Relationships among Inflammatory Biomarkers and Self-Reported Treatment-Related Symptoms in Patients Treated with Chemotherapy for Gynecologic Cancer: A Controlled Comparison
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Demographic and Clinical Data
2.2.2. Circulating Inflammatory Biomarkers
2.2.3. Treatment-Related Symptoms
2.3. Data Analyses
3. Results
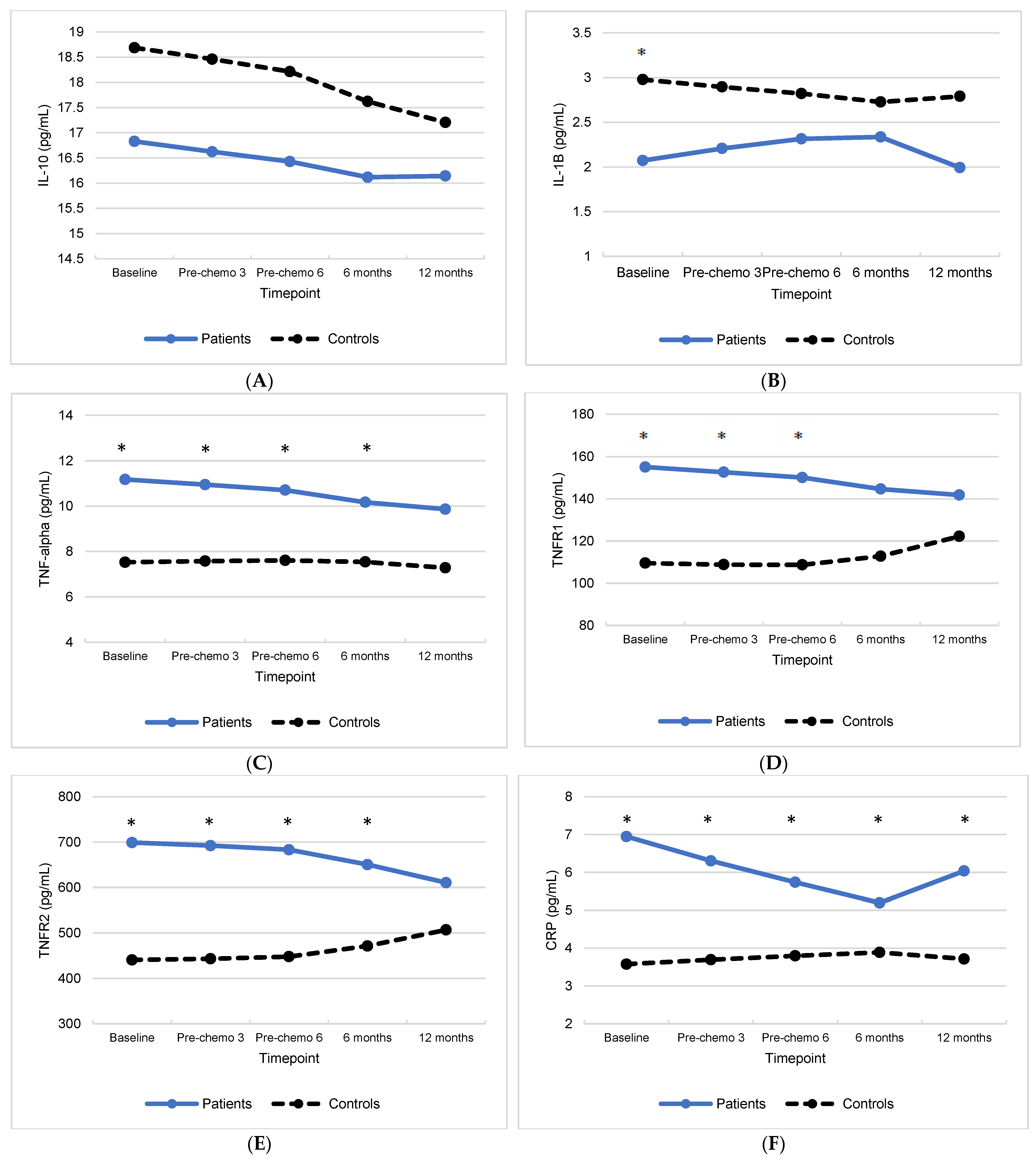
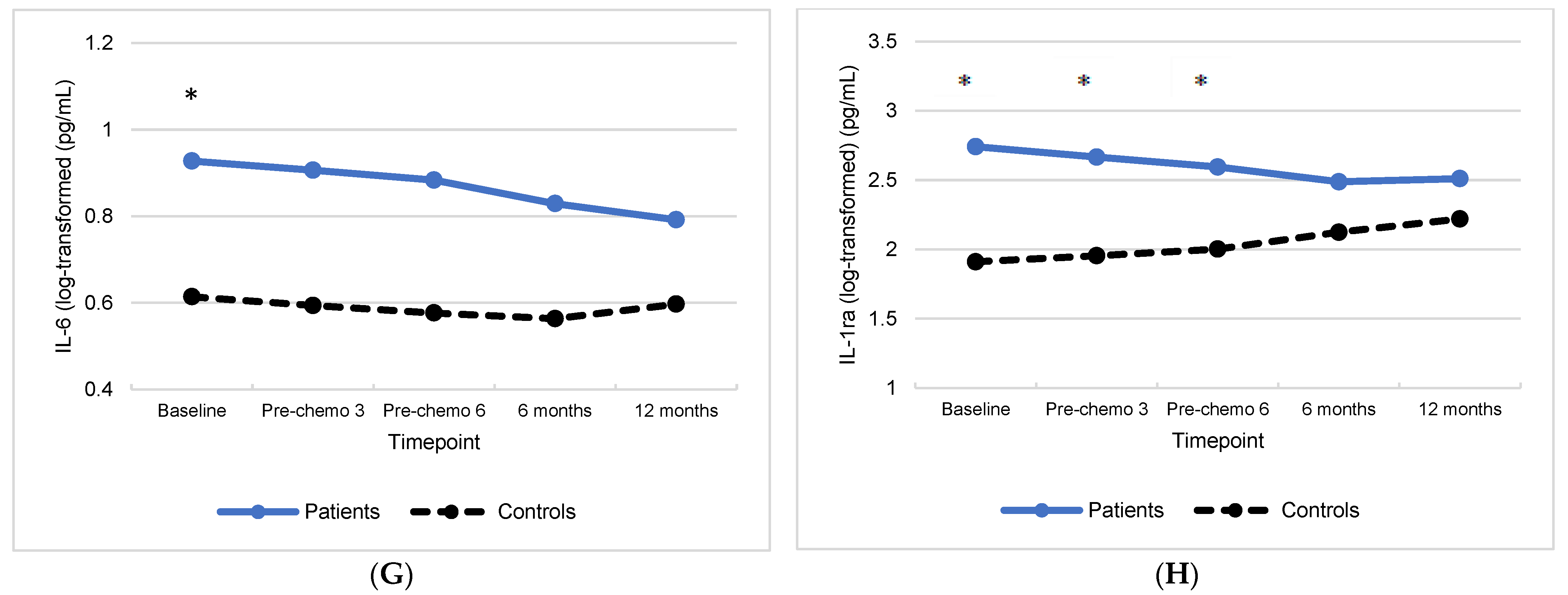
3.1. Group Differences in Inflammatory Biomarkers
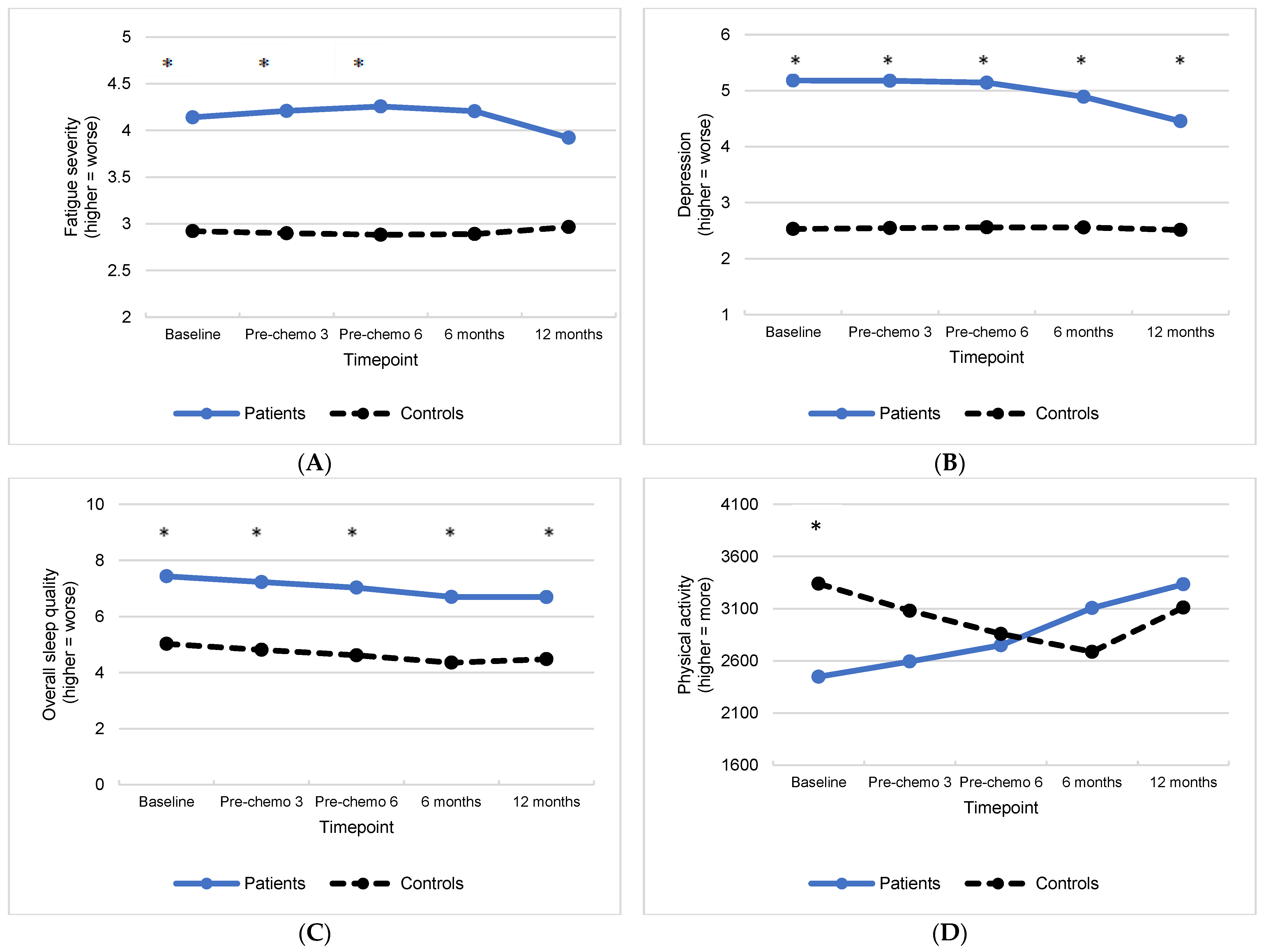
3.2. Group Differences in Treatment-Related Symptoms and Associations with Inflammation
3.2.1. Fatigue
3.2.2. Depression
3.2.3. Sleep
3.2.4. Physical Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beesley, V.L.; Price, M.A.; Butow, P.N.; Green, A.C.; Olsen, C.M.; Australian Ovarian Cancer Study Group; Australian Ovarian Cancer Study—Quality of Life Study; Webb, P.M. Physical activity in women with ovarian cancer and its association with decreased distress and improved quality of life. Psychooncology 2011, 20, 1161–1169. [Google Scholar] [PubMed]
- Stevinson, C.; Faught, W.; Steed, H.; Tonkin, K.; Ladha, A.B.; Vallance, J.K.; Capstick, V.; Schepansky, A.; Courneya, K.S. Associations between physical activity and quality of life in ovarian cancer survivors. Gynecol. Oncol. 2007, 106, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Stevinson, C.; Steed, H.; Faught, W.; Tonkin, K.; Vallance, J.K.; Ladha, A.B.; Schepansky, A.; Capstick, V.; Courneya, K.S. Physical activity in ovarian cancer survivors: Associations with fatigue, sleep, and psychosocial functioning. Int. J. Gynecol. Cancer 2009, 19, 73–78. [Google Scholar] [CrossRef]
- Pozzar, R.A.; Hammer, M.J.; Paul, S.M.; Cooper, B.A.; Kober, K.M.; Conley, Y.P.; Levine, J.D.; Miaskowski, C. Distinct sleep disturbance profiles among patients with gynecologic cancer receiving chemotherapy. Gynecol. Oncol. 2021, 163, 419–426. [Google Scholar] [CrossRef]
- Poort, H.; de Rooij, B.H.; Uno, H.; Weng, S.; Ezendam, N.P.M.; van de Poll-Franse, L.; Wright, A.A. Patterns and predictors of cancer-related fatigue in ovarian and endometrial cancers: 1-year longitudinal study. Cancer 2020, 126, 3526–3533. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.M.; Cristea, M.; DeRosa, M.; Eisenhauer, E.L.; et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 191–226. [Google Scholar] [CrossRef]
- Jim, H.S.; Jacobsen, P.B.; Phillips, K.M.; Wenham, R.M.; Roberts, W.; Small, B.J. Lagged relationships among sleep disturbance, fatigue, and depressed mood during chemotherapy. Health Psychol. 2013, 32, 768–774. [Google Scholar] [CrossRef]
- Jim, H.S.; Small, B.; Faul, L.A.; Franzen, J.; Apte, S.; Jacobsen, P.B. Fatigue, depression, sleep, and activity during chemotherapy: Daily and intraday variation and relationships among symptom changes. Ann. Behav. Med. 2011, 42, 321–333. [Google Scholar] [CrossRef]
- Bluthe, R.M.; Pawlowski, M.; Suarez, S.; Parnet, P.; Pittman, Q.; Kelley, K.W.; Dantzer, R. Synergy between tumor necrosis factor alpha and interleukin-1 in the induction of sickness behavior in mice. Psychoneuroendocrinology 1994, 19, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Borisov, A.S.; Majer, M.; Drake, D.F.; Pagnoni, G.; Woolwine, B.J.; Vogt, G.J.; Massung, B.; Miller, A.H. Activation of central nervous system inflammatory pathways by interferon-alpha: Relationship to monoamines and depression. Biol. Psychiatry 2009, 65, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Capuron, L.; Ravaud, A.; Dantzer, R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J. Clin. Oncol. 2000, 18, 2143–2151. [Google Scholar] [CrossRef]
- Capuron, L.; Ravaud, A.; Dantzer, R. Timing and specificity of the cognitive changes induced by interleukin-2 and interferon-alpha treatments in cancer patients. Psychosom. Med. 2001, 63, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Kelley, K.W.; Bluthe, R.M.; Dantzer, R.; Zhou, J.H.; Shen, W.H.; Johnson, R.W.; Broussard, S.R. Cytokine-induced sickness behavior. Brain Behav. Immun. 2003, 17 (Suppl. 1), S112–S118. [Google Scholar] [CrossRef]
- Malik, U.R.; Makower, D.F.; Wadler, S. Interferon-mediated fatigue. Cancer 2001, 92 (Suppl. S6), 1664–1668. [Google Scholar] [CrossRef]
- Swiergiel, A.H.; Burunda, T.; Patterson, B.; Dunn, A.J. Endotoxin- and interleukin-1-induced hypophagia are not affected by adrenergic, dopaminergic, histaminergic, or muscarinic antagonists. Pharmacol. Biochem. Behav. 1999, 63, 629–637. [Google Scholar] [CrossRef]
- Yirmiya, R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996, 711, 163–174. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Irwin, M.R.; Castellon, S.; Arevalo, J.; Cole, S.W. Cytokine genetic variations and fatigue among patients with breast cancer. J. Clin. Oncol. 2013, 31, 1656–1661. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.W.; Kim, Y.K. Inflammation-induced depression: Its pathophysiology and therapeutic implications. J. Neuroimmunol. 2017, 313, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Karshikoff, B.; Sundelin, T.; Lasselin, J. Role of Inflammation in Human Fatigue: Relevance of Multidimensional Assessments and Potential Neuronal Mechanisms. Front. Immunol. 2017, 8, 21. [Google Scholar] [CrossRef]
- Irwin, M.R. Sleep and inflammation: Partners in sickness and in health. Nat. Rev. Immunol. 2019, 19, 702–715. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Derry, H.M.; Fagundes, C. Inflammation: Depression fans the flames and feasts on the heat. Am. J. Psychiatry 2015, 172, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Ancoli-Israel, S.; Bower, J.E.; Capuron, L.; Irwin, M.R. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J. Clin. Oncol. 2008, 26, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Anisman, H. Cascading effects of stressors and inflammatory immune system activation: Implications for major depressive disorder. J. Psychiatry Neurosci. 2009, 34, 4–20. [Google Scholar] [PubMed]
- Muller, N.; Schwarz, M.J. The immune-mediated alteration of serotonin and glutamate: Towards an integrated view of depression. Mol. Psychiatry 2007, 12, 988–1000. [Google Scholar] [CrossRef]
- Pucak, M.L.; Kaplin, A.I. Unkind cytokines: Current evidence for the potential role of cytokines in immune-mediated depression. Int. Rev. Psychiatry 2005, 17, 477–483. [Google Scholar] [CrossRef]
- Dunn, A.J.; Wang, J.; Ando, T. Effects of cytokines on cerebral neurotransmission. Comparison with the effects of stress. Adv. Exp. Med. Biol. 1999, 461, 117–127. [Google Scholar]
- Schubert, C.; Hong, S.; Natarajan, L.; Mills, P.J.; Dimsdale, J.E. The association between fatigue and inflammatory marker levels in cancer patients: A quantitative review. Brain Behav. Immun. 2007, 21, 413–427. [Google Scholar] [CrossRef]
- Hoogland, A.I.; Jim, H.S.L.; Gonzalez, B.D.; Small, B.J.; Gilvary, D.; Breen, E.C.; Bower, J.E.; Fishman, M.; Zachariah, B.; Jacobsen, P.B. Systemic inflammation and symptomatology in patients with prostate cancer treated with androgen deprivation therapy: Preliminary findings. Cancer 2021, 127, 1476–1482. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Tao, M.L.; Hu, W.; Belin, T.R.; Sepah, S.; Cole, S.; Aziz, N. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin. Cancer Res. 2009, 15, 5534–5540. [Google Scholar] [CrossRef]
- Wang, X.S.; Williams, L.A.; Krishnan, S.; Liao, Z.; Liu, P.; Mao, L.; Shi, Q.; Mobley, G.M.; Woodruff, J.F.; Cleeland, C.S. Serum sTNF-R1, IL-6, and the development of fatigue in patients with gastrointestinal cancer undergoing chemoradiation therapy. Brain Behav. Immun. 2012, 26, 699–705. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Aziz, N.; Fahey, J.L. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom. Med. 2002, 64, 604–611. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Aziz, N.; Fahey, J.L.; Cole, S.W. T-cell homeostasis in breast cancer survivors with persistent fatigue. J. Natl. Cancer Inst. 2003, 95, 1165–1168. [Google Scholar] [CrossRef]
- Collado-Hidalgo, A.; Bower, J.E.; Ganz, P.A.; Cole, S.W.; Irwin, M.R. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin. Cancer Res. 2006, 12, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Shafqat, A.; Einhorn, L.H.; Hanna, N.; Sledge, G.W.; Hanna, A.; Juliar, B.E.; Monahan, P.; Bhatia, S. Screening studies for fatigue and laboratory correlates in cancer patients undergoing treatment. Ann. Oncol. 2005, 16, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Gelinas, C.; Fillion, L. Factors related to persistent fatigue following completion of breast cancer treatment. Oncol. Nurs. Forum 2004, 31, 269–278. [Google Scholar] [CrossRef]
- Dimeo, F.; Schmittel, A.; Fietz, T.; Schwartz, S.; Kohler, P.; Boning, D.; Thiel, E. Physical performance, depression, immune status and fatigue in patients with hematological malignancies after treatment. Ann. Oncol. 2004, 15, 1237–1242. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Irwin, M.R.; Kwan, L.; Breen, E.C.; Cole, S.W. Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? J. Clin. Oncol. 2011, 29, 3517–3522. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Beitler, J.J.; Higgins, K.A.; Conneely, K.; Dwivedi, B.; Felger, J.; Wommack, E.C.; Shin, D.M.; Saba, N.F.; Ong, L.Y.; et al. Fatigue is associated with inflammation in patients with head and neck cancer before and after intensity-modulated radiation therapy. Brain Behav. Immun. 2016, 52, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Manigault, A.W.; Ganz, P.A.; Irwin, M.R.; Cole, S.W.; Kuhlman, K.R.; Bower, J.E. Moderators of inflammation-related depression: A prospective study of breast cancer survivors. Transl. Psychiatry 2021, 11, 615. [Google Scholar] [CrossRef]
- Manigault, A.W.; Kuhlman, K.R.; Irwin, M.R.; Cole, S.W.; Ganz, P.A.; Crespi, C.M.; Bower, J.E. Vulnerability to inflammation-related depressive symptoms: Moderation by stress in women with breast cancer. Brain Behav. Immun. 2021, 94, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Sleight, A.G.; Crowder, S.L.; Skarbinski, J.; Coen, P.; Parker, N.H.; Hoogland, A.I.; Gonzalez, B.D.; Playdon, M.C.; Cole, S.; Ose, J.; et al. A New Approach to Understanding Cancer-Related Fatigue: Leveraging the 3P Model to Facilitate Risk Prediction and Clinical Care. Cancers 2022, 14, 1982. [Google Scholar] [CrossRef] [PubMed]
- Lutgendorf, S.K.; Weinrib, A.Z.; Penedo, F.; Russell, D.; DeGeest, K.; Costanzo, E.S.; Henderson, P.J.; Sephton, S.E.; Rohleder, N.; Lucci, J.A., 3rd; et al. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J. Clin. Oncol. 2008, 26, 4820–4827. [Google Scholar] [CrossRef] [PubMed]
- Witek-Janusek, L.; Gabram, S.; Mathews, H.L. Psychologic stress, reduced NK cell activity, and cytokine dysregulation in women experiencing diagnostic breast biopsy. Psychoneuroendocrinology 2007, 32, 22–35. [Google Scholar] [CrossRef]
- Palesh, O.G.; Collie, K.; Batiuchok, D.; Tilston, J.; Koopman, C.; Perlis, M.L.; Butler, L.D.; Carlson, R.; Spiegel, D. A longitudinal study of depression, pain, and stress as predictors of sleep disturbance among women with metastatic breast cancer. Biol. Psychol. 2007, 75, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.M.; Farr, L.A.; Kuhn, B.R.; Fischer, P.; Agrawal, S. Values of sleep/wake, activity/rest, circadian rhythms, and fatigue prior to adjuvant breast cancer chemotherapy. J. Pain Symptom Manag. 2007, 33, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.S.; Lee, L.F.; Ting, J. Paclitaxel up-regulates interleukin-8 synthesis in human lung carcinoma through an NF-kappaB- and AP-1-dependent mechanism. Cancer Immunol. Immunother. 2000, 49, 78–84. [Google Scholar] [CrossRef]
- White, C.M.; Martin, B.K.; Lee, L.F.; Haskill, J.S.; Ting, J. Effects of paclitaxel on cytokine synthesis by unprimed human monocytes, T lymphocytes, and breast cancer cells. Cancer Immunol. Immunother. 1998, 46, 104–112. [Google Scholar] [CrossRef]
- Bogdan, C.; Ding, A. Taxol, a microtubule-stabilizing antineoplastic agent, induces expression of tumor necrosis factor alpha and interleukin-1 in macrophages. J. Leukoc. Biol. 1992, 52, 119–121. [Google Scholar] [CrossRef]
- Mullins, D.W.; Burger, C.J.; Elgert, K.D. Paclitaxel enhances macrophage IL-12 production in tumor-bearing hosts through nitric oxide. J. Immunol. 1999, 162, 6811–6818. [Google Scholar] [CrossRef]
- Pusztai, L.; TMendoza, R.; Reuben, J.M.; Martinez, M.M.; Willey, J.S.; Lara, J.; Syed, A.; Fritsche, H.A.; Bruera, E.; Booser, D.; et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine 2004, 25, 94–102. [Google Scholar] [CrossRef]
- Wang, T.H.; Chan, Y.-H.; Chen, C.W.; Kung, W.H.; Lee, Y.S.; Wang, S.T.; Chang, T.C.; Wang, H.S. Paclitaxel (Taxol) upregulates expression of functional interleukin-6 in human ovarian cancer cells through multiple signaling pathways. Oncogene 2006, 25, 4857–4866. [Google Scholar] [CrossRef]
- Penson, R.T.; Kronish, K.; Duan, Z.; Feller, A.J.; Stark, P.; Cook, S.E.; Duska, L.R.; Fuller, A.F.; Goodman, A.K.; Nikrui, N.; et al. Cytokines IL-1beta, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNFalpha in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. Int. J. Gynecol. Cancer 2000, 10, 33–41. [Google Scholar] [CrossRef]
- Tsavaris, N.; Kosmas, C.; Vadiaka, M.; Kanelopoulos, P.; Boulamatsis, D. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br. J. Cancer 2002, 87, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Janelsins, M.C.; Mustian, K.M.; Palesh, O.G.; Mohile, S.G.; Peppone, L.J.; Sprod, L.K.; Heckler, C.E.; Roscoe, J.A.; Katz, A.W.; Williams, J.P.; et al. Differential expression of cytokines in breast cancer patients receiving different chemotherapies: Implications for cognitive impairment research. Support Care Cancer 2012, 20, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E.; Ganz, P.A.; Irwin, M.R.; Cole, S.W.; Carroll, J.; Kuhlman, K.R.; Petersen, L.; Garet, D.; Asher, A.; Hurvitz, S.A.; et al. Acute and Chronic Effects of Adjuvant Therapy on Inflammatory Markers in Breast Cancer Patients. JNCI Cancer Spectr. 2022, 6, pkac052. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.J.; Parker, B.; Dimsdale, J.E.; Sadler, G.R.; Ancoli-Israel, S. The relationship between fatigue and quality of life and inflammation during anthracycline-based chemotherapy in breast cancer. Biol. Psychol. 2005, 69, 85–96. [Google Scholar] [CrossRef]
- Wang, X.S.; Shi, Q.; Williams, L.A.; Mao, L.; Cleeland, C.S.; Komaki, R.R.; Mobley, G.M.; Liao, Z. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav. Immun. 2010, 24, 968–974. [Google Scholar] [CrossRef]
- Chou, H.L.; Chao, T.Y.; Chen, T.C.; Chu, C.M.; Hsieh, C.H.; Yao, C.T.; Janckila, A.J. The Relationship Between Inflammatory Biomarkers and Symptom Distress in Lung Cancer Patients Undergoing Chemotherapy. Cancer Nurs. 2017, 40, E1–E8. [Google Scholar] [CrossRef]
- Jacobsen, P.B.; Donovan, K.A.; Small, B.J.; Jim, H.S.; Munster, P.N.; Andrykowski, M.A. Fatigue after treatment for early stage breast cancer: A controlled comparison. Cancer 2007, 110, 1851–1859. [Google Scholar] [CrossRef]
- Lee, E.S.; Lee, M.K.; Kim, S.H.; Ro, J.S.; Kang, H.S.; Kim, S.W.; Lee, K.S.; Yun, Y.H. Health-related quality of life in survivors with breast cancer 1 year after diagnosis compared with the general population: A prospective cohort study. Ann. Surg. 2011, 253, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Arndt, V.; Merx, H.; Stegmaier, C.; Ziegler, H.; Brenner, H. Quality of life in patients with colorectal cancer 1 year after diagnosis compared with the general population: A population-based study. J. Clin. Oncol. 2004, 22, 4829–4836. [Google Scholar] [CrossRef]
- Andrykowski, M.A.; Donovan, K.A.; Laronga, C.; Jacobsen, P.B. Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer 2010, 116, 5740–5748. [Google Scholar] [CrossRef] [PubMed]
- Wefel, J.S.; Saleeba, A.K.; Buzdar, A.U.; Meyers, C.A. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer 2010, 116, 3348–3356. [Google Scholar] [CrossRef]
- Goedendorp, M.M.; Andrykowski, M.A.; Donovan, K.A.; Jim, H.; Phillips, K.; Small, B.J.; Laronga, C.; Jacobsen, J. Prolonged impact of chemotherapy on fatigue in breast cancer survivors: A longitudinal comparison with radiotherapy treated breast cancer survivors and non-cancer controls. Cancer 2011, 118, 3833–3841. [Google Scholar] [CrossRef]
- Phillips, K.; Jim, H.; Small, B.; Laronga, C.; Andrykowski, M.A.; Jacobsen, P. Cognitive functioning after cancer treatment: A three-year longitudinal comparison of breast cancer survivors treated with chemotherapy or radiation and non-cancer controls. Cancer 2011, 118, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Oswald, L.B.; Eisel, S.L.; Tometich, D.B.; Bryant, C.; Hoogland, A.I.; Small, B.J.; Apte, S.M.; Chon, H.S.; Shahzad, M.M.; Gonzalez, B.D.; et al. Cumulative burden of symptomatology in patients with gynecologic malignancies undergoing chemotherapy. Health Psychol. 2022, 41, 864–873. [Google Scholar] [CrossRef]
- Diggle, P.J.; Heagerty, P.; Liang, K.-Y.; Zeger, S.L. Analysis of Longitudinal Data; Oxford University Press: New York, NY, USA, 2022. [Google Scholar]
- Chaudhry, S.; Jin, L.; Meltzer, D. Use of a self-report-generated Charlson Comorbidity Index for predicting mortality. Med. Care 2005, 43, 607–615. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Greenberg, D.B.; Gray, J.L.; Mannix, C.M.; Eisenthal, S.; Carey, M. Treatment-related fatigue and serum interleukin-1 levels in patients during external beam irradiation for prostate cancer. J. Pain Symptom Manag. 1993, 8, 196–200. [Google Scholar] [CrossRef]
- Wratten, C.; Kilmurray, J.; Nash, S.; Seldon, M.; Hamilton, C.S.; O’Brien, P.C.; Denham, J.W. Fatigue during breast radiotherapy and its relationship to biological factors. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 160–167. [Google Scholar] [CrossRef]
- Bower, J.E. Cancer-related fatigue: Links with inflammation in cancer patients and survivors. Brain Behav. Immun. 2007, 21, 863–871. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.F.; Bower, J.E.; Cho, H.J.; Creswell, J.D.; Dimitrov, S.; Hamby, M.E.; Hoyt, M.A.; Martin, J.L.; Robles, T.F.; Sloan, E.K.; et al. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain Behav. Immun. 2009, 23, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Lutgendorf, S.K.; Logan, H.; Costanzo, E.; Lubaroff, D. Effects of acute stress, relaxation, and a neurogenic inflammatory stimulus on interleukin-6 in humans. Brain Behav. Immun. 2004, 18, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Hann, D.M.; Jacobsen, P.B.; Azzarello, L.M.; Martin, S.C.; Curran, S.L.; Fields, K.K.; Greenberg, H.; Lyman, G. Measurement of fatigue in cancer patients: Development and validation of the Fatigue Symptom Inventory. Qual. Life Res. 1998, 7, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Donovan, K.A.; Jacobsen, P.B.; Small, B.J.; Munster, P.N.; Andrykowski, M.A. Identifying clinically meaningful fatigue with the Fatigue Symptom Inventory. J. Pain Symptom Manag. 2008, 36, 480–487. [Google Scholar] [CrossRef]
- Moorey, S.; Greer, S.; Watson, M.; Gorman, C.; Rowden, L.; Tunmore, R.; Robertson, B.; Bliss, J. The factor structure and factor stability of the hospital anxiety and depression scale in patients with cancer. Br. J. Psychiatry 1991, 158, 255–259. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Hipkins, J.; Whitworth, M.; Tarrier, N.; Jayson, G. Social support, anxiety and depression after chemotherapy for ovarian cancer: A prospective study. Br. J. Health Psychol. 2004, 9, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef]
- Forde, C. Scoring the International Physical Activity Questionnaire (IPAQ); University of Dublin: Dublin, Ireland, 2018; Volume 3. [Google Scholar]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Knobf, M.T.; Kerstetter, J.; Jeon, S. Adherence to American Cancer Society Guidelines on Nutrition and Physical Activity in Female Cancer Survivors: Results From a Randomized Controlled Trial (Yale Fitness Intervention Trial). Cancer Nurs. 2019, 42, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L. Longitudinal Analysis: Modeling Within-Person Fluctuation and Change; Routledge: East Sussex, UK, 2015. [Google Scholar]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Bottazzi, B.; Riboli, E.; Mantovani, A. Aging, inflammation and cancer. Semin. Immunol. 2018, 40, 74–82. [Google Scholar] [CrossRef]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Renna, M.E.; Shrout, M.R.; Madison, A.A.; Alfano, C.M.; Povoski, S.P.; Lipari, A.M.; Carson, W.E., 3rd; Malarkey, W.B.; Kiecolt-Glaser, J.K. Depression and anxiety in colorectal cancer patients: Ties to pain, fatigue, and inflammation. Psychooncology 2022, 31, 1536–1544. [Google Scholar] [CrossRef]
- Kuhlman, K.R.; Irwin, M.R.; Ganz, P.A.; Cole, S.W.; Manigault, A.W.; Crespi, C.M.; Bower, J.E. Younger women are more susceptible to inflammation: A longitudinal examination of the role of aging in inflammation and depressive symptoms. J. Affect. Disord. 2022, 310, 328–336. [Google Scholar] [CrossRef]
- McFarland, D.C.; Doherty, M.; Atkinson, T.M.; O’Hanlon, R.; Breitbart, W.; Nelson, C.J.; Miller, A.H. Cancer-related inflammation and depressive symptoms: Systematic review and meta-analysis. Cancer 2022, 128, 2504–2519. [Google Scholar] [CrossRef]
- Sabiston, C.M.; Wrosch, C.; Castonguay, A.L.; Sylvester, B.D. Changes in physical activity behavior and C-reactive protein in breast cancer patients. Ann. Behav. Med. 2018, 52, 545–551. [Google Scholar] [CrossRef]
- Romero-Elías, M.; Álvarez-Bustos, A.; Cantos, B.; Maximiano, C.; Méndez, M.; Méndez, M.; de Pedro, C.G.; Rosado-García, S.; Sanchez-Lopez, A.J.; García-González, D.; et al. C-Reactive Protein Is Associated with Physical Fitness in Breast Cancer Survivors. J. Clin. Med. 2022, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Crowder, S.L.; Hoogland, A.I.; Welniak, T.L.; LaFranchise, E.A.; Carpenter, K.M.; Li, D.; Rotroff, D.M.; Mariam, A.; Pierce, C.M.; Fischer, S.M.; et al. Metagenomics and chemotherapy-induced nausea: A roadmap for future research. Cancer 2022, 128, 461–470. [Google Scholar] [CrossRef] [PubMed]
| Variable | Patients (n = 121) | Controls (n = 105) | p-Values |
|---|---|---|---|
| Age: M (SD) | 60.4 (10.7) | 58.0 (12.8) | 0.12 |
| Race: n (%) White | 112 (93) | 93 (89) | 0.11 |
| Education: n (%) college graduate | 45 (37) | 76 (72) | <0.0001 |
| Income: n (%) USD 40 k or more | 66 (69) | 74 (80) | 0.08 |
| Comorbidities: mean (SD) [range] | 2.4 (0.8) [2–7] | 2.2 (0.5) [2–5] | 0.04 |
| Menopausal status: n (%) | 0.01 | ||
| Pre-menopausal | 15 (13) | 27 (26) | |
| Post-menopausal | 104 (87) | 78 (74) | |
| Cancer type: n (%) | - | - | |
| Cervical | 4 (3) | ||
| Endometrial | 27 (22) | ||
| Fallopian | 5 (4) | ||
| Ovarian | 64 (53) | ||
| Peritoneal | 7 (6) | ||
| Uterine | 10 (8) | ||
| Vulvar | 1 (1) | ||
| Other | 2 (2) | ||
| Stage: n (%) | - | - | |
| 1 | 22 (19) | ||
| 2 | 13 (11) | ||
| 3 | 61 (54) | ||
| 4 | 18 (16) | ||
| Prior lines of chemotherapy: n (%) 3 or more | 14 (12) | - | - |
| Baseline | Pre-Chemotherapy Cycle 3 | Pre-Chemotherapy Cycle 6 | 6-Month Follow-Up | 12-Month Follow-Up | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients Mean (SD) | Controls Mean (SD) | Patients Mean (SD) | Controls Mean (SD) | Patients Mean (SD) | Controls Mean (SD) | Patients Mean (SD) | Controls Mean (SD) | Patients Mean (SD) | Controls Mean (SD) | |
| Biomarkers of Inflammation (pg/mL): | ||||||||||
| IL-10 | 24.4 (21.1) | 23.0 (16.7) | 22.3 (18.4) | 23.3 (14.8) | 22.7 (18.0) | 24.2 (14.8) | 23.7 (18.5) | 22.7 (15.9) | 25.7 (20.1) | 23.2 (15.6) |
| IL-1b | 3.4 (2.5) | 3.9 (2.9) | 3.4 (2.6) | 4.0 (2.7) | 3.5 (2.6) | 4.1 (2.8) | 3.4 (2.1) | 4.0 (2.8) | 3.4 (2.6) | 4.4 (3.2) |
| TNF-alpha | 16.3 (7.9) | 12.0 (6.6) | 15.4 (7.9) | 12.3 (5.7) | 15.8 (7.4) | 12.4 (7.3) | 16.0 (7.1) | 12.6 (6.8) | 16.6 (7.5) | 12.7 (6.3) |
| TNFR1 | 181.6 (107.7) | 126.0 (78.4) | 178.7 (106.5) | 126.1 (82.8) | 185.3 (105.9) | 133.7 (90.7) | 187.9 (105.1) | 132.6 (74.9) | 185.4 (100.1) | 133.5 (80.5) |
| TNFR2 | 1015.4 (580.2) | 733.2 (313.8) | 1078.3 (626.3) | 748.3 (363.6) | 1071.7 (560.6) | 757.5 (326.5) | 1077.0 (581.1) | 793.9 (348.0) | 1021.3 (474.4) | 758.5 (334.4) |
| CRP | 6.2 (4.6) | 2.9 (2.9) | 6.2 (4.7) | 3.0 (3.1) | 5.6 (4.9) | 3.0 (2.8) | 5.0 (4.6) | 2.9 (2.8) | 5.4 (5.1) | 2.5 (2.2) |
| IL-6 | 6.4 (4.8) | 4.4 (3.0) | 5.6 (3.5) | 4.5 (2.7) | 5.5 (3.4) | 4.5 (2.8) | 6.3 (5.2) | 4.6 (2.8) | 5.7 (4.3) | 4.4 (2.8) |
| IL-6 (log- transformed) | 1.5 (1.1) | 1.0 (1.4) | 1.4 (1.2) | 1.2 (1.2) | 1.4 (0.9) | 1.1 (1.4) | 1.4 (1.4) | 1.1 (1.4) | 1.5 (0.9) | 1.0 (1.4) |
| IL-1ra | 62.9 (94.2) | 55.1 (101.5) | 59.6 (84.6) | 59.7 (103.7) | 49.9 (80.1) | 50.3 (97.2) | 74.5 (132.1) | 59.3 (118.9) | 93.8 (139.1) | 54.4 (100.8) |
| IL-1ra (log- transformed) | 3.4 (1.4) | 2.5 (1.8) | 3.2 (1.4) | 2.6 (1.8) | 3.0 (1.4) | 2.3 (1.8) | 3.3 (1.5) | 2.5 (1.8) | 3.4 (1.9) | 2.4 (1.8) |
| Sickness Behaviors: | ||||||||||
| Fatigue 1 | 3.4 (1.9) | 2.5 (1.6) | 3.7 (1.7) | 2.6 (1.8) | 4.3 (2.2) | 2.4 (1.7) | 3.4 (2.0) | 2.4 (1.9) | 3.2 (2.0) | 2.6 (1.9) |
| Depression 2 | 4.7 (3.7) | 2.1 (2.2) | 5.0 (3.9) | 2.2 (2.7) | 5.6 (3.9) | 2.1 (2.6) | 3.9 (3.4) | 2.1 (2.5) | 3.9 (3.4) | 2.2 (2.4) |
| Overall Sleep Quality 3 | 7.2 (3.5) | 4.9 (2.8) | 7.4 (4.0) | 4.9 (2.7) | 6.8 (3.4) | 5.2 (3.0) | 6.9 (3.8) | 4.1 (2.9) | 6.5 (4.2) | 4.7 (3.1) |
| Physical Activity (Total METs) 4 | 1771 (2254) | 2744 (2440) | 1984 (2205) | 2700 (2825) | 2003 (3281) | 2408 (2783) | 2590 (3051) | 2455 (2808) | 2618 (3155) | 2336 (2639) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoogland, A.I.; Small, B.J.; Oswald, L.B.; Bryant, C.; Rodriguez, Y.; Gonzalez, B.D.; Li, X.; Janelsins, M.C.; Bulls, H.W.; James, B.W.; et al. Relationships among Inflammatory Biomarkers and Self-Reported Treatment-Related Symptoms in Patients Treated with Chemotherapy for Gynecologic Cancer: A Controlled Comparison. Cancers 2023, 15, 3407. https://doi.org/10.3390/cancers15133407
Hoogland AI, Small BJ, Oswald LB, Bryant C, Rodriguez Y, Gonzalez BD, Li X, Janelsins MC, Bulls HW, James BW, et al. Relationships among Inflammatory Biomarkers and Self-Reported Treatment-Related Symptoms in Patients Treated with Chemotherapy for Gynecologic Cancer: A Controlled Comparison. Cancers. 2023; 15(13):3407. https://doi.org/10.3390/cancers15133407
Chicago/Turabian StyleHoogland, Aasha I., Brent J. Small, Laura B. Oswald, Crystal Bryant, Yvelise Rodriguez, Brian D. Gonzalez, Xiaoyin Li, Michelle C. Janelsins, Hailey W. Bulls, Brian W. James, and et al. 2023. "Relationships among Inflammatory Biomarkers and Self-Reported Treatment-Related Symptoms in Patients Treated with Chemotherapy for Gynecologic Cancer: A Controlled Comparison" Cancers 15, no. 13: 3407. https://doi.org/10.3390/cancers15133407
APA StyleHoogland, A. I., Small, B. J., Oswald, L. B., Bryant, C., Rodriguez, Y., Gonzalez, B. D., Li, X., Janelsins, M. C., Bulls, H. W., James, B. W., Arboleda, B., Colon-Echevarria, C., Townsend, M. K., Tworoger, S. S., Rodriguez, P. C., Bower, J. E., Apte, S. M., Wenham, R. M., & Jim, H. S. L. (2023). Relationships among Inflammatory Biomarkers and Self-Reported Treatment-Related Symptoms in Patients Treated with Chemotherapy for Gynecologic Cancer: A Controlled Comparison. Cancers, 15(13), 3407. https://doi.org/10.3390/cancers15133407






