.; Chung, S.A.; Sokalsky, S.; Shapiro, C.M. Blocking low-wavelength light prevents nocturnal melatonin suppression with no adverse effect on performance during simulated shift work. J. Clin. Endocrinol. Metab. 2005, 90, 2755–2761. [Google Scholar] [CrossRef]
- Lockley, S.W.; Brainard, G.C.; Czeisler, C.A. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J. Clin. Endocrinol. Metab. 2003, 88, 4502–4505. [Google Scholar] [CrossRef]
- Brainard, G.C.; Hanifin, J.P.; Greeson, J.M.; Byrne, B.; Glickman, G.; Gerner, E.; Rollag, M.D. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J. Neurosci. 2001, 21, 6405–6412. [Google Scholar] [CrossRef]
- Basaria, S.; Dobs, A.S. Risks versus benefits of testosterone therapy in elderly men. Drugs Aging 1999, 15, 131–142. [Google Scholar] [CrossRef]
- Lund, B.C.; Bever-Stille, K.A.; Perry, P.J. Testosterone and andropause: The feasibility of testosterone replacement therapy in elderly men. Pharmacotherapy 1999, 19, 951–956. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Barton, D.L.; Adjei, A.A.; Loprinzi, C.L. Pathophysiology and treatment of hot flashes. Mayo Clin. Proc. 2002, 77, 1207–1218. [Google Scholar] [CrossRef]
- Stearns, V.; Ullmer, L.; Lopez, J.F.; Smith, Y.; Isaacs, C.; Hayes, D.F. Hot flashes. Lancet 2002, 360, 1851–1861. [Google Scholar] [CrossRef]
- Holzbeierlein, J.M.; McLaughlin, M.D.; Thrasher, J.B. Complications of androgen deprivation therapy for prostate cancer. Curr. Opin. Urol. 2004, 14, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Quella, S.K.; Loprinzi, C.L.; Sloan, J.; Novotny, P.; Perez, E.A.; Burch, P.A.; Antolak, S.J., Jr.; Pisansky, T.M. Pilot evaluation of venlafaxine for the treatment of hot flashes in men undergoing androgen ablation therapy for prostate cancer. J. Urol. 1999, 162, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, C.L.; Pisansky, T.M.; Fonseca, R.; Sloan, J.A.; Zahasky, K.M.; Quella, S.K.; Novotny, P.J.; Rummans, T.A.; Dumesic, D.A.; Perez, E.A. Pilot evaluation of venlafaxine hydrochloride for the therapy of hot flashes in cancer survivors. J. Clin. Oncol. 1998, 16, 2377–2381. [Google Scholar] [CrossRef] [PubMed]
- Burté, C. Comment je prescris une hormonothérapie substitutive chez un patient présentant un déficit en testostérone? Progrès Urol.—FMC 2022, 32, 60–63. [Google Scholar] [CrossRef]
- Bhasin, S.; Ozimek, N. Optimizing diagnostic accuracy and treatment decisions in men with testosterone deficiency. Endocr. Pract. 2021, 27, 1252–1259. [Google Scholar] [CrossRef]
- Østergren, P.B.; Kistorp, C.; Bennedbaek, F.N.; Faber, J.; Sønksen, J.; Fode, M. The use of exercise interventions to overcome adverse effects of androgen deprivation therapy. Nat. Rev. Urol. 2016, 13, 353–364. [Google Scholar] [CrossRef]
- Rot, I.; Wassersug, R.J.; Walker, L.M. What do urologists think patients need to know when starting on androgen deprivation therapy? The perspective from Canada versus countries with lower gross domestic product. Transl. Androl. Urol. 2016, 5, 235. [Google Scholar] [CrossRef]
- Dimopoulou, C.; Ceausu, I.; Depypere, H.; Lambrinoudaki, I.; Mueck, A.; Pérez-López, F.R.; Rees, M.; van der Schouw, Y.T.; Senturk, L.M.; Simonsini, T.; et al. EMAS position statement: Testosterone replacement therapy in the aging male. Maturitas 2016, 84, 94–99. [Google Scholar] [CrossRef]
- Bourke, L.; Gilbert, S.; Hooper, R.; Steed, L.A.; Joshi, M.; Catto, J.W.; Saxton, J.M.; Rosario, D.J. Lifestyle changes for improving disease-specific quality of life in sedentary men on long-term androgen-deprivation therapy for advanced prostate cancer: A randomised controlled trial. Eur. Urol. 2014, 65, 865–872. [Google Scholar] [CrossRef]
- Cormie, P.; Newton, R.U.; Taaffe, D.R.; Spry, N.; Joseph, D.; Akhlil Hamid, M.; Galvao, D.A. Exercise maintains sexual activity in men undergoing androgen suppression for prostate cancer: A randomized controlled trial. Prostate Cancer Prostatic Dis. 2013, 16, 170–175. [Google Scholar] [CrossRef]
- Culos-Reed, S.N.; Robinson, J.W.; Lau, H.; Stephenson, L.; Keats, M.; Norris, S.; Kline, G.; Faris, P. Physical activity for men receiving androgen deprivation therapy for prostate cancer: Benefits from a 16-week intervention. Support. Care Cancer 2010, 18, 591–599. [Google Scholar] [CrossRef]
- Gooren, L.J. Androgens and male aging: Current evidence of safety and efficacy. Asian J. Androl. 2010, 12, 136. [Google Scholar] [CrossRef]
- Tung, D.S.; Cunningham, G.R. Androgen deficiency in men. Endocrinologist 2007, 17, 101–115. [Google Scholar] [CrossRef]
- Buvat, J.; Maggi, M.; Guay, A.; Torres, L.O. Testosterone deficiency in men: Systematic review and standard operating procedures for diagnosis and treatment. J. Sex. Med. 2013, 10, 245–284. [Google Scholar] [CrossRef]
- Bhasin, S.; Basaria, S. Diagnosis and treatment of hypogonadism in men. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 251–270. [Google Scholar] [CrossRef]
- Kumari, M.; Badrick, E.; Ferrie, J.; Perski, A.; Marmot, M.; Chandola, T. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J. Clin. Endocrinol. Metab. 2009, 94, 4801–4809. [Google Scholar] [CrossRef]
- Walker, W.H.; Borniger, J.C. Molecular mechanisms of cancer-induced sleep disruption. Int. J. Mol. Sci. 2019, 20, 2780. [Google Scholar] [CrossRef]
- Mondal, S.; Edwards, S.; Wibowo, E.; Ahmed, H.; Wassersug, R.J.; Ellis, J.; Isaac, M.; Dimitriou, D.; Mangar, S. Evaluating Patterns and Factors Related to Sleep Disturbances in Prostate Cancer Patients. Healthcare 2022, 10, 832. [Google Scholar] [CrossRef]
| References | Citation Burstness | Publication Year | Burst Begin | Burst End | Duration | Centrality | Sigma |
|---|---|---|---|---|---|---|---|
| Scher et al. [39] | 9.5844 | 2012 | 2013 | 2019 | 6 | 0.04 | 1.45 |
| Bhasin et al. [40] | 8.4489 | 2010 | 2012 | 2015 | 3 | 0.01 | 1.07 |
| Beer et al. [41] | 7.7534 | 2014 | 2015 | 2023 | 8 | 0.04 | 1.32 |
| Basaria et al. [42] | 7.7256 | 2010 | 2011 | 2016 | 5 | 0.20 | 4.07 |
| Sih et al. [43] | 7.6022 | 1997 | 1999 | 2005 | 6 | 0.02 | 1.21 |
| Harman et al. [44] | 6.7064 | 2001 | 2002 | 2008 | 6 | 0.05 | 1.38 |
| Ryan et al. [45] | 6.4786 | 2013 | 2014 | 2019 | 5 | 0.00 | 1.02 |
| Amory et al. [46] | 6.2562 | 2004 | 2005 | 2010 | 5 | 0.04 | 1.31 |
| Snyder et al. [47] | 5.9516 | 1999 | 2003 | 2006 | 3 | 0.00 | 1.01 |
| Bhasin et al. [48] | 5.8709 | 2006 | 2007 | 2010 | 3 | 0.02 | 1.10 |
| Cluster ID | Size | Silhouette | Mean Year | LLR Label | Suggested Label |
|---|---|---|---|---|---|
| 0 | 100 | 0.969 | 2002 | clinical practice guidelines | androgen deficiency |
| 1 | 58 | 0.931 | 2013 | metastatic castration-resistant prostate cancer | metastatic castration-resistant prostate cancer |
| 2 | 50 | 0.990 | 2008 | testosterone deficiency | diagnosis and treatment of hypogonadism |
| 3 | 35 | 0.967 | 2015 | prostate cancer risk | night work and prostate cancer risk |
| 5 | 26 | 0.985 | 2000 | cross-sectional comparison | insomnia and fatigue in cancer patients |
| 6 | 24 | 0.996 | 2000 | hot flashes | hot flashes |
| 9 | 23 | 0.981 | 2009 | health consequence | chronodisruption, light exposure, and cancer |
| 10 | 19 | 0.977 | 2010 | oncological treatment | assessment of circadian rhythms in oncology treatments |
| 19 | 11 | 0.990 | 1994 | elderly men | testosterone therapy in elderly men |
| 24 | 9 | 0.941 | 2012 | androgen deprivation therapy | androgen deprivation therapy |
| 25 | 7 | 0.998 | 2015 | preference | sleep management |
| 33 | 6 | 1.000 | 2017 | practical recommendation | management of testosterone deficiency |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangar, S.; Abbadasari, M.; Carollo, A.; Esposito, G.; Ahmed, H.; Shah, T.; Dimitriou, D. Understanding Sleep Disturbances in Prostate Cancer—A Scientometric Analysis of Sleep Assessment, Aetiology, and Its Impact on Quality of Life. Cancers 2023, 15, 3485. https://doi.org/10.3390/cancers15133485
Mangar S, Abbadasari M, Carollo A, Esposito G, Ahmed H, Shah T, Dimitriou D. Understanding Sleep Disturbances in Prostate Cancer—A Scientometric Analysis of Sleep Assessment, Aetiology, and Its Impact on Quality of Life. Cancers. 2023; 15(13):3485. https://doi.org/10.3390/cancers15133485
Chicago/Turabian StyleMangar, Stephen, Monica Abbadasari, Alessandro Carollo, Gianluca Esposito, Hashim Ahmed, Taimur Shah, and Dagmara Dimitriou. 2023. "Understanding Sleep Disturbances in Prostate Cancer—A Scientometric Analysis of Sleep Assessment, Aetiology, and Its Impact on Quality of Life" Cancers 15, no. 13: 3485. https://doi.org/10.3390/cancers15133485
APA StyleMangar, S., Abbadasari, M., Carollo, A., Esposito, G., Ahmed, H., Shah, T., & Dimitriou, D. (2023). Understanding Sleep Disturbances in Prostate Cancer—A Scientometric Analysis of Sleep Assessment, Aetiology, and Its Impact on Quality of Life. Cancers, 15(13), 3485. https://doi.org/10.3390/cancers15133485






