CDK4/6 Inhibitors in the First-Line Treatment of Postmenopausal Women with HR+/HER2− Advanced or Metastatic Breast Cancer: An Updated Network Meta-Analysis and Cost-Effectiveness Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Network Meta-Analysis (NMA)
2.1.1. Study Eligibility and Selection
2.1.2. Data Collection and Assessment of the Risk of Bias
2.1.3. Statistical Analysis
2.2. Cost-Effectiveness Analysis (CEA)
2.2.1. Overview
2.2.2. Base-Case Analyses Population and Interventions
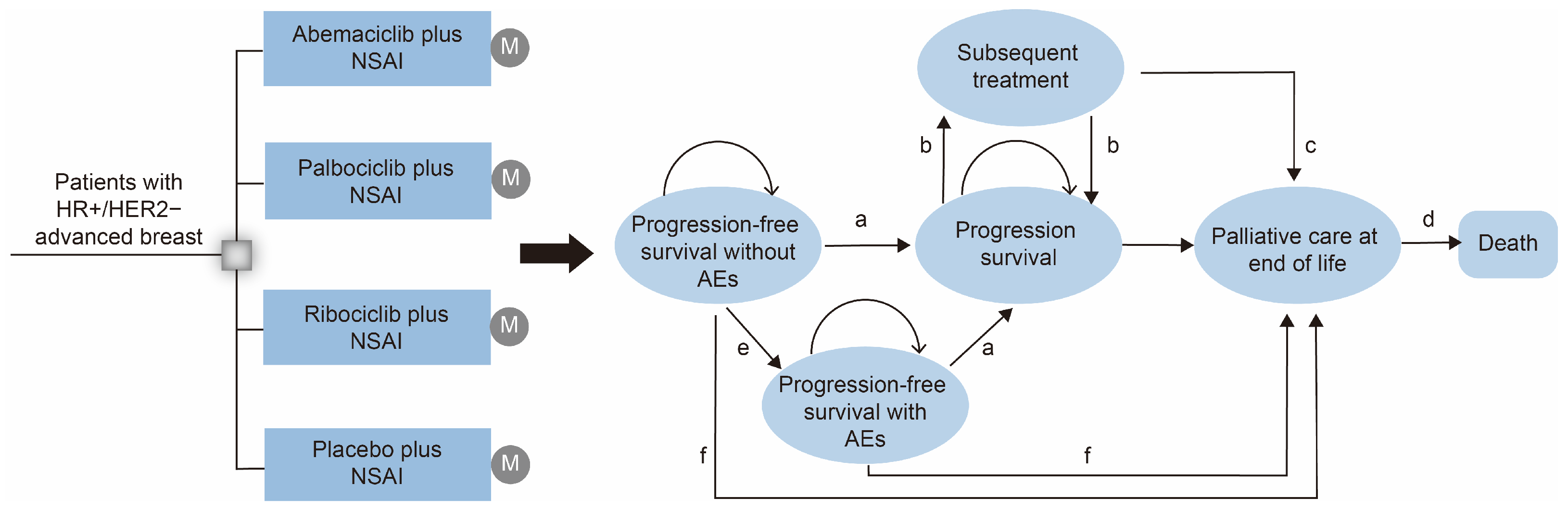
2.2.3. Model Structures
2.2.4. Transition Probabilities
2.2.5. Costs and Utilities Inputs
2.2.6. Sensitivity Analyses
3. Results
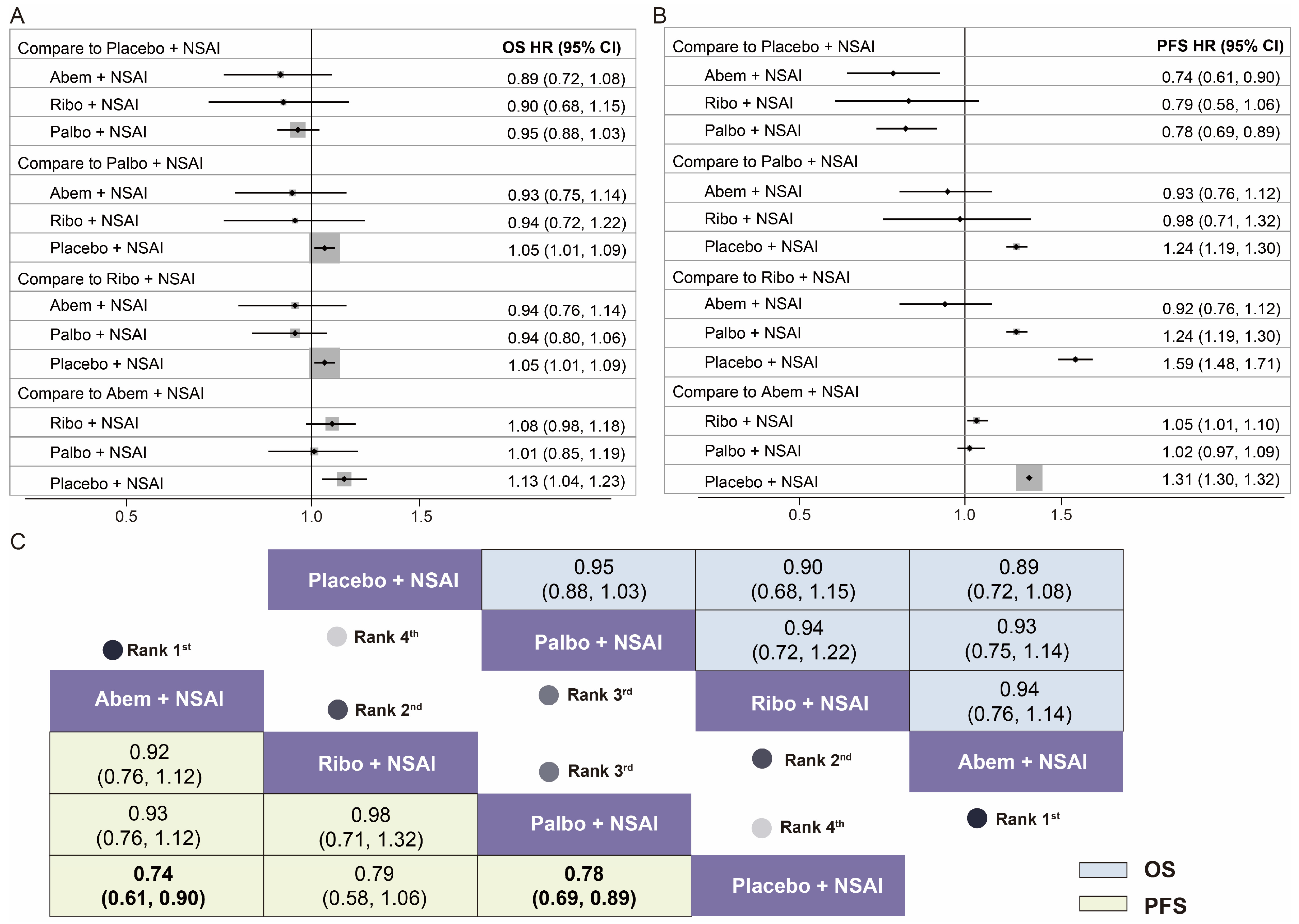
3.1. Network Meta-Analysis (NMA)
3.2. Cost-Effectiveness Analysis (CEA)
3.2.1. Baseline Results
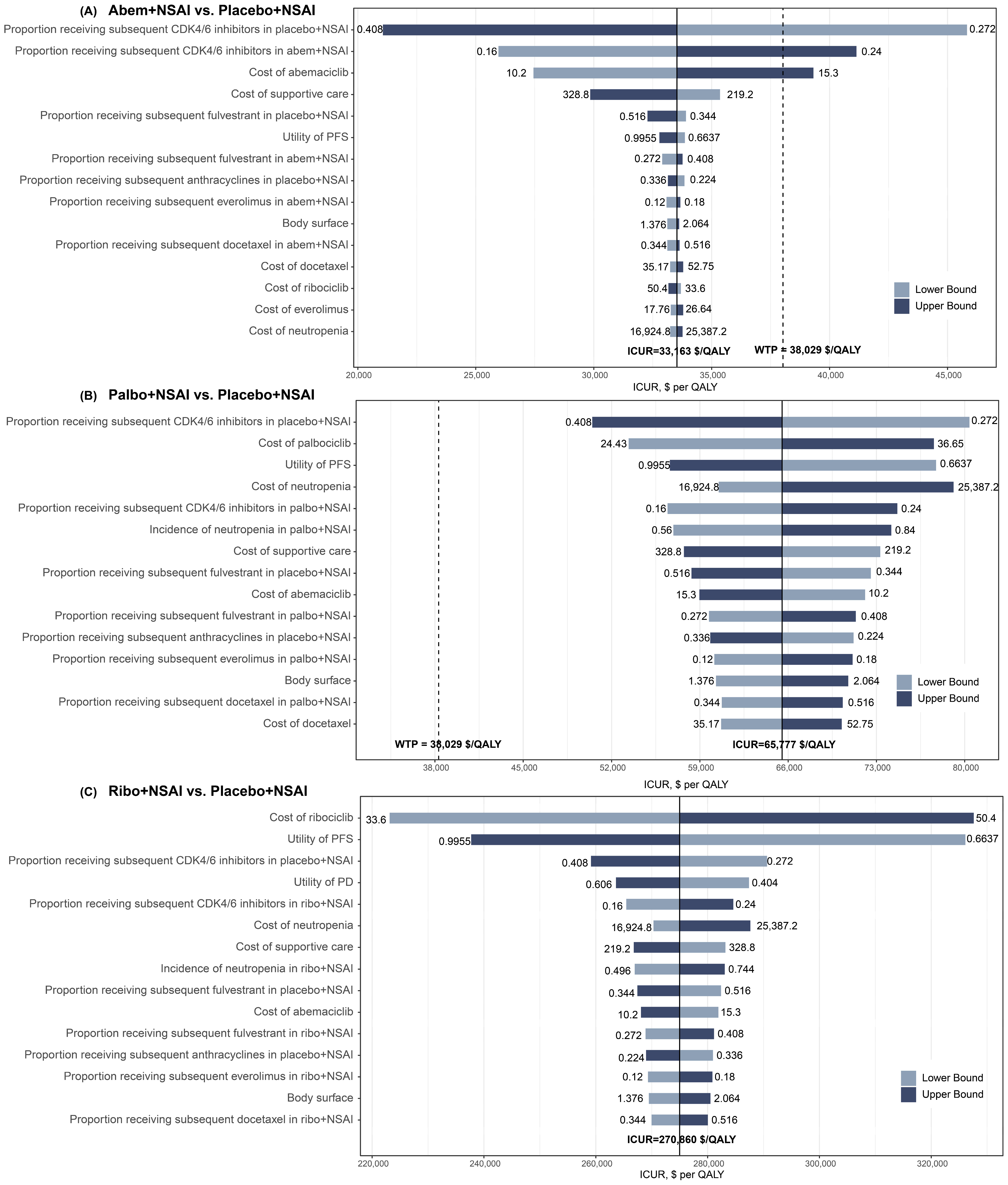
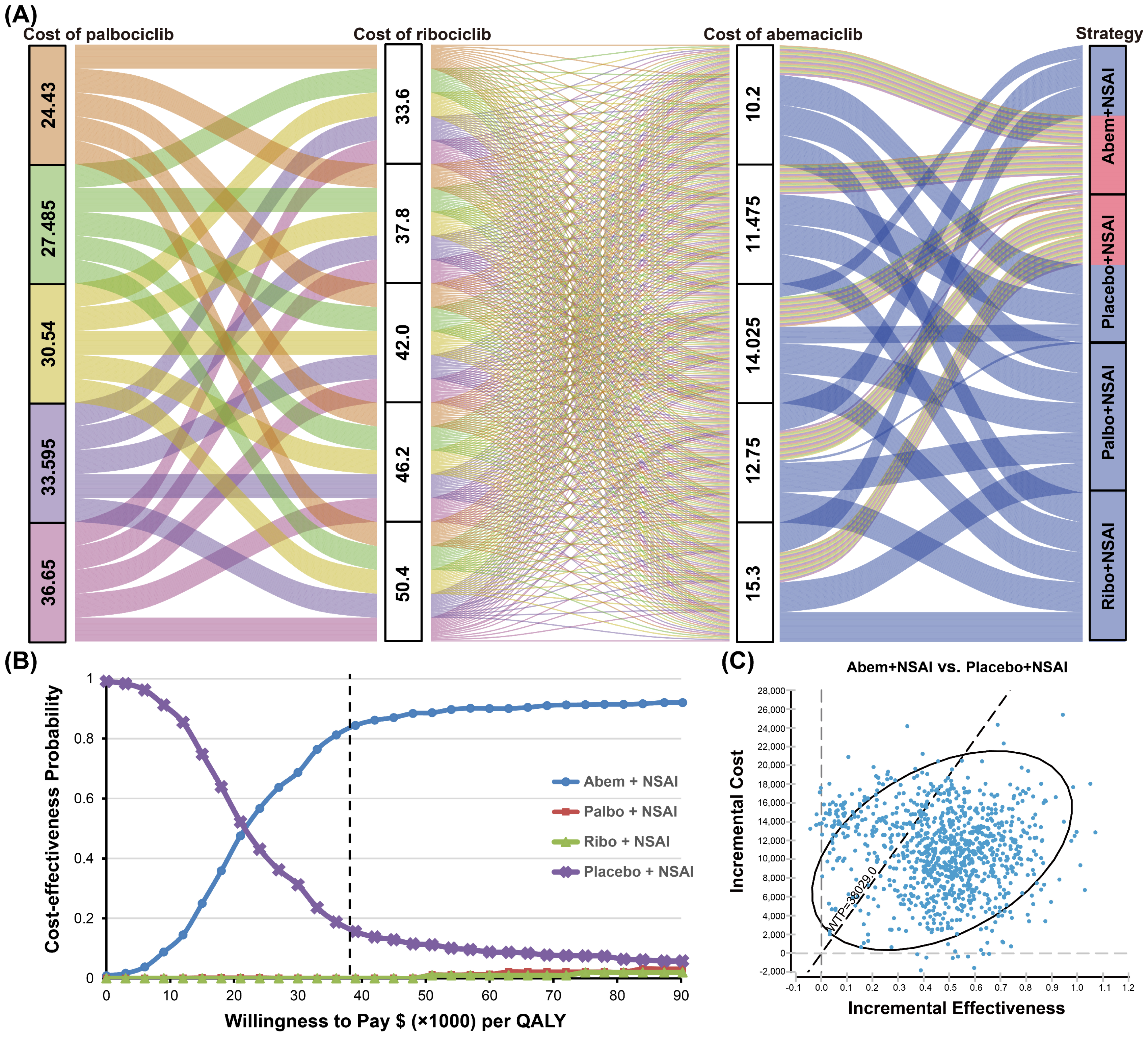
3.2.2. Sensitivity Analyses
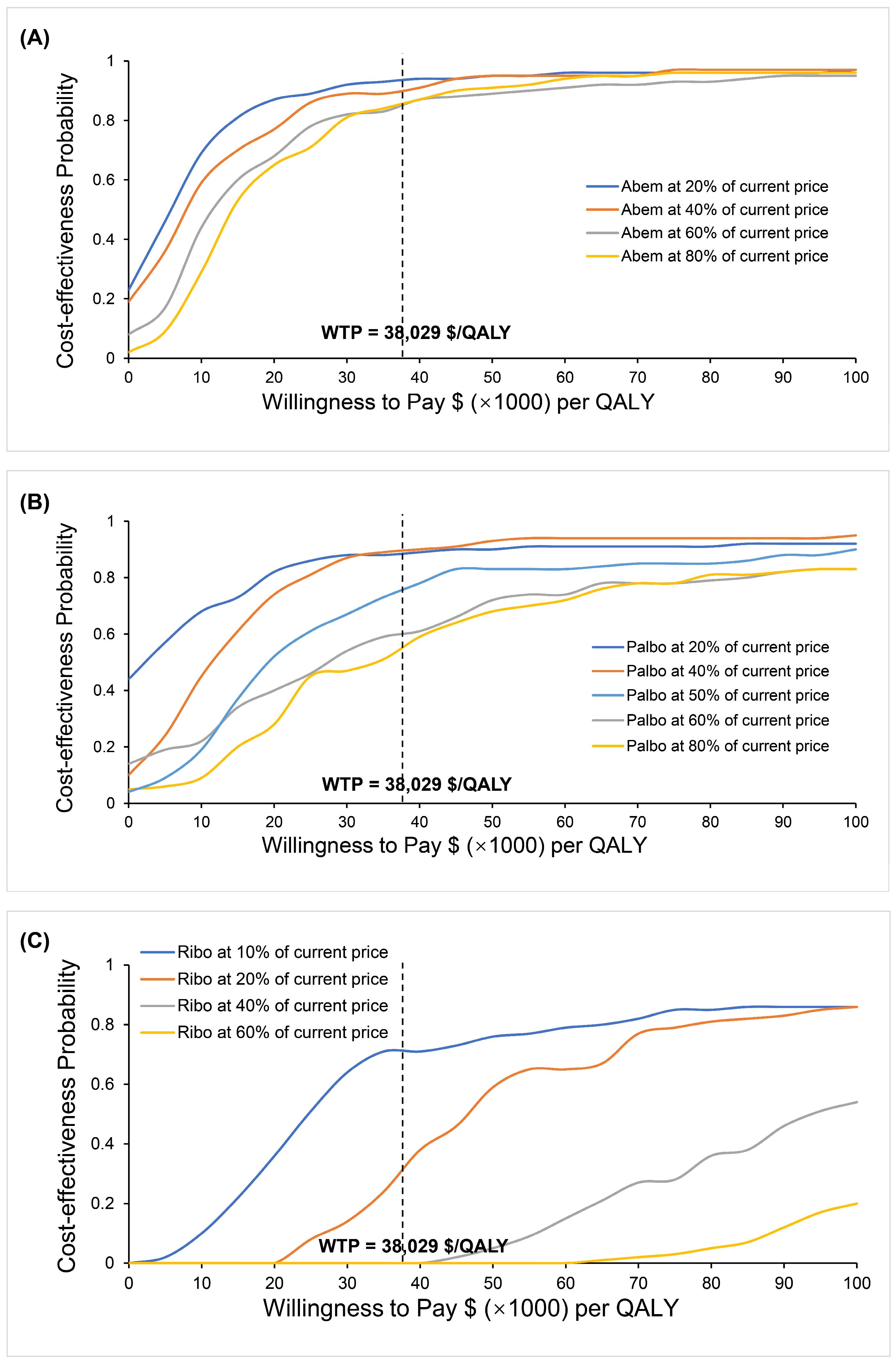
3.2.3. Variations in the Cost of CDK4/6 Inhibitors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.; Martínez-Pérez, C.; Meehan, J.; Gray, M.; Webber, V.; Dixon, J.M.; Turnbull, A.K. Current trends in the treatment of HR+/HER2+ breast cancer. Future Oncol. 2021, 17, 1665–1681. [Google Scholar] [CrossRef]
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.C.; Manso, L.; et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef]
- Xu, B.; Hu, X.; Li, W.; Sun, T.; Shen, K.; Wang, S.; Cheng, Y.; Zhang, Q.; Cui, S.; Tong, Z.; et al. Palbociclib plus letrozole versus placebo plus letrozole in Asian postmenopausal women with oestrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: Primary results from PALOMA-4. Eur. J. Cancer 2022, 175, 236–245. [Google Scholar] [CrossRef]
- Turner, N.C.; Ro, J.; André, F.; Loi, S.; Verma, S.; Iwata, H.; Harbeck, N.; Loibl, S.; Huang Bartlett, C.; Zhang, K.; et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2015, 373, 209–219. [Google Scholar] [CrossRef]
- Bell, T.; Crown, J.P.; Lang, I.; Bhattacharyya, H.; Zanotti, G.; Randolph, S.; Kim, S.; Huang, X.; Huang Bartlett, C.; Finn, R.S.; et al. Impact of palbociclib plus letrozole on pain severity and pain interference with daily activities in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer as first-line treatment. Curr. Med. Res. Opin. 2016, 32, 959–965. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Q.Y.; Zhang, P.; Tong, Z.; Sun, T.; Li, W.; Ouyang, Q.; Hu, X.; Cheng, Y.; Yan, M.; et al. LBA16—Dalpiciclib plus letrozole or anastrozole as first-line treatment for HR+/HER2− advanced breast cancer (DAWNA-2): A phase III trial. Ann. Oncol. 2022, 33 (Suppl. S7), S808–S869. [Google Scholar] [CrossRef]
- Rugo, H.S.; Finn, R.S.; Diéras, V.; Ettl, J.; Lipatov, O.; Joy, A.A.; Harbeck, N.; Castrellon, A.; Iyer, S.; Lu, D.R.; et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res. Treat. 2019, 174, 719–729. [Google Scholar] [CrossRef]
- Finn, R.S.; Rugo, H.S.; Dieras, V.C.; Harbeck, N.; Im, S.A.; Gelmon, K.A.; Walshe, J.M.; Martin, M.; Chavez Mac Gregor, M.; Bananis, E.; et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2−ABC): Analyses from PALOMA-2. J. Clin. Oncol. 2022, 40 (Suppl. S17), LBA1003. [Google Scholar]
- Rugo, H.S.; Brufsky, A.; Liu, X.; Li, B.; McRoy, L.; Chen, C.; Layman, R.M.; Cristofanilli, M.; Torres, M.A.; Curigliano, G.; et al. Real-world study of overall survival with palbociclib plus aromatase inhibitor in HR+/HER2- metastatic breast cancer. NPJ Breast Cancer 2022, 8, 114. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018, 29, 1541–1547. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2022, 386, 942–950. [Google Scholar] [CrossRef]
- Johnston, S.; Martin, M.; Di Leo, A.; Im, S.A.; Awada, A.; Forrester, T.; Frenzel, M.; Hardebeck, M.C.; Cox, J.; Barriga, S.; et al. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019, 5, 5. [Google Scholar] [CrossRef]
- Goetz, M.P.; Toi, M.; Huober, J.; Sohn, J.; Tredan, O.; Park, I.H.; Campone, M.; Chen, S.C.; Sanchez, L.M.; Paluch-Shimon, S.; et al. LBA15-MONARCH 3: Interim overall survival (OS) results of abemaciclib plus a nonsteroidal aromatase inhibitor (NSAI) in patients (pts) with HR+, HER2- advanced breast cancer (ABC). Ann. Oncol. 2022, 33 (Suppl. S7), S808–S869. [Google Scholar] [CrossRef]
- Husereau, D.; Drummond, M.; Augustovski, F.; de Bekker-Grob, E.; Briggs, A.H.; Carswell, C.; Caulley, L.; Chaiyakunapruk, N.; Greenberg, D.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 Explanation and Elaboration: A Report of the ISPOR CHEERS II Good Practices Task Force. Value Health J. Int. Soc. Pharm. Outcomes Res. 2022, 25, 10–31. [Google Scholar] [CrossRef]
- Eichler, H.G.; Kong, S.X.; Gerth, W.C.; Mavros, P.; Jönsson, B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: How are cost-effectiveness thresholds expected to emerge? Value Health J. Int. Soc. Pharm. Outcomes Res. 2004, 7, 518–528. [Google Scholar] [CrossRef]
- Finn, R.S.; Crown, J.P.; Ettl, J.; Schmidt, M.; Bondarenko, I.M.; Lang, I.; Pinter, T.; Boer, K.; Patel, R.; Randolph, S.; et al. Efficacy and safety of palbociclib in combination with letrozole as first-line treatment of ER-positive, HER2-negative, advanced breast cancer: Expanded analyses of subgroups from the randomized pivotal trial PALOMA-1/TRIO-18. Breast Cancer Res. 2016, 18, 67. [Google Scholar] [CrossRef]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Long, E.F. Cost-effectiveness analysis of palbociclib or ribociclib in the treatment of advanced hormone receptor-positive, HER2-negative breast cancer. Breast Cancer Res. Treat. 2019, 175, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Han, J.; Wang, Z.; Chen, J. Immune checkpoint inhibition in first-line treatment for recurrent or metastatic nasopharyngeal carcinoma: A CAPTAIN-1st and JUPITER-02 trial-based cost-effectiveness analysis. Oral Oncol. 2022, 128, 105842. [Google Scholar] [CrossRef] [PubMed]
- Mistry, R.; May, J.R.; Suri, G.; Young, K.; Brixner, D.; Oderda, G.; Biskupiak, J.; Tang, D.; Bhattacharyya, S.; Mishra, D.; et al. Cost-Effectiveness of Ribociclib plus Letrozole Versus Palbociclib plus Letrozole and Letrozole Monotherapy in the First-Line Treatment of Postmenopausal Women with HR+/HER2- Advanced or Metastatic Breast Cancer: A U.S. Payer Perspective. J. Manag. Care Spec. Pharm. 2018, 24, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Galve-Calvo, E.; González-Haba, E.; Gostkorzewicz, J.; Martínez, I.; Pérez-Mitru, A. Cost-effectiveness analysis of ribociclib versus palbociclib in the first-line treatment of HR+/HER2- advanced or metastatic breast cancer in Spain. Clin. Outcomes Res. 2018, 10, 773–790. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, M.W.; Henley, W. Improved curve fits to summary survival data: Application to economic evaluation of health technologies. BMC Med. Res. Methodol. 2011, 11, 139. [Google Scholar] [CrossRef]
- Yang, G.; Wang, Y.; Zeng, Y.; Gao, G.F.; Liang, X.; Zhou, M.; Wan, X.; Yu, S.; Jiang, Y.; Naghavi, M.; et al. Rapid health transition in China, 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet 2013, 381, 1987–2015. [Google Scholar] [CrossRef]
- Bank of China. Foreign Exchange Rate. Available online: https://www.boc.cn/sourcedb/whpj/ (accessed on 18 January 2023).
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2022, 20, 691–722. [Google Scholar] [CrossRef]
- Finn, R.S.; Crown, J.P.; Lang, I.; Boer, K.; Bondarenko, I.M.; Kulyk, S.O.; Ettl, J.; Patel, R.; Pinter, T.; Schmidt, M.; et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015, 16, 25–35. [Google Scholar] [CrossRef]
- Suri, G.; Chandiwana, D.; Lee, A.; Mistry, R. Cost-effectiveness analysis of ribociclib plus letrozole versus palbociclib plus letrozole in the United Kingdom. J. Health Econ. Outcomes Res. 2019, 6, 20–31. [Google Scholar] [CrossRef]
- Mamiya, H.; Tahara, R.K.; Tolaney, S.M.; Choudhry, N.K.; Najafzadeh, M. Cost-effectiveness of palbociclib in hormone receptor-positive advanced breast cancer. Ann. Oncol. 2017, 28, 1825–1831. [Google Scholar] [CrossRef]
- Matter-Walstra, K.; Schwenkglenks, M.; Dedes, K.J. Cost-effectiveness of palbociclib plus letrozole versus letrozole alone as a first-line treatment in women with oestrogen receptor-positive, HER2-negative, advanced breast cancer. Revised results for the Swiss health care setting. Breast Cancer Res. Treat. 2017, 163, 635. [Google Scholar] [CrossRef]
- Raphael, J.; Helou, J.; Pritchard, K.I.; Naimark, D.M. Palbociclib in hormone receptor positive advanced breast cancer: A cost-utility analysis. Eur. J. Cancer 2017, 85, 146–154. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, Y.; Ma, J.; Tan, C.; Zeng, X.; Peng, L. Ribociclib in hormone-receptor-positive advanced breast cancer: Establishing a value-based cost in China. Breast 2019, 43, 1–6. [Google Scholar] [CrossRef]
- Huang, X.; Lin, S.; Rao, X.; Zeng, D.; Wang, H.; Weng, X.; Huang, P. First-line Treatment with Ribociclib plus Endocrine Therapy for Premenopausal Women with Hormone-receptor-positive Advanced Breast Cancer: A Cost-effectiveness Analysis. Clin. Breast Cancer 2021, 21, e479–e488. [Google Scholar] [CrossRef]
- Giuliani, J.; Bonetti, A. The introduction of a third CDK4/6 inhibitor does not change the cost-effectiveness profile in first and subsequent-lines after progression or relapse during previous endocrine therapy in patients with hormone receptor positive (HR+)/human epidermal receptor-2 negative (HER-2) advanced or metastatic breast cancer. J. Oncol. Pharm. Pract. Off. Publ. Int. Soc. Oncol. Pharm. Pract. 2020, 26, 1486–1491. [Google Scholar]
- Masurkar, P.P.; Damgacioglu, H.; Deshmukh, A.A.; Trivedi, M.V. Cost Effectiveness of CDK4/6 Inhibitors in the First-Line Treatment of HR+/HER2- Metastatic Breast Cancer in Postmenopausal Women in the USA. PharmacoEconomics 2023, 41, 709–718. [Google Scholar] [CrossRef]
| Variable | Baseline Value (Range) | Reference | Distribution |
|---|---|---|---|
| Placebo + NSAI OS survival model | λ = 0.001027, γ = 1.814 | [6,9,11,12,13,14,15,16,17] | - |
| Placebo + NSAI PFS survival model HR for OS | λ = 0.045913, γ = 1.008831 | [6,9,11,12,13,14,15,16,17] | - |
| Abem + NSAI vs. Placebo + NSAI | 0.89 (0.72–1.08) | [6,9,11,12,13,14,15,16,17] | - |
| Ribo + NSAI vs. Placebo + NSAI | 0.90 (0.68–1.15) | [6,9,11,12,13,14,15,16,17] | - |
| Palbo + NSAI vs. Placebo + NSAI HR for PFS | 0.95 (0.88–1.03) | [6,9,11,12,13,14,15,16,17] | - |
| Abem + NSAI vs. Placebo + NSAI | 0.74 (0.61–0.90) | [6,9,11,12,13,14,15,16,17] | - |
| Ribo + NSAI vs. Placebo + NSAI | 0.79 (0.58–1.06) | [6,9,11,12,13,14,15,16,17] | - |
| Palbo + NSAI vs. Placebo + NSAI | 0.78 (0.69–0.89) | [6,9,11,12,13,14,15,16,17] | - |
| Background mortality rate | Age specific | [27] | - |
| Palbo + NSAI, Ribo + NSAI, and Abem + NSAI Subsequent therapy proportion | |||
| Exemestane | 0.260 (0.208–0.312) | [6,9,11,12,13,14,15,16,17] | Beta (407, 1159) |
| NSAI | 0.180 (0.144–0.216) | [6,9,11,12,13,14,15,16,17] | Beta (282, 1284) |
| Fulvestrant | 0.340(0.272–0.408) | [6,9,11,12,13,14,15,16,17] | Beta (532, 1034) |
| Tamoxifen | 0.140 (0.112–0.168) | [6,9,11,12,13,14,15,16,17] | Beta (219, 1347) |
| Everolimus | 0.150 (0.120–0.180) | [6,9,11,12,13,14,15,16,17] | Beta (234, 1332) |
| Anthracyclines | 0.190 (0.152–0.228) | [6,9,11,12,13,14,15,16,17] | Beta (297, 1269) |
| Capecitabine | 0.340 (0.272–0.408) | [6,9,11,12,13,14,15,16,17] | Beta (532, 1034) |
| Gemcitabine | 0.050 (0.040–0.060) | [6,9,11,12,13,14,15,16,17] | Beta (78, 1488) |
| Docetaxel | 0.430 (0.344–0.516) | [6,9,11,12,13,14,15,16,17] | Beta (673, 893) |
| Vinorelbine | 0.150 (0.120–0.180) | [6,9,11,12,13,14,15,16,17] | Beta (234, 1332) |
| CDK4/6 inhibitors | 0.200 (0.160–0.240) | [6,9,11,12,13,14,15,16,17] | Beta (313, 1253) |
| Placebo + NSAI Subsequent therapy proportion | |||
| Exemestane | 0.350 (0.280–0.420) | [6,9,11,12,13,14,15,16,17] | Beta (375, 697) |
| NSAI | 0.250 (0.200–0.300) | [6,9,11,12,13,14,15,16,17] | Beta (268, 804) |
| Fulvestrant | 0.430 (0.344–0.516) | [6,9,11,12,13,14,15,16,17] | Beta (460, 612) |
| Tamoxifen | 0.220 (0.176–0.264) | [6,9,11,12,13,14,15,16,17] | Beta (236, 835) |
| Everolimus | 0.170 (0.136–0.204) | [6,9,11,12,13,14,15,16,17] | Beta (182, 890) |
| Anthracyclines | 0.280 (0.224–0.336) | [6,9,11,12,13,14,15,16,17] | Beta (300, 772) |
| Capecitabine | 0.420 (0.336–0.504) | [6,9,11,12,13,14,15,16,17] | Beta (450, 622) |
| Gemcitabine | 0.100 (0.880–0.120) | [6,9,11,12,13,14,15,16,17] | Beta (107, 965) |
| Docetaxel | 0.390 (0.312–0.468) | [6,9,11,12,13,14,15,16,17] | Beta (419, 653) |
| Vinorelbine | 0.080 (0.064–0.096) | [6,9,11,12,13,14,15,16,17] | Beta (86, 986) |
| CDK4/6 inhibitors | 0.340 (0.272–0.408) | [6,9,11,12,13,14,15,16,17] | Beta (364, 708) |
| Palbo + NSAI AEs incidence (Grade 3 or higher) | |||
| Anemia | 0.053 (0.042–0.064) | [6,11,12,13] | Beta (37, 659) |
| Thrombocytopenia | 0.032 (0.025–0.038) | [6,11,12,13] | Beta (22, 674) |
| Neutropenia | 0.700 (0.560–0.840) | [6,11,12,13] | Beta (487, 209) |
| Leukopenia | 0.267 (0.214–0.320) | [6,11,12,13] | Beta (186, 510) |
| Nausea | 0.004 (0.003–0.005) | [6,11,12,13] | Beta (3, 693) |
| Diarrhea | 0.016 (0.013–0.019) | [6,11,12,13] | Beta (11, 685) |
| Fatigue | 0.022 (0.017–0.026) | [6,11,12,13] | Beta (15, 681) |
| Hepatobiliary toxicity | 0.020 (0.016–0.024) | [6,11,12,13] | Beta (14, 682) |
| Infection | 0.016 (0.013–0.019) | [6,11,12,13] | Beta (11, 685) |
| Vomiting | 0.0030 (0.0024–0.0036) | [6,11,12,13] | Beta (2, 694) |
| Ribo + NSAI AEs incidence (Grade 3 or higher) | |||
| Anemia | 0.024 (0.019–0.029) | [9,14,15] | Beta (8, 326) |
| Neutropenia | 0.620 (0.496–0.744) | [9,14,15] | Beta (207, 127) |
| Leukopenia | 0.213 (0.170–0.256) | [9,14,15] | Beta (71, 263) |
| Nausea | 0.024 (0.019–0.029) | [9,14,15] | Beta (8, 326) |
| Diarrhea | 0.024 (0.019–0.029) | [9,14,15] | Beta (8, 326) |
| Fatigue | 0.030 (0.024–0.036) | [9,14,15] | Beta (10, 324) |
| Hepatobiliary toxicity | 0.150 (0.120–0.180) | [9,14,15] | Beta (50, 284) |
| Infection | 0.042 (0.034–0.050) | [9,14,15] | Beta (14, 320) |
| Vomiting | 0.036 (0.029–0.043) | [9,14,15] | Beta (12, 322) |
| Abem + NSAI AEs incidence (Grade 3 or higher) | |||
| Leukopenia | 0.100 (0.080–0.120) | [16,17] | Beta (52, 480) |
| Anemia | 0.080 (0.064–0.096) | [16,17] | Beta (42, 490) |
| Neutropenia | 0.231 (0.185–0.277) | [16,17] | Beta (123, 409) |
| Thrombocytopenia | 0.050 (0.040–0.060) | [16,17] | Beta (11, 521) |
| Nausea | 0.008 (0.006–0.010) | [16,17] | Beta (4, 528) |
| Diarrhea | 0.073 (0.059–0.088) | [16,17] | Beta (39, 493) |
| Fatigue | 0.013 (0.010–0.016) | [16,17] | Beta (7, 525) |
| Hepatobiliary toxicity | 0.077 (0.062–0.092) | [16,17] | Beta (41, 491) |
| Infection | 0.030 (0.024–0.036) | [16,17] | Beta (16, 517) |
| Vomiting | 0.017 (0.014–0.020) | [16,17] | Beta (9, 525) |
| Placebo + NSAI AEs incidence (Grade 3 or higher) | |||
| Anemia | 0.015 (0.012–0.018) | [6,9,11,12,13,14,15,16,17] | Beta (16, 1056) |
| Thrombocytopenia | 0.004 (0.003–0.005) | [6,9,11,12,13,14,15,16,17] | Beta (4, 1068) |
| Neutropenia | 0.015 (0.012–0.018) | [6,9,11,12,13,14,15,16,17] | Beta (16, 1056) |
| Leukopenia | 0.006 (0.005–0.007) | [6,9,11,12,13,14,15,16,17] | Beta (7, 1065) |
| Nausea | 0.008 (0.007–0.010) | [6,9,11,12,13,14,15,16,17] | Beta (8, 1064) |
| Diarrhea | 0.007 (0.006–0.009) | [6,9,11,12,13,14,15,16,17] | Beta (8, 1064) |
| Fatigue | 0.005 (0.004–0.006) | [6,9,11,12,13,14,15,16,17] | Beta (5, 1067) |
| Hepatobiliary toxicity | 0.018 (0.015–0.022) | [6,9,11,12,13,14,15,16,17] | Beta (19, 1053) |
| Infection | 0.018 (0.015–0.022) | [6,9,11,12,13,14,15,16,17] | Beta (19, 1053) |
| Vomiting | 0.008 (0.007–0.010) | [6,9,11,12,13,14,15,16,17] | Beta (8, 1064) |
| Variable | Baseline Value (Range) | Reference | Distribution |
|---|---|---|---|
| Drug cost per dosage unit, $ | |||
| Palbociclib (125 mg) | 30.54 (24.43–36.65) | Local database | Gamma (96.00, 3.14) |
| Ribociclib (200 mg) | 42.00 (33.60–50.40) | Gamma (96.04, 2.29) | |
| Abemaciclib (150 mg) | 12.75 (10.20–15.30) | Gamma (96.04, 7.53) | |
| Letrozole (2.5 mg) | 0.32 (0.26–0.38) | Gamma (96.04, 300.13) | |
| Anastrozole (1 mg) | 0.42 (0.34–0.50) | Gamma (96.04, 228.67) | |
| Supportive care | 274 (219.20–328.80) | [22,23,24,25] | Gamma (96.04, 0.35) |
| Imaging/Surveillance | 176.49 (141.19–211.79) | [22,23,24,25] | Gamma (96.03, 0.54) |
| Laboratory test | 82.59 (66.07–99.11) | [22,23,24,25] | Gamma (96.02, 1.16) |
| End of life care | 9032 (7225–10,838) | [22,23,24,25] | Gamma (96.04, 0.01) |
| AEs cost, $ | |||
| Anemia | 6434 (5147.2–7720.8) | [22,23,24,25] | Gamma (96.04, 0.015) |
| Thrombocytopenia | 3551 (2841.36–4262.04) | [22,23,24,25] | Gamma (96.04, 0.027) |
| Neutropenia | 21,156 (16,924.8–25,387.2) | [22,23,24,25] | Gamma (96.04, 0.005) |
| Leukopenia | 21,156 (16,924.8–25,387.2) | [22,23,24,25] | Gamma (96.04, 0.005) |
| Diarrhea | 7377 (5901.6–8852.4) | [22,23,24,25] | Gamma (96.04, 0.013) |
| Hepatobiliary toxicity | 7516 (6012.8–9019.2) | [22,23,24,25] | Gamma (96.04, 0.012) |
| Fatigue | 6908 (5526.4–8289.6) | [22,23,24,25] | Gamma (96.04, 0.014) |
| Infection | 10,128 (8102.4–12,153.6) | [22,23,24,25] | Gamma (96.04, 0.009) |
| Nausea | 6182 (4945.6–7418.4) | [22,23,24,25] | Gamma (96.04, 0.016) |
| Vomiting | 5246 (4196.8–6295.2) | [22,23,24,25] | Gamma (96.04, 0.018) |
| Pulmonary embolism | 10,036 (8028.8–12,043.2) | [22,23,24,25] | Gamma (96.04, 0.010) |
| Discount rate, % | 3 | [22,23,24,25] | Beta (0.03, 0.97) |
| Body Weight (kg) | 65 (32.5–97.5) | [22,23,24,25] | Gamma (15.37, 0.24) |
| Body surface area (m2) | 1.72 (1.376–2.064) | [22,23,24,25] | Gamma (96.04, 55.84) |
| Utility | |||
| Progression-free state (PR/CR) | 0.8345 (0.6676–1.00) | [24,25] | Beta (0.8345, 0.1655) |
| Progression-free state (SD) | 0.8296 (0.6637–0.9955) | [24,25] | Beta (0.8296, 0.1704) |
| Progression state (PD) | 0.5050 (0.404–0.606) | [24,25] | Beta (0.505, 0.495) |
| Strategy | Abem + NSAI | Palbo + NSAI | Ribo + NSAI | Placebo + NSAI |
|---|---|---|---|---|
| Cost, $ | ||||
| Progression-free survival | 40,164 | 48,202 | 109,132 | 8683 |
| Overall | 83,345 | 91,134 | 152,001 | 70,743 |
| QALYs | ||||
| Progression-free survival | 2.22 | 2.16 | 2.15 | 1.92 |
| Overall | 4.16 | 4.09 | 4.08 | 3.78 |
| LYs | 6.51 | 6.43 | 6.40 | 6.00 |
| ICUR, $/QALY a | 33,163 | 65,777 | 270,860 | — |
| ICER, $/LY a | 24,710 | 47,421 | 203,145 | — |
| INHB, QALY a | 0.05 | −0.23 | −1.84 | — |
| INMB, $ a | 1849 | −8602 | −69,849 | — |
| Groups | Vs. Abem + NSAI | vs. Palbo + NSAI | Vs. Ribo + NSAI | Vs. Placebo + NSAI | |
|---|---|---|---|---|---|
| Abem + NSAI | ICUR, $/QALY | — | Dominate | Dominate | 33,163 |
| INHB, QALY | — | 0.27 | 1.89 | 0.05 | |
| INMB, $ | — | 10,451 | 71,698 | 1849 | |
| Palbo + NSAI | ICUR, $/QALY | Dominated | — | Dominate | 65,777 |
| INHB, QALY | −0.27 | — | 1.61 | −0.23 | |
| INMB, $ | −10,451 | — | 61,247 | −8602 | |
| Ribo + NSAI | ICUR, $/QALY | Dominated | Dominated | — | 270,860 |
| INHB, QALY | −1.89 | −1.61 | — | −1.84 | |
| INMB, $ | −71,698 | −61,247 | — | −69,849 | |
| Placebo + NSAI | ICUR, $/QALY | Dominated | Dominated | Dominated | — |
| INHB, QALY | −0.05 | 0.23 | 1.84 | — | |
| INMB, $ | −1849 | 8602 | 69,849 | — | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, N.; Han, J.; Liu, Z.; He, J.; Tian, K.; Chen, N. CDK4/6 Inhibitors in the First-Line Treatment of Postmenopausal Women with HR+/HER2− Advanced or Metastatic Breast Cancer: An Updated Network Meta-Analysis and Cost-Effectiveness Analysis. Cancers 2023, 15, 3386. https://doi.org/10.3390/cancers15133386
Zeng N, Han J, Liu Z, He J, Tian K, Chen N. CDK4/6 Inhibitors in the First-Line Treatment of Postmenopausal Women with HR+/HER2− Advanced or Metastatic Breast Cancer: An Updated Network Meta-Analysis and Cost-Effectiveness Analysis. Cancers. 2023; 15(13):3386. https://doi.org/10.3390/cancers15133386
Chicago/Turabian StyleZeng, Ni, Jiaqi Han, Zijian Liu, Jinlan He, Kun Tian, and Nianyong Chen. 2023. "CDK4/6 Inhibitors in the First-Line Treatment of Postmenopausal Women with HR+/HER2− Advanced or Metastatic Breast Cancer: An Updated Network Meta-Analysis and Cost-Effectiveness Analysis" Cancers 15, no. 13: 3386. https://doi.org/10.3390/cancers15133386
APA StyleZeng, N., Han, J., Liu, Z., He, J., Tian, K., & Chen, N. (2023). CDK4/6 Inhibitors in the First-Line Treatment of Postmenopausal Women with HR+/HER2− Advanced or Metastatic Breast Cancer: An Updated Network Meta-Analysis and Cost-Effectiveness Analysis. Cancers, 15(13), 3386. https://doi.org/10.3390/cancers15133386





