Local Treatment of Hepatocellular Carcinoma with Oligometastases: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Inclusion Criteria and Search Strategy
2.2. Data Collection
2.3. Quality Assessment
2.4. Effect Measures and Data Synthesis
3. Results
3.1. Study Selection and Characteristics
3.2. Risk of Bias Assessment
3.3. Clinical Information and Synthesized Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pastorino, U.; Buyse, M.; Friedel, G.; Ginsberg, R.J.; Girard, P.; Goldstraw, P.; Johnston, M.; McCormack, P.; Pass, H.; Putnam, J.B., Jr. Long-term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J. Thorac. Cardiovasc. Surg. 1997, 113, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Chalkidou, A.; Macmillan, T.; Grzeda, M.T.; Peacock, J.; Summers, J.; Eddy, S.; Coker, B.; Patrick, H.; Powell, H.; Berry, L.; et al. Stereotactic ablative body radiotherapy in patients with oligometastatic cancers: A prospective, registry-based, single-arm, observational, evaluation study. Lancet Oncol. 2021, 22, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Weichselbaum, R.R.; Hellman, S. Oligometastases revisited. Nat. Rev. Clin. Oncol. 2011, 8, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Bahig, H.; Palma, D.A. Oligometastases: Emerging Evidence. J. Clin. Oncol. 2022, 40, 4250–4260. [Google Scholar] [CrossRef]
- Gomez, D.R.; Tang, C.; Zhang, J.; Blumenschein, G.R., Jr.; Hernandez, M.; Lee, J.J.; Ye, R.; Palma, D.A.; Louie, A.V.; Camidge, D.R. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non–small-cell lung cancer: Long-term results of a multi-institutional, phase II, randomized study. J. Clin. Oncol. 2019, 37, 1558–1565. [Google Scholar] [CrossRef]
- Iyengar, P.; Wardak, Z.; Gerber, D.E.; Tumati, V.; Ahn, C.; Hughes, R.S.; Dowell, J.E.; Cheedella, N.; Nedzi, L.; Westover, K.D. Consolidative radiotherapy for limited metastatic non–small-cell lung cancer: A phase 2 randomized clinical trial. JAMA Oncol. 2018, 4, e173501. [Google Scholar] [CrossRef]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; DeBruycker, A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Claeys, T. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase II trial. J. Clin. Oncol. 2018, 36, 446–453. [Google Scholar] [CrossRef]
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Ritchie, A.W.; Attard, G.; Chowdhury, S.; Cross, W. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomised controlled phase 3 trial. Lancet 2018, 392, 2353–2366. [Google Scholar] [CrossRef]
- Rim, C.H.; Shin, I.-S.; Park, S.; Lee, H.Y. Benefits of local consolidative treatment in oligometastases of solid cancers: A stepwise-hierarchical pooled analysis and systematic review. NPJ Precis. Oncol. 2021, 5, 2. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Aino, H.; Sumie, S.; Niizeki, T.; Kuromatsu, R.; Tajiri, N.; Nakano, M.; Satani, M.; Yamada, S.; Okamura, S.; Shimose, S. Clinical characteristics and prognostic factors for advanced hepatocellular carcinoma with extrahepatic metastasis. Mol. Clin. Oncol. 2014, 2, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Abd El Aziz, M.A.; Tartaglia, N.; Ramai, D.; Mohan, B.P.; Cotsoglou, C.; Pusceddu, S.; Giacomelli, L.; Ambrosi, A.; Sacco, R. Microwave Ablation Versus Radiofrequency Ablation for Treatment of Hepatocellular Carcinoma: A Meta-Analysis of Randomized Controlled Trials. Cancers 2020, 12, 3796. [Google Scholar] [CrossRef]
- Granito, A.; Facciorusso, A.; Sacco, R.; Bartalena, L.; Mosconi, C.; Cea, U.V.; Cappelli, A.; Antonino, M.; Modestino, F.; Brandi, N.; et al. TRANS-TACE: Prognostic Role of the Transient Hypertransaminasemia after Conventional Chemoembolization for Hepatocellular Carcinoma. J. Pers. Med. 2021, 11, 1041. [Google Scholar] [CrossRef] [PubMed]
- Rim, C.H.; Kim, H.J.; Seong, J. Clinical feasibility and efficacy of stereotactic body radiotherapy for hepatocellular carcinoma: A systematic review and meta-analysis of observational studies. Radiother Oncol. 2019, 131, 135–144. [Google Scholar] [CrossRef]
- Pan, T.; Xie, Q.-K.; Lv, N.; Li, X.-S.; Mu, L.-W.; Wu, P.-H.; Zhao, M. Percutaneous CT-guided radiofrequency ablation for lymph node oligometastases from hepatocellular carcinoma: A propensity score–matching analysis. Radiology 2017, 282, 259–270. [Google Scholar] [CrossRef]
- Kim, K.; Kim, T.H.; Kim, T.H.; Seong, J. Efficacy of local therapy for oligometastatic hepatocellular carcinoma: A propensity score matched analysis. J. Hepatocell. Carcinoma 2021, 8, 35. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Su, D.; Wu, B.; Shi, L. Cost-effectiveness of Atezolizumab Plus Bevacizumab vs Sorafenib as First-Line Treatment of Unresectable Hepatocellular Carcinoma. JAMA Netw. Open 2021, 4, e210037. [Google Scholar] [CrossRef]
- Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ott. Ott. Hosp. Res. Inst. 2011, 2, 1–12. [Google Scholar]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Ch 24.6: Synthesis of Results from Non-randomized Studies. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Chen, J.; Lu, S.; Zhang, Y.; Xu, L.; Chen, J.; Wang, J.; Chen, M.; Zhang, R.; Zhou, Z. Sorafenib monotherapy versus sorafenib combined with regional therapies for hepatocellular carcinoma patients with pulmonary oligometastases: A propensity score-matched analysis. J. Cancer 2018, 9, 1745. [Google Scholar] [CrossRef] [PubMed]
- Cochran, W.G. The Combination of Estimates from Different Experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Jo, I.Y.; Park, H.C.; Kim, E.S.; Yeo, S.-G.; Kim, M.; Seong, J.; Kim, J.W.; Kim, T.H.; Yoon, W.S.; Jeong, B.K. Stereotactic ablative radiotherapy for pulmonary oligometastases from primary hepatocellular carcinoma: A multicenter and retrospective analysis (KROG 17-08). Jpn. J. Clin. Oncol. 2022, 52, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Park, S.; Rim, C.H.; Choi, C.; Seong, J. Improved oncologic outcomes by ablative radiotherapy in patients with bone metastasis from hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2021, 147, 2693–2700. [Google Scholar] [CrossRef]
- Lyu, N.; Kong, Y.; Pan, T.; Mu, L.; Sun, X.; Li, S.; Deng, H.; Lai, J.; Zhao, M. Survival benefits of computed tomography-guided thermal ablation for adrenal metastases from hepatocellular carcinoma. Int. J. Hyperth. 2019, 36, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Matoba, M.; Tsuchiya, H.; Kondo, T.; Ota, K. Stereotactic body radiotherapy delivered with IMRT for oligometastatic regional lymph node metastases in hepatocellular carcinoma: A single-institutional study. J. Radiat. Res. 2020, 61, 776–783. [Google Scholar] [CrossRef]
- Mu, L.; Sun, L.; Pan, T.; Lyu, N.; Li, S.; Li, X.; Wang, J.; Xie, Q.; Deng, H.; Zheng, L. Percutaneous CT-guided radiofrequency ablation for patients with extrahepatic oligometastases of hepatocellular carcinoma: Long-term results. Int. J. Hyperth. 2018, 34, 59–67. [Google Scholar] [CrossRef]
- Omae, K.; Hiraki, T.; Gobara, H.; Iguchi, T.; Fujiwara, H.; Matsui, Y.; Toyooka, S.; Nagasaka, T.; Kanazawa, S. Long-term survival after radiofrequency ablation of lung oligometastases from five types of primary lesions: A retrospective evaluation. J. Vasc. Interv. Radiol. 2016, 27, 1362–1370. [Google Scholar] [CrossRef]
- Song, S.Y.; Je, H.U.; Choi, W.; Jwa, E.J.; Kim, J.H.; Yoon, S.M.; Kim, S.S.; Choi, E.K. Stereotactic Ablative radiotherapy for pulmonary oligometastasis from hepatocellular carcinoma. J. Thorac. Oncol. 2013, 8, S540–S541. [Google Scholar]
- Giannini, E.G.; Farinati, F.; Ciccarese, F.; Pecorelli, A.; Rapaccini, G.L.; Di Marco, M.; Benvegnù, L.; Caturelli, E.; Zoli, M.; Borzio, F. Prognosis of untreated hepatocellular carcinoma. Hepatology 2015, 61, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Zeeneldin, A.A.; Salem, S.E.; Darwish, A.D.; El-Gammal, M.M.; Hussein, M.M.; Saadeldin, M. Untreated hepatocellular carcinoma in Egypt: Outcome and prognostic factors. J. Hepatocell. Carcinoma 2015, 2, 3. [Google Scholar]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Rim, C.H.; Cho, W.K.; Lee, J.H.; Kim, Y.S.; Suh, Y.-G.; Kim, K.H.; Chie, E.K.; Ahn, Y.C. Role of Local Treatment for Oligometastasis: A Comparability-Based Meta-Analysis. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2022, 54, 953–969. [Google Scholar] [CrossRef]
- Wen, T.; Jin, C.; Facciorusso, A.; Donadon, M.; Han, H.-S.; Mao, Y.; Dai, C.; Cheng, S.; Zhang, B.; Peng, B.; et al. Multidisciplinary management of recurrent and metastatic hepatocellular carcinoma after resection: An international expert consensus. Hepatobiliary Surg. Nutr. 2018, 7, 353–371. [Google Scholar] [CrossRef]
- Rim, C.H.; Lee, J.S.; Kim, S.Y.; Seong, J. Comparison of radiofrequency ablation and ablative external radiotherapy for the treatment of intrahepatic malignancies: A hybrid meta-analysis. JHEP Rep. 2022, 5, 100594. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Byun, H.K.; Seong, J. Irradiation-Related Lymphopenia for Bone Metastasis from Hepatocellular Carcinoma. Liver Cancer 2019, 8, 468–479. [Google Scholar] [CrossRef]
- Rim, C.H.; Park, S.; Shin, I.S.; Yoon, W.S. Is the Concurrent Use of Sorafenib and External Radiotherapy Feasible for Advanced Hepatocellular Carcinoma? A Meta-Analysis. Cancers 2021, 13, 2912. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.-S.; Rim, C.H. Updating Perspectives on Meta-Analyses in the Field of Radiation Oncology. Medicina 2021, 57, 117. [Google Scholar] [CrossRef] [PubMed]
- Shrier, I.; Boivin, J.-F.; Steele, R.J.; Platt, R.W.; Furlan, A.; Kakuma, R.; Brophy, J.; Rossignol, M. Should meta-analyses of interventions include observational studies in addition to randomized controlled trials? A critical examination of underlying principles. Am. J. Epidemiol. 2007, 166, 1203–1209. [Google Scholar] [CrossRef] [PubMed]

| Author | No. of Patients | Affiliation | Study Design | Recruiting Year | Metastatic Organ | OM No. | OM Definition | CPC A (%) | Other Metastasis (%) |
|---|---|---|---|---|---|---|---|---|---|
| Pan | 46 (RFA) | Sun Yats sen center, Taiwan | PSM Retrospective cohort | 2004–2013 | lymph node | 2.22 ± 1.35 | NA | 95.7 | 26.1 |
| 73 (non-RFA) | 2.74 ± 1.37 | 93.5 | 37.0 | ||||||
| Chen | 34 (combined) | Sun Yat sen center, China | PSM Retrospective cohort | 2013–2016 | lung | ≤5 | 94.1 | 17.6 | |
| 34 (sorafenib alone) | 88.2 | 17.6 | |||||||
| Kim | 36 (combination of local, systemic therapies) | Yonsei University College of Medicine, Korea | PSM Retrospective cohort | 2008–2015 | lung | ≤4 | 100.0 | ||
| 22 (systemic therapy alone) | 100.0 | ||||||||
| Omae | 16 | Okayama University Medical School, Japan | Case series | 2001–2013 | lung | single 43.8% | ≤5 | 37.5 | |
| Mu | 79 | Sun Yat sen center, China | Case series | 2004–2015 | lung 56%; lymph node 13% | single 67.1% | ≤3 | 98.7 | 15.2 |
| Lyu | 18 | Sun Yat sen center, China | Case series | 2004–2015 | adrenal | ≤2 | |||
| Matoba | 15 | Kanazawa Medical University, Japan | Case series | 2014–2017 | lymph node | single 66.7% | ≤5 | 33.3 | |
| Kim | 59 | Yonsei University College of Medicine, Korea | Case series | 1992–2019 | bone | ≤5 lesions | ≤5 | ||
| Jo | 58 | 9 centers, Korea | Case series | 2011–2018 | lung | NA | |||
| Song | 37 | Asan hospital, Korea | Case series | 2006–2011 | lung | ≤5 |
| Author | No. of Patients | Study Design | Treatment Method | Primary Controlled | Follow-Up Period | Overall Survival (M, Median) | Factors Affecting Survival | Toxicity (Grade ≥ 3) |
|---|---|---|---|---|---|---|---|---|
| Pan | 46 (RFA) | PSM Retrospective cohort | RFA (sorafenib 26%) vs. non-RFA (sorafenib 20%) | 54.4% | 14 | M13.0 months 1/2y: 58.3/12.6 (p = 0.001) | Controlled primary disease, Local treatment, Other metastasis | No grade ≥ 3 toxicity |
| 73 (non-RFA) | 50.0% | 13.8 | M7.8 months 1/2y: 17.9/0 (p = 0.001) | |||||
| Chen | 34 (combined) | PSM Retrospective cohort | sorafenib & local therapies (TACE, RFA, 125I) vs. sorafenib | 35.3% | M18.4 months 1/2y: 61.7/46% | AFP, Local treatment, Macrovascular invasion | ||
| 34 (sorafenib alone) | 26.5% | M7.4 months 1/2y: 35.7/23.7% (p = 0.015) | ||||||
| Kim | 36 (combination of local, systemic therapies) | PSM Retrospective cohort | Local (surgery or RT) vs. systemic therapies | 1/2y: 91.6/66.6% (p < 0.001) | AFP, Local treatment | 1 case (2.8%) of grade 3 pneumonitis after surgery | ||
| 22 (systemic therapy alone) | 1/2y: 68.4/31.2% (p < 0.001) | No grade ≥ 3 toxicity | ||||||
| Omae | 16 | Case series | RFA (systemic therapy 36%) | 25.0% | 26 | M26 months 1/2y: 94/66% (p = 0.85) | No mortality related to RFA | |
| Mu | 79 | Case series | RFA (sorafenib 17.7%) | 28 | M33.5 months 1/2y: 91/70% | AFP (p = 0.054), CPC, Time to metastasis | 9 cases of pneumothorax (9.5%, 9/95) necessitate chest tube insertion | |
| Lyu | 18 | Case series | RFA | 17~18 | M21.8 months 0.5/1/2y: 88.9, 66.7, 44.4% (p = 0.043) | CPC | 8 of 33 sessions of grade 3 hypertension, which was restored after treatment) No grade ≥ 4 toxicity | |
| Matoba | 15 | Case series | SBRT (sorafenib 13.3%) | 18.1 | 1/2y: 73.3/28.6% | CPC (univariate analysis) | No grade ≥ 3 toxicity | |
| Kim | 59 | Case series | HFRT | 12 | MOS 9.8 mo 1/2y: 39.3, 22.9% (p < 0.001) | Controlled primary disease, Extraosseous metastasis | ||
| Jo | 58 | Case series | SBRT (chemotherapy, 36.2%) | MOS 16.3 months (1/2y: 65.5, 41.4%) MPFS 4.9 months (1/2y: 22.4, 13.5%) | CPC, Controlled primary disease, RT response | 3 cases of grade 4 Leukopenia (5.2%); 2 cases of grade 3 pneumonia (3.4%), 1 case of leukopenia (1.7%), 1 case of dermatitis (1.7%) | ||
| Song | 37 | Case series | SBRT | 19.9 | M19.9 months 1/3y: 67.6/35.5 | Treatment modality | No significant RT complication |
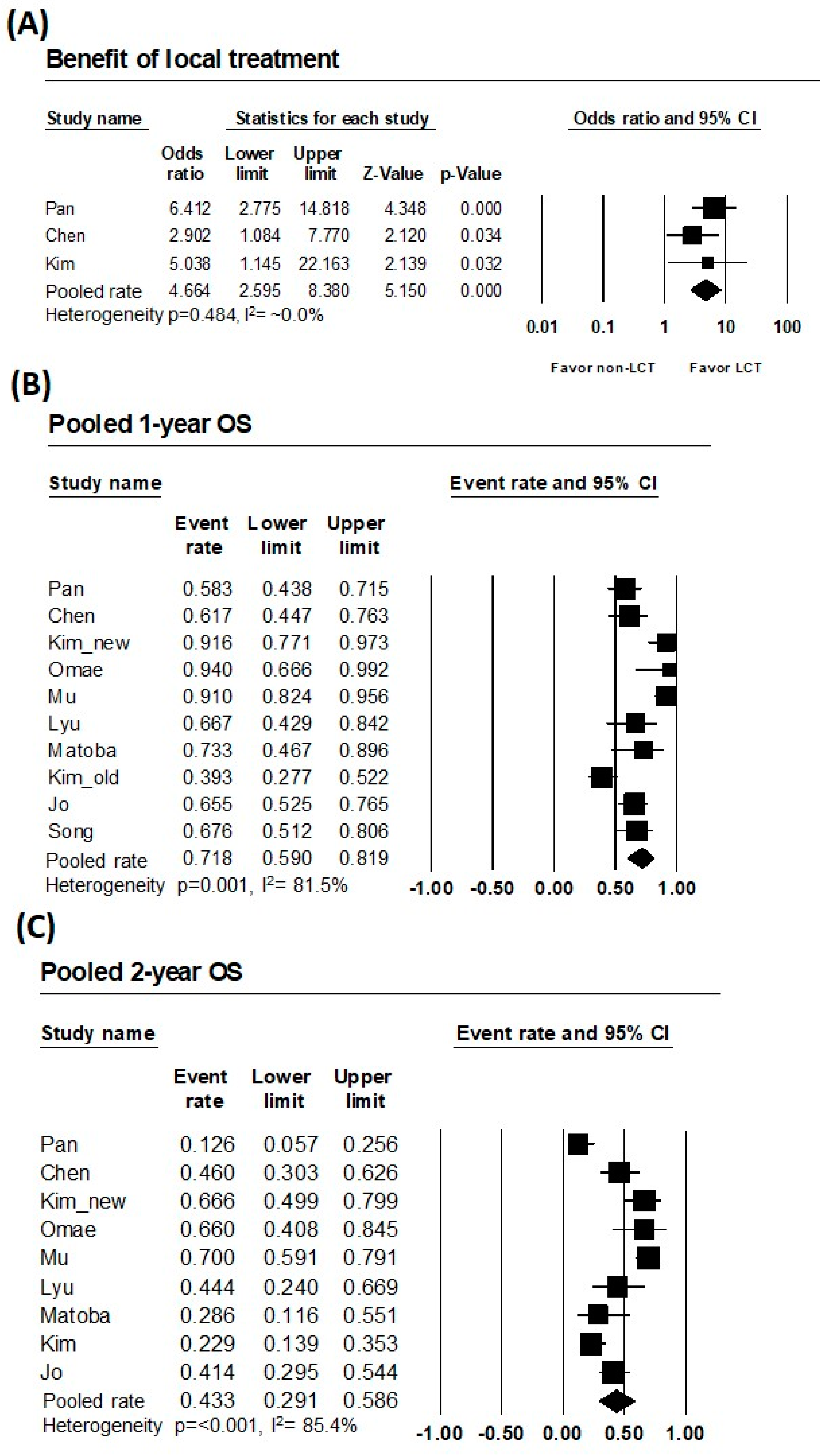
| Subject | No. of Studies | No. of Cases | Heterogeneity p | I2 (%) | Heterogeneity Assessment | Pooled Rate (95% CI) |
|---|---|---|---|---|---|---|
| Comparative OR | 3 | 245 | 0.484 | ~0.0% | Very low | 4.664 (2.595–8.380) |
| 1-year OS | 10 | 398 | 0.001 | 81.5% | High | 71.8% (59.0–81.9) |
| 2-year OS | 9 | 361 | <0.001 | 85.4% | High | 43.3% (29.1–58.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Lee, J.; Rim, C.H. Local Treatment of Hepatocellular Carcinoma with Oligometastases: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 3467. https://doi.org/10.3390/cancers15133467
Kim S, Lee J, Rim CH. Local Treatment of Hepatocellular Carcinoma with Oligometastases: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(13):3467. https://doi.org/10.3390/cancers15133467
Chicago/Turabian StyleKim, Sooyeon, Jungsue Lee, and Chai Hong Rim. 2023. "Local Treatment of Hepatocellular Carcinoma with Oligometastases: A Systematic Review and Meta-Analysis" Cancers 15, no. 13: 3467. https://doi.org/10.3390/cancers15133467
APA StyleKim, S., Lee, J., & Rim, C. H. (2023). Local Treatment of Hepatocellular Carcinoma with Oligometastases: A Systematic Review and Meta-Analysis. Cancers, 15(13), 3467. https://doi.org/10.3390/cancers15133467







