Evaluation of Efficacy of ALK Inhibitors According to Body Mass Index in ALK Rearranged NSCLC Patients—A Retrospective Observational Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Classifications
2.3. Statistical Analysis
3. Results
3.1. Patients Characteristics
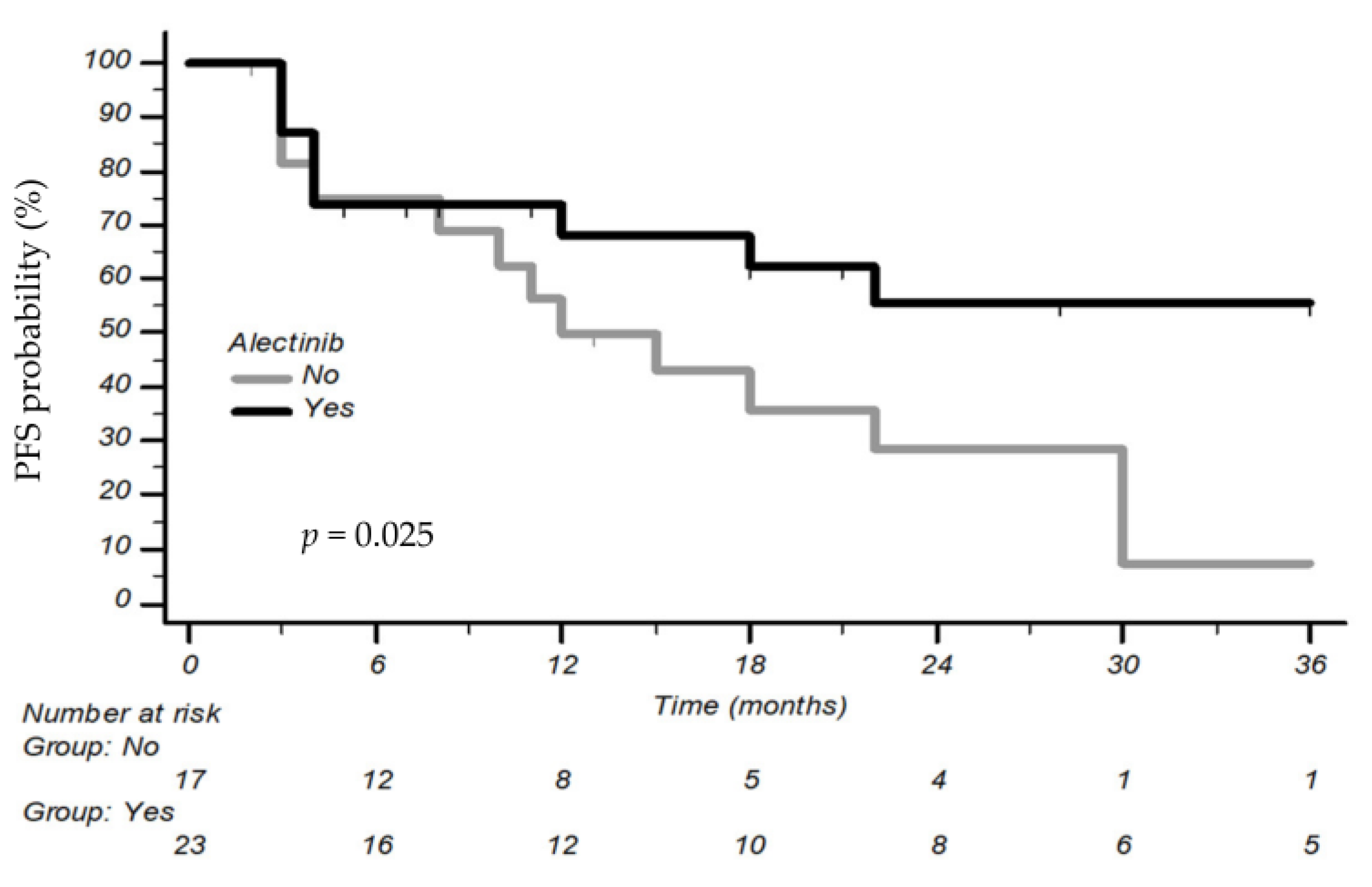
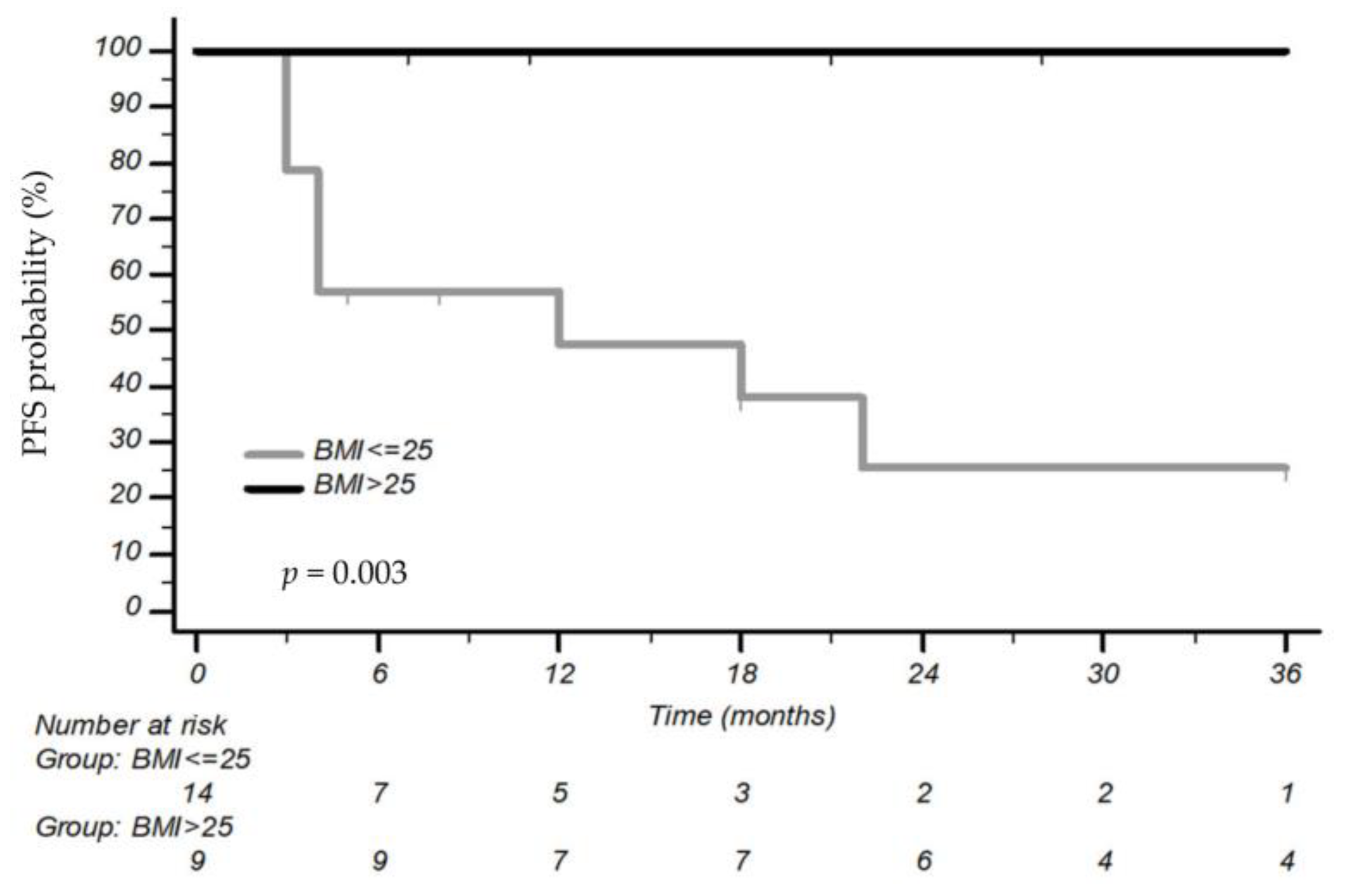
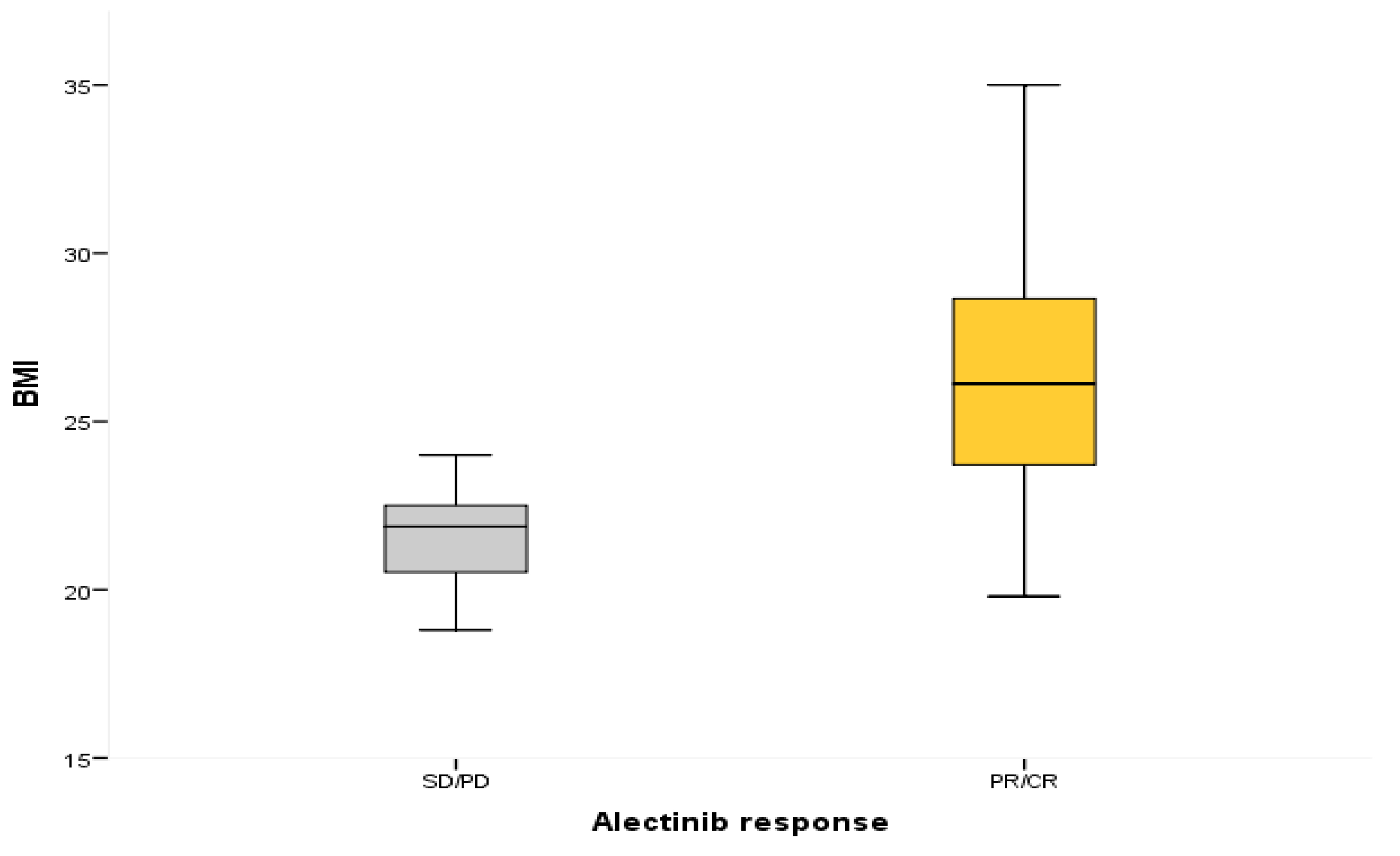
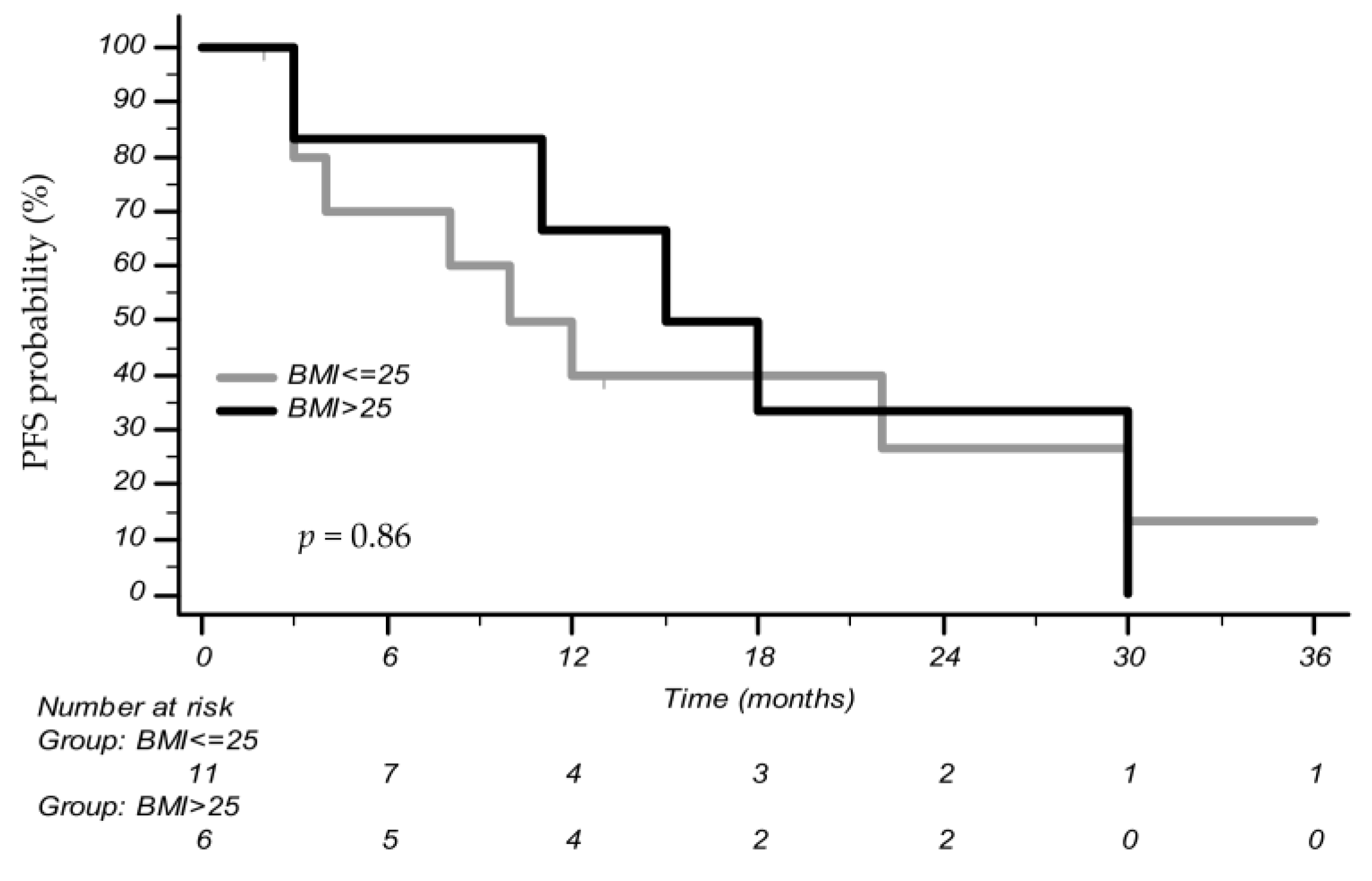
3.2. Association between BMI and Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L. Lung Cancer Statistics. Adv. Exp. Med. Biol. 2016, 893, 1–19. [Google Scholar] [CrossRef]
- Ordóñez-Mena, J.M.; Schöttker, B. Consortium on Health and Ageing: Network of Cohorts in Europe and the United States (CHANCES). Quantification of the smoking-associated cancer risk with rate advancement periods: Meta-analysis of individual participant data from cohorts of the CHANCES consortium. BMC Med. 2016, 14, 62. [Google Scholar] [CrossRef]
- Toh, C.K.; Gao, F. Never-smokers with lung cancer: Epidemiologic evidence of a distinct disease entity. J. Clin. Oncol. 2006, 24, 2245–2251. [Google Scholar] [CrossRef]
- Couraud, S.; Souquet, P.J. French Cooperative Intergroup IFCT. BioCAST/IFCT-1002: Epidemiological and molecular features of lung cancer in never-smokers. Eur. Respir. J. 2015, 45, 1403–1414. [Google Scholar] [CrossRef]
- Melosky, B.; Cheema, P. Canadian perspectives: Update on inhibition of ALK-positive tumours in advanced non-small-cell lung cancer. Curr. Oncol. 2018, 25, 317–328. [Google Scholar] [CrossRef]
- Wong, D.W.; Leung, E.L. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 2009, 115, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Von Laffert, M.; Warth, A. Multicenter immunohistochemical ALK-testing of non–small-cell lung cancer shows high concordance after harmonization of techniques and interpretation criteria. J. Thorac. Oncol. 2014, 9, 1685–1692. [Google Scholar] [CrossRef]
- Johung, K.L.; Yeh, N. Extended survival and prognostic factors for patients with ALK-rearranged non–small-cell lung cancer and brain metastasis. J. Clin. Oncol. 2016, 34, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Mok, T. PROFILE 1014 Investigators. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef]
- Peters, S.; Camidge, D.R. Alectinib versus crizotinib in untreated ALK-positive non–small-cell lung cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef]
- Gadgeel, S.; Peters, S. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann. Oncol. 2018, 29, 2214–2222. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Kim, H.R. Brigatinib versus Crizotinib in ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2027–2039. [Google Scholar] [CrossRef]
- Solomon, B.J.; Besse, B. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: Results from a global phase 2 study. Lancet Oncol. 2018, 19, 1654–1667. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Bauer. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 2020, 383, 2018–2029. [Google Scholar] [CrossRef]
- Marinelli, D.; Siringo, M. Non-small-cell lung cancer: How to manage ALK-, ROS1- and NTRK-rearranged disease. Drugs Context 2022, 11, 2022-3-1. [Google Scholar] [CrossRef]
- Eckel, R.H.; Grundy, S.M. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Lohmann, A.E.; Goodwin, P.J. Association of Obesity-Related Metabolic Disruptions With Cancer Risk and Outcome. J. Clin. Oncol. 2016, 34, 4249–4255. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Gucalp, A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef]
- Petrelli, F.; Cortellini, A. Association of Obesity With Survival Outcomes in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e213520. [Google Scholar] [CrossRef]
- Ligibel, J.A.; Alfano, C.M. American Society of Clinical Oncology position statement on obesity and cancer. J. Clin. Oncol. 2014, 32, 3568–3574. [Google Scholar] [CrossRef]
- Greenlee, H.; Unger, J.M. Association between Body Mass Index and Cancer Survival in a Pooled Analysis of 22 Clinical Trials. Cancer Epidemiol. Biomark. Prev. 2017, 26, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Shepshelovich, D.; Xu, W. Body Mass Index (BMI), BMI Change, and Overall Survival in Patients with SCLC and NSCLC: A Pooled Analysis of the International Lung Cancer Consortium. J. Thorac. Oncol. 2019, 14, 1594–1607. [Google Scholar] [CrossRef] [PubMed]
- Gelibter, A.; Occhipinti, M. Status of correlation between BMI and response to immunocheck-point inhibitor in advanced non-small-cell lung cancer. Lung Cancer Manag. 2020, 9, LMT26. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Ricciuti, B. Baseline BMI and BMI variation during first line pembrolizumab in NSCLC patients with a PD-L1 expression ≥ 50%: A multicenter study with external validation. J. Immunother. Cancer 2020, 8, e001403. [Google Scholar] [CrossRef]
- Ding, Z.Y.; Chen, Y.Y. Obesity has an impact on the efficacy of EGFR-TKI in NSCLC patients harbouring EGFR mutation: A real-world study. Ann. Oncol. 2019, 30, ii49–ii50. [Google Scholar] [CrossRef]
- De Giglio, A.; Di Federico, A. The prognostic role of body-mass index (BMI) for advanced EGFR positive non-small cell lung cancer (NSCLC) patients treated with osimertinib. J. Clin. Oncol. 2021, 39 (Suppl. S15), e21078. [Google Scholar] [CrossRef]
- Ono, T.; Igawa, S. Evaluation of osimertinib efficacy according to body surface area and body mass index in patients with non-small cell lung cancer harboring an EGFR mutation: A prospective observational study. Thorac. Cancer 2019, 10, 880–889. [Google Scholar] [CrossRef]
- Takeda, T.; Yamada, T. Prognostic Markers of Survival among Japanese Patients with Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer Receiving First-Line Alectinib. Diagnostics 2021, 11, 2170. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, G. Causal relationships between body mass index, smoking and lung cancer: Univariable and multivariable Mendelian randomization. Int. J. Cancer 2021, 148, 1077–1086. [Google Scholar] [CrossRef]
- Wang, N.F.; Tang, H.M. Prolonged progression-free survival and overall survival are associated with diabetes mellitus but inversely associated with levels of blood glucose in patients with lung cancer. Chin. Med. J. 2020, 133, 786–791. [Google Scholar] [CrossRef]
- Tsai, M.J.; Yang, C.J. Metformin decreases lung cancer risk in diabetic patients in a dose-dependent manner. Lung Cancer 2014, 86, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Jia, Y. Concurrent use of metformin enhances the efficacy of EGFR-TKIs in patients with advanced EGFR-mutant non-small cell lung cancer-an option for overcoming EGFR-TKI resistance. Transl. Lung Cancer Res. 2021, 10, 1277–1291. [Google Scholar] [CrossRef]
- Chen, H.; Lin, C. Metformin reduces HGF-induced resistance to alectinib via the inhibition of Gab1. Cell Death Dis. 2020, 11, 111. [Google Scholar] [CrossRef]
- Abdel-Rahman, O. Pre-diagnostic body mass index trajectory in relationship to lung cancer incidence and mortality; findings from the PLCO trial. Expert Rev. Respir. Med. 2019, 13, 1029–1035. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, Z. Updates on the pathogenesis of advanced lung cancer-induced cachexia. Thorac. Cancer 2019, 10, 8–16. [Google Scholar] [CrossRef]
- Card, P.; Schussler, O. Pre-Disease and Pre-Surgery BMI, Weight Loss and Sarcopenia Impact Survival of Resected Lung Cancer Independently of Tumor Stage. Cancers 2020, 12, 266. [Google Scholar] [CrossRef]
- Matsunaga, T.; Suzuki, K. Body Mass Index as a Prognostic Factor in Resected Lung Cancer: Obesity or Underweight, Which Is the Risk Factor? Thorac. Cardiovasc. Surg. 2015, 63, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Ricciuti, B. Prognostic effect of body mass index in patients with advanced NSCLC treated with chemoimmunotherapy combinations. J. Immunother. Cancer 2022, 10, e004374. [Google Scholar] [CrossRef] [PubMed]
- Sutandyo, N.; Hanafi, A.R. Overweight and Obesity are Associated with Poorer Survival Among Patients with Advanced Non-Small Cell Lung Cancer Receiving Platinum-Based Chemotherapy. Int. J. Gen. Med. 2023, 16, 85–93. [Google Scholar] [CrossRef]
- Chen, Y.M.; Lai, C.H. Body Mass Index, Weight Loss, and Mortality Risk in Advanced-Stage Non-Small Cell Lung Cancer Patients: A Focus on EGFR Mutation. Nutrients 2021, 13, 3761. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Fares, A.F. The relationship between body-mass index and overall survival in non-small cell lung cancer by sex, smoking status, and race: A pooled analysis of 20,937 International lung Cancer consortium (ILCCO) patients. Lung Cancer 2021, 152, 58–65. [Google Scholar] [CrossRef]
- Scott, H.R.; McMillan, D.C.; Forrest, L.M.; Brown, D.J.F.; McArdle, C.S.; Milroy, R. The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small cell lung cancer. Br. J. Cancer 2002, 87, 264–267. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, S.P.; Pruis, M.A.; Sikkema, B.J.; Mohseni, M.; Veerman, G.D.M.; Paats, M.S.; Dumoulin, D.W.; Smit, E.F.; Schols, A.M.J.; Mathijssen, R.H.; et al. Analysis of Serious Weight Gain in Patients Using Alectinib for ALK-Positive Lung Cancer. J. Thorac. Oncol. 2023. [Google Scholar] [CrossRef] [PubMed]

| Variable | Level | Overall (N = 40) |
|---|---|---|
| Sex | Female | 25 (62.5%) |
| Male | 15 (37.5%) | |
| PS | 0 | 13 (32.5%) |
| 1 | 25 (62.5%) | |
| 2 | 2 (5%) | |
| BMI | Low | 25 (62.5%) |
| High | 15 (37.5%) | |
| Smoking status | Non-smoker | 21 (52.5%) |
| Smoker | 7 (17.5%) | |
| Former smoker | 12 (30%) | |
| DM2 | Yes | 7 (17.5%) |
| No | 33 (82.5%) | |
| Metformin | Yes | 4 (10%) |
| No | 36 (90%) | |
| Steroids | Yes | 21 (52.5%) |
| No | 19 (47.5%) | |
| Antiepileptic drugs | Yes | 16 (40%) |
| No | 24 (60%) | |
| Visceral metastasis | Yes | 32 (80%) |
| No | 8 (20%) | |
| CNS metastasis | Yes | 20 (50%) |
| No | 20 (50%) | |
| Number of metastases | ≤3 | 13 (32.5%) |
| >3 | 27 (67.5%) | |
| I line | Alectinib | 23 |
| Crizotinib | 10 | |
| Brigatinib | 2 | |
| Chemotherapy | 3 | |
| Immunotherapy | 2 | |
| II line | No treatment | 23 |
| Alectinib | 9 | |
| Crizotinib | 2 | |
| Brigatinib | 1 | |
| Lorlatinib | 2 | |
| Ceritinib | 1 | |
| Chemotherapy | 2 | |
| III line | No treatment | 33 |
| Alectinib | 2 | |
| Lorlatinib | 4 | |
| Chemotherapy | 1 |
| Variables | PFS Univariate Analysis | OS Univariate Analysis |
|---|---|---|
| (a) | ||
| Smoking status | HR 1.85 (CI 95% 0.81–4.22) p = 0.15 | HR 1.36 (CI 95% 0.49–3.81) p = 0.56 |
| Yes vs. No | ||
| Age at metastatic stage | HR 1.06 (CI 95% 0.98–1.03) p = 0.74 | HR 1.03 (CI 95% 0.99–1.07) p = 0.22 |
| Number of metastases | HR 1.45 (CI 95% 0.58–3.67) p = 0.43 | HR 1.02 (CI 95% 0.36–2.91) p = 0.96 |
| Visceral metastasis | HR 1.85 (CI 95% 0.54–6.26) p = 0.33 | HR 3.68 (CI 95% 0.49–27.9) p = 0.21 |
| Yes vs. No | ||
| CNS metastasis | HR 1.61 (CI 95% 0.72–3.58) p = 0.24 | HR 1.08 (CI 95% 0.41–2.85) p = 0.87 |
| Yes vs. No | ||
| PS0 vs. PS1/2 | HR 0.22 (CI 95% 0.07–0.65) p = 0.007 | HR 0.21 (CI 95% 0.06–0.71) p = 0.013 |
| BMI high vs. BMI low | HR 0.35 (CI 95% 0.15–0.84) p = 0.019 | HR 0.27 (CI 95% 0.09–0.80) p = 0.020 |
| First-line ALKi | HR 2.35 (CI 95% 1.05–5.25) p = 0.038 | - |
| Others vs. Alectinib | ||
| (b) | ||
| PS0 vs. PS1/2 | HR 0.24 (CI 95% 0.08–0.73) p = 0.012 | HR 0.15 (CI 95% 0.04–0.55) p = 0.014 |
| BMI high vs. BMI low | HR 0.39 (CI 95% 0.16–0.96) p = 0.042 | HR 0.18 (CI 95% 0.05–0.61) p = 0.006 |
| First-line ALKi | HR 2.01 (CI 95% 0.89–4.5) p = 0.094 | - |
| Others vs. Alectinib | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siringo, M.; Gentile, G.; Caponnetto, S.; Sperduti, I.; Santini, D.; Cortesi, E.; Gelibter, A.J. Evaluation of Efficacy of ALK Inhibitors According to Body Mass Index in ALK Rearranged NSCLC Patients—A Retrospective Observational Study. Cancers 2023, 15, 3422. https://doi.org/10.3390/cancers15133422
Siringo M, Gentile G, Caponnetto S, Sperduti I, Santini D, Cortesi E, Gelibter AJ. Evaluation of Efficacy of ALK Inhibitors According to Body Mass Index in ALK Rearranged NSCLC Patients—A Retrospective Observational Study. Cancers. 2023; 15(13):3422. https://doi.org/10.3390/cancers15133422
Chicago/Turabian StyleSiringo, Marco, Gabriella Gentile, Salvatore Caponnetto, Isabella Sperduti, Daniele Santini, Enrico Cortesi, and Alain Jonathan Gelibter. 2023. "Evaluation of Efficacy of ALK Inhibitors According to Body Mass Index in ALK Rearranged NSCLC Patients—A Retrospective Observational Study" Cancers 15, no. 13: 3422. https://doi.org/10.3390/cancers15133422
APA StyleSiringo, M., Gentile, G., Caponnetto, S., Sperduti, I., Santini, D., Cortesi, E., & Gelibter, A. J. (2023). Evaluation of Efficacy of ALK Inhibitors According to Body Mass Index in ALK Rearranged NSCLC Patients—A Retrospective Observational Study. Cancers, 15(13), 3422. https://doi.org/10.3390/cancers15133422






