Could Metformin and Resveratrol Support Glioblastoma Treatment? A Mechanistic View at the Cellular Level
Abstract
Simple Summary
Abstract
1. Metformin and Resveratrol in Glioblastoma
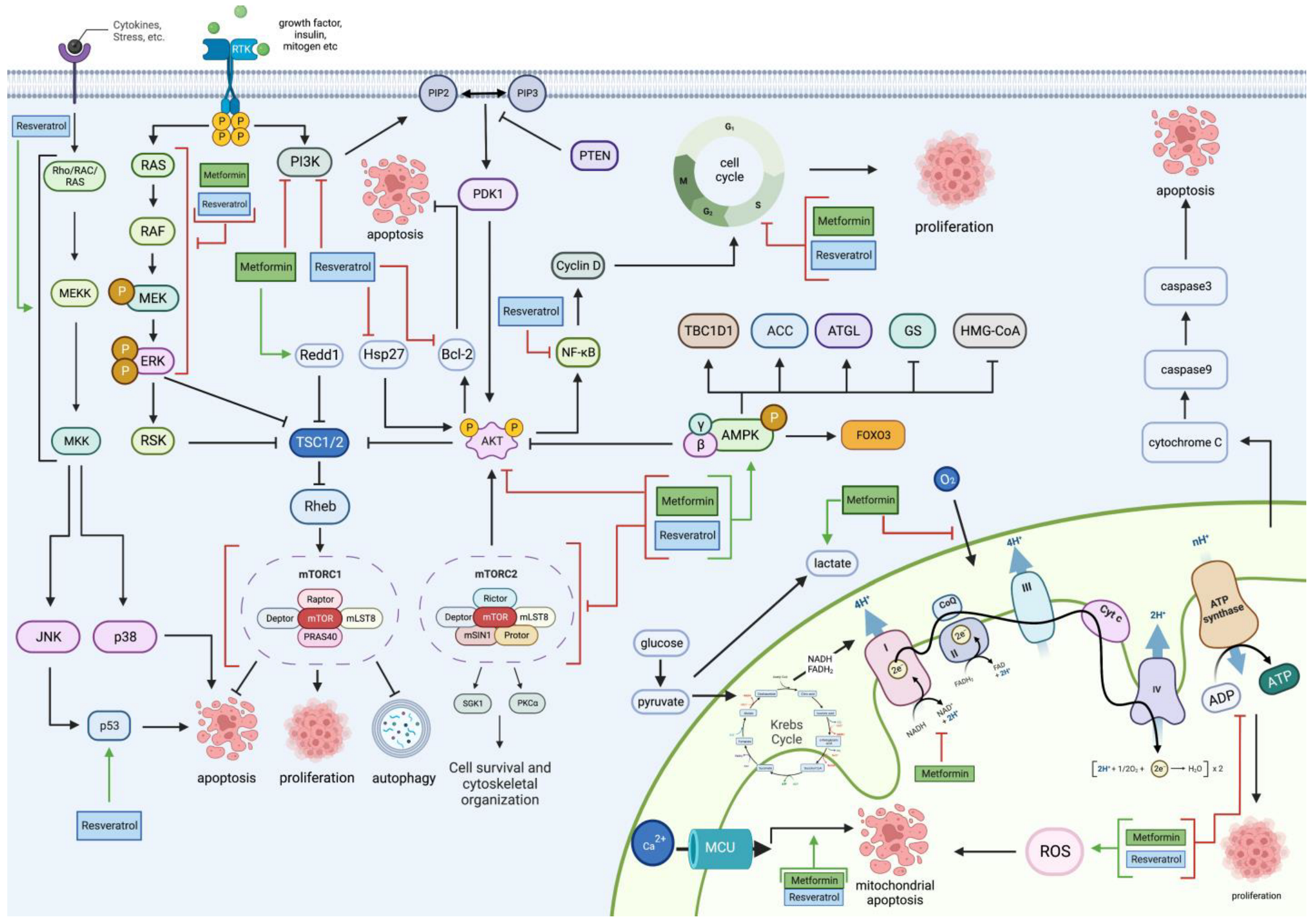
2. Metformin and Resveratrol on Glioblastoma’s Proliferative and Apoptotic Pathways
2.1. PI3K/Akt Pathway
2.2. mTOR Pathway
2.3. RAS/RAF/MAPK Pathway
2.4. AMPK Pathway
2.5. Mitochondrial Pathway
2.6. In Vivo
3. Metformin and Resveratrol on Glucose in Glioblastoma
4. Clinical Considerations and Relevance
4.1. Bioavailibility
4.2. Delivery
4.3. Clinical Trials
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | Acetyl-CoA carboxylase |
| AKT | Protein Kinase B |
| AMPK | Adenosine Monophosphate-Activated Protein Kinase |
| ATGL | Adipose triglyceride lipase |
| Bcl-2 | B Cell Lymphoma 2 Proteins |
| COX-2 | Cyclooxygenase-2 |
| EGFR | Epidermal growth factor receptor |
| FASN | Fatty acid synthase |
| FOXO3 | Forkhead box protein O3 |
| HSF1 | Heat shock factor 1 |
| Hsp27 | small heat shock protein |
| JNK1/2/3 | c-Jun N-terminal protein kinase |
| MAPK | Mitogen-Activated Protein Kinase |
| MET | Metformin |
| mTOR | Mammalian Target of Rapamycin |
| NF-κB | Nuclear factor kappa beta pathway |
| PI3K | Phosphatidylinositol 3-Kinase |
| RAF/RAS/MAPK/MEK/ERK | Rapidly accelerated fibrosarcoma/Rat sarcoma/Mitogen activated protein kinase/ Mitogen activated protein kinase kinase/Extracellular signal regulated kinases. |
| RES | Resveratrol |
| ROS | Reactive Oxygen Species |
| SIRT1 | NAD-dependent deacetylase sirtuin-1 |
| TMZ | Temozolomide |
| VEGF | Vascular endothelial growth factors |
References
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and Molecular Prognostic Review of Glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and Other Central Nervous System Tumor Statistics, 2021. CA Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Xu, J.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009–2013. Neuro Oncol. 2016, 18, v1–v75. [Google Scholar] [CrossRef]
- Samec, M.; Liskova, A.; Koklesova, L.; Samuel, S.M.; Zhai, K.; Buhrmann, C.; Varghese, E.; Abotaleb, M.; Qaradakhi, T.; Zulli, A.; et al. Flavonoids against the Warburg Phenotype—Concepts of Predictive, Preventive and Personalised Medicine to Cut the Gordian Knot of Cancer Cell Metabolism. EPMA J. 2020, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Koklesova, L.; Liskova, A.; Samec, M.; Qaradakhi, T.; Zulli, A.; Smejkal, K.; Kajo, K.; Jakubikova, J.; Behzadi, P.; Pec, M.; et al. Genoprotective Activities of Plant Natural Substances in Cancer and Chemopreventive Strategies in the Context of 3P Medicine. EPMA J. 2020, 11, 261–287. [Google Scholar] [CrossRef] [PubMed]
- Samec, M.; Liskova, A.; Kubatka, P.; Uramova, S.; Zubor, P.; Samuel, S.M.; Zulli, A.; Pec, M.; Bielik, T.; Biringer, K.; et al. The Role of Dietary Phytochemicals in the Carcinogenesis via the Modulation of MiRNA Expression. J. Cancer Res. Clin. Oncol. 2019, 145, 1665–1679. [Google Scholar] [CrossRef]
- Hardie, D.G. A New Understanding of Metformin. In Comprehensive Pharmacology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 280–300. [Google Scholar] [CrossRef]
- Takhwifa, F.; Aninditha, T.; Setiawan, H.; Sauriasari, R. The Potential of Metformin as an Antineoplastic in Brain Tumors: A Systematic Review. Heliyon 2021, 7, E06558. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an Anticancer Agent: A Review. Crit. Rev. Food Sci. Nutr. 2017, 58, 1428–1447. [Google Scholar] [CrossRef]
- Bao, Z.; Chen, K.; Krepel, S.; Tang, P.; Gong, W.; Zhang, M.; Liang, W.; Trivett, A.; Zhou, M.; Wang, J.M. High Glucose Promotes Human Glioblastoma Cell Growth by Increasing the Expression and Function of Chemoattractant and Growth Factor Receptors. Transl. Oncol. 2019, 12, 1155–1163. [Google Scholar] [CrossRef]
- Tseng, H.W.; Li, S.C.; Tsai, K.W. Metformin Treatment Suppresses Melanoma Cell Growth and Motility through Modulation of MicroRNA Expression. Cancers 2019, 11, 209. [Google Scholar] [CrossRef]
- Chen, K.; Qian, W.; Jiang, Z.; Cheng, L.; Li, J.; Sun, L.; Zhou, C.; Gao, L.; Lei, M.; Yan, B.; et al. Metformin Suppresses Cancer Initiation and Progression in Genetic Mouse Models of Pancreatic Cancer. Mol. Cancer 2017, 16, 131. [Google Scholar] [CrossRef]
- Wu, X.P.; Xiong, M.; Xu, C.S.; Duan, L.N.; Dong, Y.Q.; Luo, Y.; Niu, T.H.; Lu, C.R. Resveratrol Induces Apoptosis of Human Chronic Myelogenous Leukemia Cells in Vitro through P38 and JNK-Regulated H2AX Phosphorylation. Acta Pharmacol. Sin. 2015, 36, 353–361. [Google Scholar] [CrossRef]
- Kueck, A.; Opipari, A.W.; Griffith, K.A.; Tan, L.; Choi, M.; Huang, J.; Wahl, H.; Liu, J.R. Resveratrol Inhibits Glucose Metabolism in Human Ovarian Cancer Cells. Gynecol. Oncol. 2007, 107, 450–457. [Google Scholar] [CrossRef]
- Würth, R.; Pattarozzi, A.; Gatti, M.; Bajetto, A.; Corsaro, A.; Parodi, A.; Sirito, R.; Massollo, M.; Marini, C.; Zona, G.; et al. Metformin Selectively Affects Human Glioblastoma Tumor-Initiating Cell Viability. Cell Cycle 2012, 12, 145–156. [Google Scholar] [CrossRef]
- Hassan, M.A.; Fakhoury, I.; El Masri, Z.; Ghazale, N.; Dennaoui, R.; Atat, O.E.; Kanaan, A.; El-Sibai, M. Metformin Treatment Inhibits Motility and Invasion of Glioblastoma Cancer Cells. Anal. Cell. Pathol. 2018, 2018, 5917470. [Google Scholar] [CrossRef]
- Sesen, J.; Dahan, P.; Scotland, S.J.; Saland, E.; Dang, V.T.; Lemarié, A.; Tyler, B.M.; Brem, H.; Toulas, C.; Moyal, E.C.J.; et al. Metformin Inhibits Growth of Human Glioblastoma Cells and Enhances Therapeutic Response. PLoS ONE 2015, 10, e0123721. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Zhu, K.; Song, L.; Tao, M.; Huang, P.; Pan, Y. Resveratrol Inhibits Glioma Cell Growth via Targeting LRIG1. JBUON 2018, 23, 403–409. [Google Scholar]
- Jiao, Y.; Li, H.; Liu, Y.; Guo, A.; Xu, X.; Qu, X.; Wang, S.; Zhao, J.; Li, Y.; Cao, Y. Resveratrol Inhibits the Invasion of Glioblastoma-Initiating Cells via Down-Regulation of the PI3K/Akt/NF-ΚB Signaling Pathway. Nutrients 2015, 7, 4383–4402. [Google Scholar] [CrossRef]
- Clark, P.A.; Bhattacharya, S.; Elmayan, A.; Darjatmoko, S.R.; Thuro, B.A.; Yan, M.B.; van Ginkel, P.R.; Polans, A.S.; Kuo, J.S. Resveratrol Targeting of AKT and P53 in Glioblastoma and Glioblastoma Stem-like Cells to Suppress Growth and Infiltration. J. Neurosurg. 2017, 126, 1448–1460. [Google Scholar] [CrossRef]
- Önay Uçar, E.; Şengelen, A. Resveratrol and SiRNA in Combination Reduces Hsp27 Expression and Induces Caspase-3 Activity in Human Glioblastoma Cells. Cell Stress Chaperones 2019, 24, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Filippi-Chiela, E.C.; Villodre, E.S.; Zamin, L.L.; Lenz, G. Autophagy Interplay with Apoptosis and Cell Cycle Regulation in the Growth Inhibiting Effect of Resveratrol in Glioma Cells. PLoS ONE 2011, 6, e20849. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Dai, F.; Yu, K.; Jia, Z.; Zhang, A.; Huang, Q.; Kang, C.; Jiang, H.; Pu, P. Resveratrol Inhibits Glioma Cell Growth via Targeting Oncogenic MicroRNAs and Multiple Signaling Pathways. Int. J. Oncol. 2015, 46, 1739–1747. [Google Scholar] [CrossRef]
- Xia, C.; Liu, C.; He, Z.; Cai, Y.; Chen, J. Metformin Inhibits Cervical Cancer Cell Proliferation by Modulating PI3K/Akt-Induced Major Histocompatibility Complex Class I-Related Chain A Gene Expression. J. Exp. Clin. Cancer Res. 2020, 39, 127. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Lee, M.; Park, M.; Kim, S.Y.; Shim, M.S.; Lee, C.Y.; Choi, D.H.; Cho, Y. Metformin and Dichloroacetate Suppress Proliferation of Liver Cancer Cells by Inhibiting MTOR Complex 1. Int. J. Mol. Sci. 2021, 22, 10027. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Jia, K.; Dong, Y.; Ma, W. [Corrigendum] Metformin Inhibits the Proliferation of A431 Cells by Modulating the PI3K/Akt Signaling Pathway. Exp. Ther. Med. 2022, 24, 445. [Google Scholar] [CrossRef]
- Innets, B.; Thongsom, S.; Petsri, K.; Racha, S.; Yokoya, M.; Moriue, S.; Chaotham, C.; Chanvorachote, P. Akt/MTOR Targeting Activity of Resveratrol Derivatives in Non-Small Lung Cancer. Molecules 2022, 27, 8268. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Cao, N.; Li, Z.; Han, J.; Li, L. Resveratrol, an Activator of SIRT1, Induces Protective Autophagy in Non-Small-Cell Lung Cancer via Inhibiting Akt/MTOR and Activating P38-MAPK. Onco. Targets Ther. 2018, 11, 7777–7786. [Google Scholar] [CrossRef]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. MTOR Signaling Pathway and MTOR Inhibitors in Cancer: Progress and Challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Xiong, Z.S.; Gong, S.F.; Si, W.; Jiang, T.; Li, Q.L.; Wang, T.J.; Wang, W.J.; Wu, R.Y.; Jiang, K. Effect of Metformin on Cell Proliferation, Apoptosis, Migration and Invasion in A172 Glioma Cells and Its Mechanisms. Mol. Med. Rep. 2019, 20, 887–894. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, G.; Xie, G.; Zhao, L.; Chen, Y.; Yu, H.; Zhang, Z.; Li, C.; Li, Y.; Yu, Z.; et al. Metformin and Temozolomide Act Synergistically to Inhibit Growth of Glioma Cells and Glioma Stem Cells in Vitro and in Vivo. Oncotarget 2015, 6, 32930–32943. [Google Scholar] [CrossRef]
- Lee, J.E.; Lim, J.H.; Hong, Y.K.; Yang, S.H. High-Dose Metformin Plus Temozolomide Shows Increased Antitumor Effects in Glioblastoma In Vitro and In Vivo Compared with Monotherapy. Cancer Res. Treat. 2018, 50, 1331–1342. [Google Scholar] [CrossRef]
- Yuan, Y.; Xue, X.; Guo, R.B.; Sun, X.L.; Hu, G. Resveratrol Enhances the Antitumor Effects of Temozolomide in Glioblastoma via ROS-Dependent AMPK-TSC-MTOR Signaling Pathway. CNS Neurosci. Ther. 2012, 18, 536–546. [Google Scholar] [CrossRef]
- Jiang, H.; Shang, X.; Wu, H.; Gautam, S.C.; Al-Holou, S.; Li, C.; Kuo, J.; Zhang, L.; Chopp, M. Resveratrol Downregulates PI3K/Akt/MTOR Signaling Pathways in Human U251 Glioma Cells. J. Exp. Ther. Oncol. 2009, 8, 25. [Google Scholar]
- Guarnaccia, L.; Navone, S.E.; Masseroli, M.M.; Balsamo, M.; Caroli, M.; Valtorta, S.; Moresco, R.M.; Campanella, R.; Schisano, L.; Fiore, G.; et al. Effects of Metformin as Add-On Therapy against Glioblastoma: An Old Medicine for Novel Oncology Therapeutics. Cancers 2022, 14, 1412. [Google Scholar] [CrossRef]
- Carmignani, M.; Volpe, A.R.; Aldea, M.; Soritau, O.; Irimie, A.; Florian, I.S.; Tomuleasa, C.; Baritchii, A.; Petrushev, B.; Crisan, G.; et al. Glioblastoma Stem Cells: A New Target for Metformin and Arsenic Trioxide. J. Biol. Regul. Homeost. Agents 2014, 28, 1–15. [Google Scholar]
- Jung, J.S.; Woo, J.S. Resveratrol Induces Cell Death through ROS-Dependent MAPK Activation in A172 Human Glioma Cells. J. Life Sci. 2016, 26, 212–219. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK Signalling Pathway Coordinates Cell Growth, Autophagy and Metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Mazurek, M.; Litak, J.; Kamieniak, P.; Kulesza, B.; Jonak, K.; Baj, J.; Grochowski, C. Metformin as Potential Therapy for High-Grade Glioma. Cancers 2020, 12, 210. [Google Scholar] [CrossRef]
- Sato, A.; Sunayama, J.; Okada, M.; Watanabe, E.; Seino, S.; Shibuya, K.; Suzuki, K.; Narita, Y.; Shibui, S.; Kayama, T.; et al. Glioma-Initiating Cell Elimination by Metformin Activation of FOXO3 via AMPK. Stem Cells Transl. Med. 2012, 1, 811. [Google Scholar] [CrossRef]
- Xing, J.; Wang, Z.; Xu, H.; Liu, C.; Wei, Z.; Zhao, L.; Ren, L. Pak2 Inhibition Promotes Resveratrol-Mediated Glioblastoma A172 Cell Apoptosis via Modulating the AMPK-YAP Signaling Pathway. J. Cell. Physiol. 2020, 235, 6563–6573. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Carling, D.; Guichard, C.; Ferre, P.; Foufelle, F. AMP-Activated Protein Kinase Inhibits the Glucose-Activated Expression of Fatty Acid Synthase Gene in Rat Hepatocytes. J. Biol. Chem. 1998, 273, 14767–14771. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A Nutrient and Energy Sensor That Maintains Energy Homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ho, W.S.; Lu, R. Targeting Mitochondrial Oxidative Phosphorylation in Glioblastoma Therapy. Neuromol. Med. 2022, 24, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.W.; Chang, P.C.; Chen, H.Y.; Hueng, D.Y.; Li, Y.F.; Huang, S.M. Exploring the Mechanism of Adjuvant Treatment of Glioblastoma Using Temozolomide and Metformin. Int. J. Mol. Sci. 2022, 23, 8171. [Google Scholar] [CrossRef]
- Valtorta, S.; Dico, A.L.; Raccagni, I.; Gaglio, D.; Belloli, S.; Politi, L.S.; Martelli, C.; Diceglie, C.; Bonanomi, M.; Ercoli, G.; et al. Metformin and Temozolomide, a Synergic Option to Overcome Resistance in Glioblastoma Multiforme Models. Oncotarget 2017, 8, 113090–113104. [Google Scholar] [CrossRef]
- Isakovic, A.; Harhaji, L.; Stevanovic, D.; Markovic, Z.; Sumarac-Dumanovic, M.; Starcevic, V.; Micic, D.; Trajkovic, V. Dual Antiglioma Action of Metformin: Cell Cycle Arrest and Mitochondria-Dependent Apoptosis. Cell. Mol. Life Sci. 2007, 64, 1290–1302. [Google Scholar] [CrossRef]
- Öztürk, Y.; Günaydın, C.; Yalçın, F.; Nazıroğlu, M.; Braidy, N. Resveratrol Enhances Apoptotic and Oxidant Effects of Paclitaxel through TRPM2 Channel Activation in DBTRG Glioblastoma Cells. Oxid. Med. Cell. Longev. 2019, 2019, 4619865. [Google Scholar] [CrossRef]
- Li, J.; Qin, Z.; Liang, Z. The Prosurvival Role of Autophagy in Resveratrol-Induced Cytotoxicity in Human U251 Glioma Cells. BMC Cancer 2009, 9, 215. [Google Scholar] [CrossRef]
- Wang, L.; Long, L.; Wang, W.; Liang, Z. Resveratrol, a Potential Radiation Sensitizer for Glioma Stem Cells Both in Vitro and in Vivo. J. Pharmacol. Sci. 2015, 129, 216–225. [Google Scholar] [CrossRef]
- McKelvey, K.J.; Wilson, E.B.; Short, S.; Melcher, A.A.; Biggs, M.; Diakos, C.I.; Howell, V.M. Glycolysis and Fatty Acid Oxidation Inhibition Improves Survival in Glioblastoma. Front. Oncol. 2021, 11, 570. [Google Scholar] [CrossRef]
- Supabphol, S.; Seubwai, W.; Wongkham, S.; Saengboonmee, C. High Glucose: An Emerging Association between Diabetes Mellitus and Cancer Progression. J. Mol. Med. 2021, 99, 1175–1193. [Google Scholar] [CrossRef]
- Bielecka-Wajdman, A.M.; Ludyga, T.; Smyk, D.; Smyk, W.; Mularska, M.; Świderek, P.; Majewski, W.; Mullins, C.S.; Linnebacher, M.; Obuchowicz, E. Glucose Influences the Response of Glioblastoma Cells to Temozolomide and Dexamethasone. Cancer Control 2022, 29, 1–15. [Google Scholar] [CrossRef]
- Wu, X.; Luo, Q.; Liu, Z. Ubiquitination and Deubiquitination of MCL1 in Cancer: Deciphering Chemoresistance Mechanisms and Providing Potential Therapeutic Options. Cell Death Dis. 2020, 11, 556. [Google Scholar] [CrossRef]
- Hatanpaa, K.J.; Burma, S.; Zhao, D.; Habib, A.A. Epidermal Growth Factor Receptor in Glioma: Signal Transduction, Neuropathology, Imaging, and Radioresistance. Neoplasia 2010, 12, 675–684. [Google Scholar] [CrossRef]
- Piperi, C.; Papavassiliou, K.A.; Papavassiliou, A.G. Pivotal Role of STAT3 in Shaping Glioblastoma Immune Microenvironment. Cells 2019, 8, 1398. [Google Scholar] [CrossRef]
- Lu, V.M.; Goyal, A.; Vaughan, L.S.; McDonald, K.L. The Impact of Hyperglycemia on Survival in Glioblastoma: A Systematic Review and Meta-Analysis. Clin. Neurol. Neurosurg. 2018, 170, 165–169. [Google Scholar] [CrossRef]
- Mayer, A.; Vaupel, P.; Struss, H.G.; Giese, A.; Stockinger, M.; Schmidberger, H. Strong Adverse Prognostic Impact of Hyperglycemic Episodes during Adjuvant Chemoradiotherapy of Glioblastoma Multiforme. Strahlenther. Und Onkol. 2014, 190, 933–938. [Google Scholar] [CrossRef]
- Tieu, M.T.; Lovblom, L.E.; McNamara, M.G.; Mason, W.; Laperriere, N.; Millar, B.A.; Ménard, C.; Kiehl, T.R.; Perkins, B.A.; Chung, C. Impact of Glycemia on Survival of Glioblastoma Patients Treated with Radiation and Temozolomide. J. Neurooncol. 2015, 124, 119–126. [Google Scholar] [CrossRef]
- Adeberg, S.; Bernhardt, D.; Foerster, R.; Bostel, T.; Koerber, S.A.; Mohr, A.; Koelsche, C.; Rieken, S.; Debus, J. The Influence of Hyperglycemia during Radiotherapy on Survival in Patients with Primary Glioblastoma. Acta Oncol. 2015, 55, 201–207. [Google Scholar] [CrossRef]
- Chaichana, K.L.; McGirt, M.J.; Woodworth, G.F.; Datoo, G.; Tamargo, R.J.; Weingart, J.; Olivi, A.; Brem, H.; Quinones-Hinojosa, A. Persistent Outpatient Hyperglycemia Is Independently Associated with Survival, Recurrence and Malignant Degeneration Following Surgery for Hemispheric Low Grade Gliomas. Neurol. Res. 2010, 32, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Hagan, K.; Bhavsar, S.; Arunkumar, R.; Grasu, R.; Dang, A.; Carlson, R.; Cowles, C.; Arnold, B.; Potylchansky, Y.; Rahlfs, T.F.; et al. Association between Perioperative Hyperglycemia and Survival in Patients with Glioblastoma. J. Neurosurg. Anesthesiol. 2017, 29, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Derr, R.L.; Ye, X.; Islas, M.U.; Desideri, S.; Saudek, C.D.; Grossman, S.A. Association between Hyperglycemia and Survival in Patients with Newly Diagnosed Glioblastoma. J. Clin. Oncol. 2009, 27, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Krishnaswamy, G.; Karnad, A.; Peiris, A.N. Insulin: A Novel Factor in Carcinogenesis. Am. J. Med. Sci. 2002, 323, 140–145. [Google Scholar] [CrossRef]
- Kenechukwu, F.C.; Isaac, G.T.; Nnamani, D.O.; Momoh, M.A.; Attama, A.A. Enhanced Circulation Longevity and Pharmacodynamics of Metformin from Surface-Modified Nanostructured Lipid Carriers Based on Solidified Reverse Micellar Solutions. Heliyon 2022, 8, e09100. [Google Scholar] [CrossRef]
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.K.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.M.; et al. Clinical Pharmacokinetics of Metformin. Clin. Pharm. 2011, 50, 81–99. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-Activated Protein Kinase in Mechanism of Metformin Action. J. Clin. Investig. 2001, 108, 1167. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Lei, H.; Zhang, D. Recent Progress in Nanotechnology-Based Drug Carriers for Resveratrol Delivery. Drug Deliv. 2023, 30, 2174206. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High Absorption but Very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Zenaro, E.; Piacentino, G.; Constantin, G. The Blood-Brain Barrier in Alzheimer’s Disease. Neurobiol. Dis. 2017, 107, 41. [Google Scholar] [CrossRef]
- Łabuzek, K.; Suchy, D.; Gabryel, B.; Bielecka, A.; Liber, S.; Okopień, B. Quantification of Metformin by the HPLC Method in Brain Regions, Cerebrospinal Fluid and Plasma of Rats Treated with Lipopolysaccharide. Pharmacol. Rep. 2010, 62, 956–965. [Google Scholar] [CrossRef]
- Shimazu, R.; Anada, M.; Miyaguchi, A.; Nomi, Y.; Matsumoto, H. Evaluation of Blood-Brain Barrier Permeability of Polyphenols, Anthocyanins, and Their Metabolites. J. Agric. Food Chem. 2021, 69, 11676–11686. [Google Scholar] [CrossRef]
- Wilcock, C.; Bailey, C.J. Accumulation of Metformin by Tissues of the Normal and Diabetic Mouse. Xenobiotica 1994, 24, 49–57. [Google Scholar] [CrossRef]
- Kafoud, A.; Salahuddin, Z.; Ibrahim, R.S.; Al-Janahi, R.; Mazurakova, A.; Kubatka, P.; Büsselberg, D. Potential Treatment Options for Neuroblastoma with Polyphenols through Anti-Proliferative and Apoptotic Mechanisms. Biomolecules 2023, 13, 563. [Google Scholar] [CrossRef]
- Poonia, N.; Lather, V.; Narang, J.K.; Beg, S.; Pandita, D. Resveratrol-Loaded Folate Targeted Lipoprotein-Mimetic Nanoparticles with Improved Cytotoxicity, Antioxidant Activity and Pharmacokinetic Profile. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 111016. [Google Scholar] [CrossRef]
- Argenziano, M.; Ansari, I.A.; Muntoni, E.; Spagnolo, R.; Scomparin, A.; Cavalli, R. Lipid-Coated Nanocrystals as a Tool for Improving the Antioxidant Activity of Resveratrol. Antioxidants 2022, 11, 1007. [Google Scholar] [CrossRef]
- Ebrahimi, H.; Kazem Nezhad, S.; Farmoudeh, A.; Babaei, A.; Ebrahimnejad, P.; Akbari, E.; Siahposht-Khachaki, A. Design and Optimization of Metformin-Loaded Solid Lipid Nanoparticles for Neuroprotective Effects in a Rat Model of Diffuse Traumatic Brain Injury: A Biochemical, Behavioral, and Histological Study. Eur. J. Pharm. Biopharm. 2022, 181, 122–135. [Google Scholar] [CrossRef]
- Hong, L.; Li, X.; Bao, Y.; Duvall, C.L.; Zhang, C.; Chen, W.; Peng, C. Preparation, Preliminary Pharmacokinetic and Brain Targeting Study of Metformin Encapsulated W/O/W Composite Submicron Emulsions Promoted by Borneol. Eur. J. Pharm. Sci. 2019, 133, 160–166. [Google Scholar] [CrossRef]
- Felker, J.; Agnihotri, S. Hurdling over the Blood–Brain Barrier with Exosome Technology. Neuro Oncol. 2022, 24, 1884–1885. [Google Scholar] [CrossRef]
- Zhan, Q.; Yi, K.; Cui, X.; Li, X.; Yang, S.; Wang, Q.; Fang, C.; Tan, Y.; Li, L.; Xu, C.; et al. Blood Exosomes-Based Targeted Delivery of CPLA2 SiRNA and Metformin to Modulate Glioblastoma Energy Metabolism for Tailoring Personalized Therapy. Neuro Oncol. 2022, 24, 1871–1883. [Google Scholar] [CrossRef]
- Vijayakumar, M.R.; Vajanthri, K.Y.; Balavigneswaran, C.K.; Mahto, S.K.; Mishra, N.; Muthu, M.S.; Singh, S. Pharmacokinetics, Biodistribution, in Vitro Cytotoxicity and Biocompatibility of Vitamin E TPGS Coated Trans Resveratrol Liposomes. Colloids Surf. B Biointerfaces 2016, 145, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Jalili, C.; Kiani, A.; Gholami, M.; Bahrehmand, F.; Fakhri, S.; Kakehbaraei, S.; Kakebaraei, S. Brain Targeting Based Nanocarriers Loaded with Resveratrol in Alzheimer’s Disease: A Review. IET Nanobiotechnol. 2023, 17, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Y.; Li, Z.; Wu, X.; Mei, J.; Zheng, G. Brain Targeted Peptide-Functionalized Chitosan Nanoparticles for Resveratrol Delivery: Impact on Insulin Resistance and Gut Microbiota in Obesity-Related Alzheimer’s Disease. Carbohydr. Polym. 2023, 310, 120714. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Kitanaka, C.; Miyakita, Y.; Tanaka, S.; Sonoda, Y.; Mishima, K.; Ishikawa, E.; Takahashi, M.; Yanagisawa, S.; Ohashi, K.; et al. Metformin with Temozolomide for Newly Diagnosed Glioblastoma: Results of Phase I Study and a Brief Review of Relevant Studies. Cancers 2022, 14, 4222. [Google Scholar] [CrossRef]
- Maraka, S.; Groves, M.D.; Mammoser, A.G.; Melguizo-Gavilanes, I.; Conrad, C.A.; Tremont-Lukats, I.W.; Loghin, M.E.; O’Brien, B.J.; Puduvalli, V.K.; Sulman, E.P.; et al. Phase 1 Lead-in to a Phase 2 Factorial Study of Temozolomide Plus Memantine, Mefloquine, and Metformin as Postradiation Adjuvant Therapy for Newly Diagnosed Glioblastoma. Cancer 2019, 125, 424. [Google Scholar] [CrossRef]
- Porper, K.; Shpatz, Y.; Plotkin, L.; Pechthold, R.G.; Talianski, A.; Champ, C.E.; Furman, O.; Shimoni-Sebag, A.; Symon, Z.; Amit, U.; et al. A Phase I Clinical Trial of Dose-Escalated Metabolic Therapy Combined with Concomitant Radiation Therapy in High-Grade Glioma. J. Neurooncol. 2021, 153, 487–496. [Google Scholar] [CrossRef]
- Boocock, D.J.; Faust, G.E.S.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I Dose Escalation Pharmacokinetic Study in Healthy Volunteers of Resveratrol, a Potential Cancer Chemopreventive Agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef]
- Patel, K.R.; Brown, V.A.; Jones, D.J.L.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical Pharmacology of Resveratrol and Its Metabolites in Colorectal Cancer Patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I Randomized, Double-Blind Pilot Study of Micronized Resveratrol (SRT501) in Patients with Hepatic Metastases—Safety, Pharmacokinetics, and Pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef]
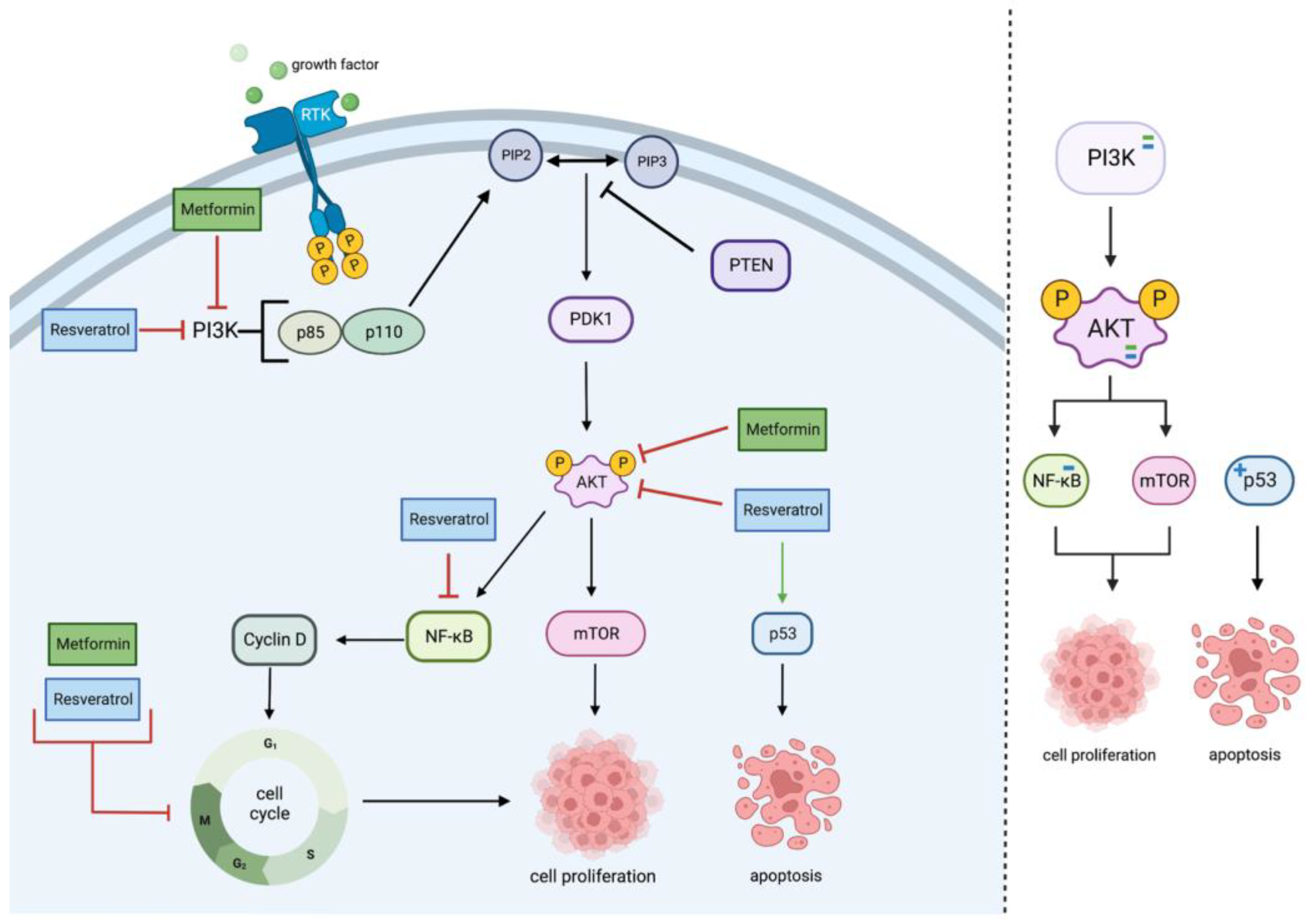
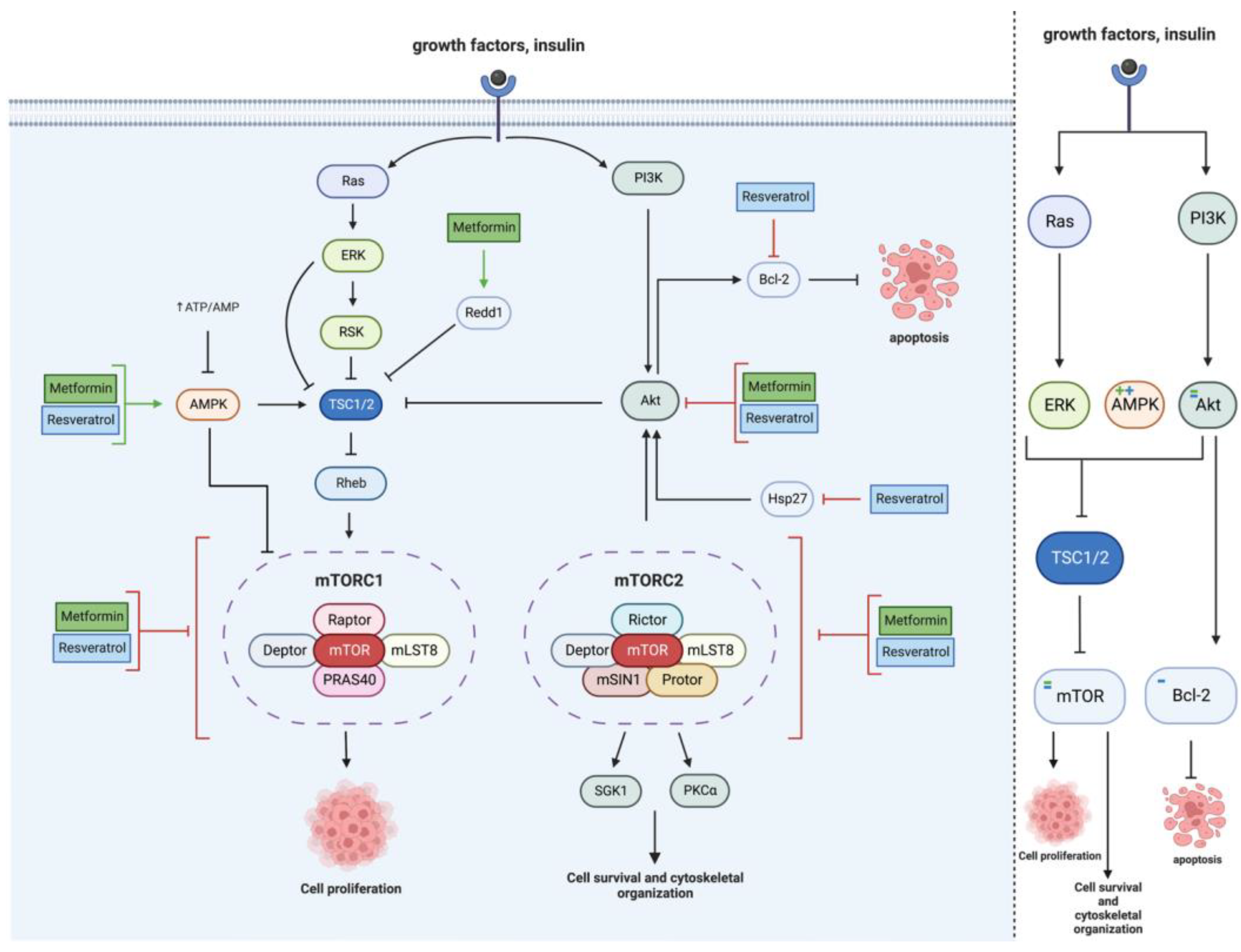
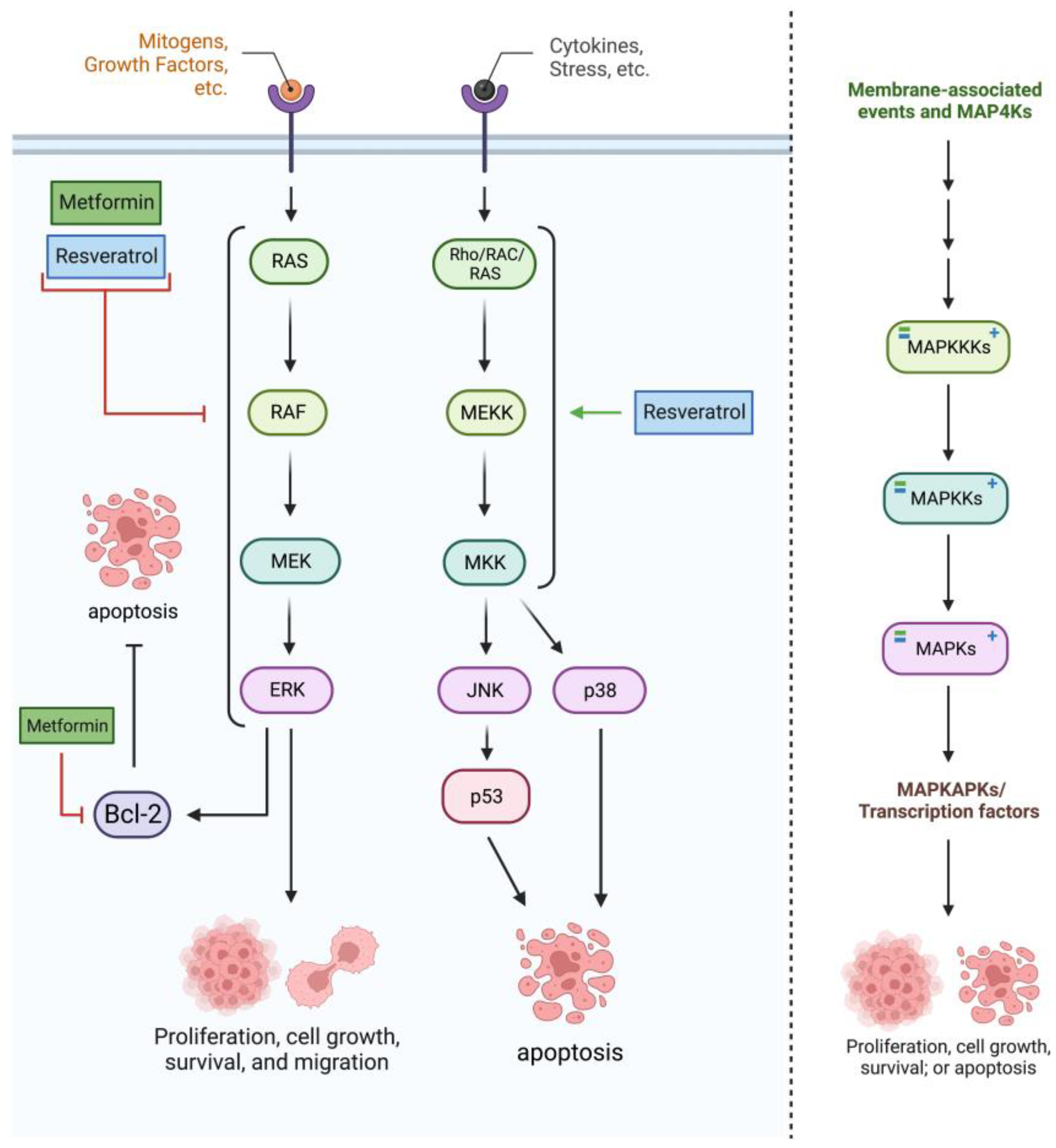
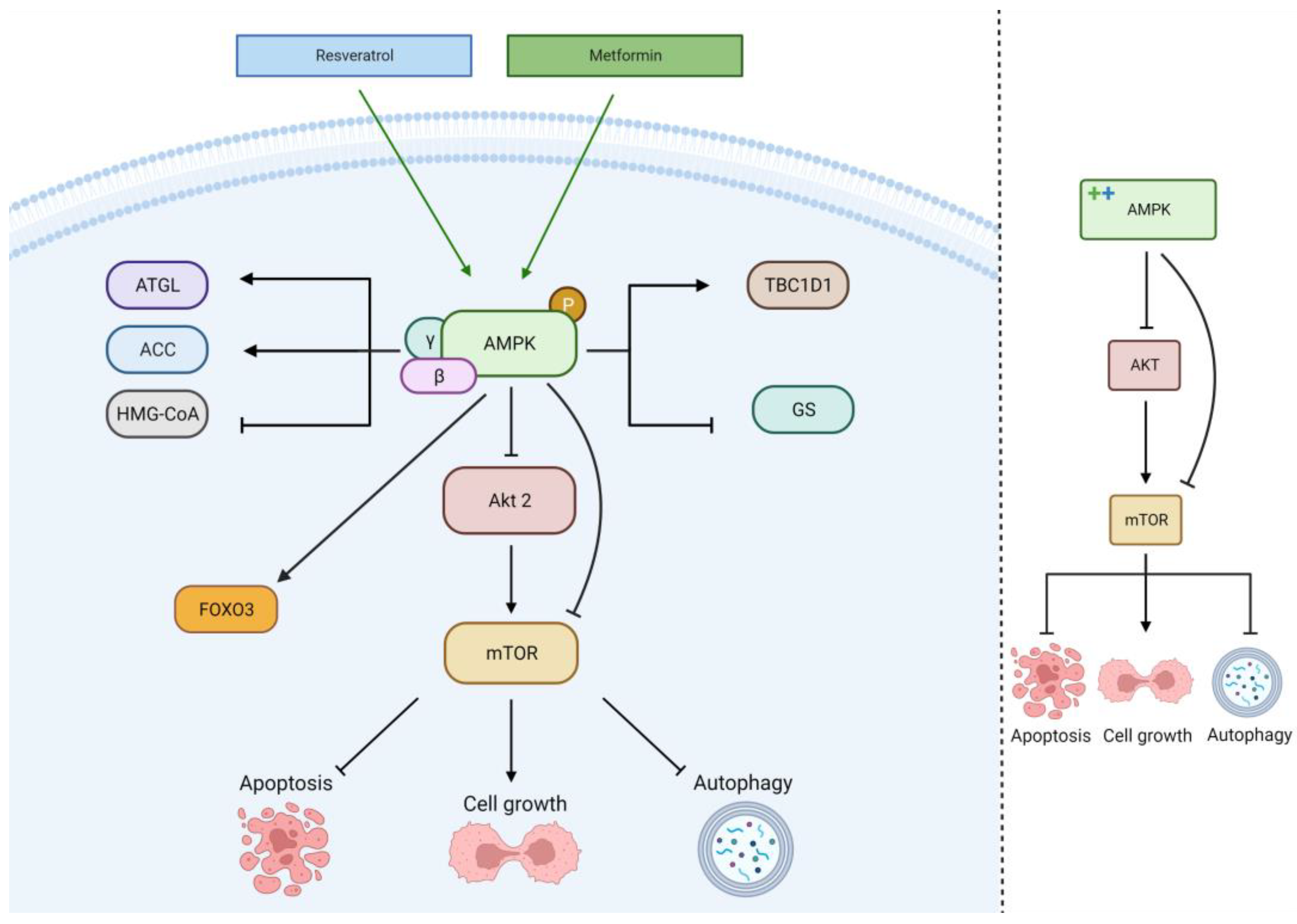

| Cell Line | Incubation Concentration | Results | References |
|---|---|---|---|
| Metformin | |||
| SF268 | 2.5 mM for 24 h | ↓ phosphorylation of Akt | [17] |
| ↓ cellular invasion | |||
| ↓ migration | |||
| GBM1–4 | GBM1: 9.2 mM GBM2: 4.9 mM GBM3: 9.0 mM GBM4: 9.4 mM for 48 h | ↓ phosphorylation of Akt | [16] |
| ↓ cell survival | |||
| ↓ proliferation | |||
| U87, LN18, U251, SF767 | 1, 5, 10 mM for 6 days | ↓ Akt phosphorylation | [18] |
| ↓ PI3K pathway | |||
| ↓ cell proliferation | |||
| ↓ G1 phase | |||
| ↑ cells in G0 phase | |||
| Resveratrol | |||
| U251 | 100 mM for 48 h | ↑ LRIG1 | [19] |
| ↓ EGFR | |||
| ↑ apoptosis | |||
| ↓ cell proliferation | |||
| GIC 400, 411, 412 | 20 μM for 48 h | ↓ Akt phosphorylation | [20] |
| ↓ NF-κB | |||
| ↓ cell invasion | |||
| GSC (44-GSC) U87 | 0, 5, 25, 50, 100 μM for 4–48 h | ↓ AKT protein activation | [21] |
| ↑ expression of p53 | |||
| ↓ cell proliferation | |||
| ↓ cell migration | |||
| ↑ apoptosis | |||
| U87 | N/A | ↓ PI3K/AKT | [22] |
| ↓ NF-κB | |||
| ↑ SIRT1- dependent apoptosis | |||
| ↓ cell proliferation | |||
| U87, U138, U251 | 30 µM or 100 µM for 48 h | ↓ PI3K class III | [23] |
| ↓ number of cells undergoing autophagy | |||
| ↓ number of mature autophagosomes formed per cell | |||
| ↑ S-G2/M cell cycle arrest |
| Cell Line | Incubation Concentration | Results | References |
|---|---|---|---|
| Metformin | |||
| A172 | 0, 0.1, 1, 10 mM for 24–72 h | ↑ apoptosis | [31] |
| ↑ AMPK and pAMPK | |||
| ↓ proliferation | |||
| ↓ mTOR/Bcl-2 | |||
| ↓ invasion | |||
| ↓ migration | |||
| U87 U251 A172 | 5, 10, 20 mM for 24–72 h | ↓ mTOR phosphorylation | [33] |
| ↑ AMPK phosphorylation | |||
| ↓ proliferation | |||
| ↑ apoptosis | |||
| U87 LN18 U251 SF767 | 10 mM for 48 h | ↓ mTOR phosphorylation | [18] |
| ↑ Redd1 | |||
| ↓ proliferation | |||
| ↑ apoptosis | |||
| ↑ autophagy | |||
| U87 U251 | 10 mM for 0–48 h 0–20 mM for 48 h | ↓ Akt/mTOR pathway | [32] |
| ↓ phosphorylated mTOR | |||
| ↓ proliferation | |||
| ↑ apoptosis | |||
| Resveratrol | |||
| SHG44 | 10 µM for 72 h | ↑ ROS production | [34] |
| ↑ AMPK | |||
| ↓ mTOR | |||
| ↓ Bcl-2 | |||
| ↑ apoptosis | |||
| ↓ proliferation | |||
| U251 | 100 μM for 24 h | ↓ phosphorylated Akt | [35] |
| ↓ phosphorylated mTOR | |||
| ↑ caspase-3 | |||
| ↑ apoptosis | |||
| U87 | 10 or 15 μM for 48 h | ↓ mTOR | [22] |
| ↓ HSF1 | |||
| ↓ Hsp27 expression | |||
| ↓ proliferation | |||
| ↑ apoptosis |
| Cell Line | Incubation Concentration | Results | References |
|---|---|---|---|
| Metformin | |||
| GBM tissue samples | 5 mM, 10 mM, 20 mM, 50 mM | ↓ RAF/RAS/MAPK/MEK/ERK | [36] |
| ↓ Bcl-2 | |||
| ↓ viability | |||
| ↓ proliferation | |||
| ↑ apoptosis | |||
| GSC | N/A | ↑ MAPK | [37] |
| ↑ autophagy | |||
| ↑ apoptosis | |||
| Resveratrol | |||
| A172 | 100 µΜ | ↑ ROS-induced activation of MAPK subfamily | [38] |
| ↑ apoptosis |
| Cell Line | Incubation Concentration | Results | References |
|---|---|---|---|
| Metformin | |||
| A172 | 0, 0.1, 1, 10 mM for 24, 48, 72 h | ↑ AMPK phosphorylation | [31] |
| ↑ Bax expression | |||
| ↑ apoptosis | |||
| ↓ proliferation | |||
| U87 U251 A172 | 5, 10, 20 mM for 24, 48, 72 h | ↑ AMPK phosphorylation | [33] |
| U87 LN18 U251 SF767 | 10 mM for 48 h | ↑ AMPK phosphorylation | [18] |
| U87 U251 | 10 mM for 0–48 h 0–2 0 mM for 48 h | ↑ AMPK phosphorylation | [32] |
| ↓ proliferation | |||
| ↑ apoptosis | |||
| GICs | 1 mM | ↑ AMPK phosphorylation | [41] |
| ↑ FOXO3 activation | |||
| Resveratrol | |||
| A172 | N/A | ↓ AMPK and YAP transcription | [42] |
| ↓ cell viability | |||
| ↑ apoptosis | |||
| SHG44 | 10 µM for 72 h | ↑ ROS production | [34] |
| ↑ AMPK phosphorylation | |||
| ↓ mTOR | |||
| ↑ apoptosis | |||
| ↑ G2/M arrest |
| Cell Line | Incubation Concentration | Results | References |
|---|---|---|---|
| Metformin | |||
| U87 LN18 U251 SF767 | 10 mM for 48 h | ↓ oxygen consumption | [18] |
| ↓ mitochondrial dependent ATP production | |||
| ↑ glycolytic ATP production | |||
| ↑ lactate production | |||
| ↓ ETC1 activity | |||
| U87MG LNZ308 LN229 | 0, 25, 50, 75, 100, 125 mM for 24 h | ↓ PGC-1α | [46] |
| ↓ mtTFA | |||
| ↑ ROS | |||
| ↓ mitochondrial biogenesis | |||
| ↓ mitochondrial membrane potential | |||
| U251 | 4 mM for 24 h | ↑ ROS production | [48] |
| ↑ mitochondrial depolarization | |||
| ↑ apoptosis | |||
| U251 T98G | 10 mM for 24, 48, 72 h | ↑ glucose consumption | [47] |
| ↑ lactate production | |||
| Resveratrol | |||
| DBTRG | 50 µM for 24 h | ↑ Ca2+ influx | [49] |
| ↑ mitochondrial apoptosis | |||
| ↑ caspase 3 activity | |||
| ↑ ROS production | |||
| ↑ cell sensitivity | |||
| U251 | 150 µM for 6–72 h | ↑ collapsed mitochondria membrane potential | [50] |
| ↑ apoptosis | |||
| N/A | N/A | ↓ mitochondrial-dependent ATP production | [45] |
| Cell Line | Applied Concentration | Results | References |
|---|---|---|---|
| Metformin | |||
| Athymic nude mice inoculated with U87 cells | 2 mg/25 g/day for 4 weeks | ↑ phosphorylated AMPK | [33] |
| ↓ Fatty acid synthase (FASN) | |||
| ↓ tumor growth | |||
| ↑ survival in models | |||
| NU/NU athymic mice injected with U87 and LN18 cells | 200 mL of 300 mg/kg/day for 30 days | ↑ active caspase-3 | [18] |
| ↓ Ki67 | |||
| ↓ tumor growth | |||
| ↓ cell proliferation | |||
| ↑ cell death | |||
| Female nude mice injected with U251 or T98G cells | 250 mg/kg/day for 21 days | ↓ tumor volume only when combined with (400 mg/kg) TMZ | [47] |
| ↓ tumor growth when combined with (400 mg/kg) TMZ | |||
| Resveratrol | |||
| BALB/cA nude mice injected with SHG44 cells | Oral administration 40 mg/kg | ↓ tumor volume when combined with (68 mg/kg) TMZ | [34] |
| ↓ Ki-67 staining index when combined with (68 mg/kg) TMZ | |||
| BALB/cA nude mice injected with U87 cells | 0.1 mg/mL or 50 mg/kg or 5 injections of 200 mL of 5 mg over 2 weeks | ↓ tumor volume | [21] |
| ↓ tumor growth | |||
| BALB/cA nude mice injected with SU-2 cells | 150 mg/kg | ↓ tumor growth | [51] |
| ↓ Bcl-2 | |||
| ↑ apoptosis | |||
| ↑ autophagy | |||
| Rat models with C6 glioma | Oral administration RES 8 mg/kg/day | ↑ survival in models | [24] |
| ↓ tumor growth | |||
| ↑ number of apoptotic cells | |||
| ↓ EGFR, NF-κB, COX-2 and VEGF |
| Cell Line | Incubation Concentration | Results | References |
|---|---|---|---|
| U87 | 25 mM glucose for 24, 48, 72 h | ↑ cell proliferation | [11] |
| ↑ cell survival | |||
| ↑ tumorigenesis | |||
| ↑ Bcl-2 | |||
| ↑ Mcl-1 | |||
| ↑ NF-κB phosphorylation | |||
| ↑ FPR1 | |||
| ↑ EGFR | |||
| ↑ VEGF | |||
| T98G HROG02 HROG17 | 4.5 g/L glucose for 48 h | ↑ cell viability | [54] |
| ↑ GBM cell division | |||
| ↑ Dispersal | |||
| U87 U251 T98G | 5, 10, 40 mg/mL | ↑ glycolytic activity | [52] |
| ↑ expression of PDK1, PDK3, ECH, and HADH | |||
| N/A | N/A | ↑ ERK | [53] |
| ↑ STAT3 | |||
| ↑ EGF | |||
| ↑ EGFR | |||
| ↑ ROS production | |||
| ↑ NF-ĸB | |||
| ↑ cell proliferation | |||
| ↑ anti-apoptosis | |||
| ↑ VEGF | |||
| ↑ Warburg effect | |||
| ↑ impaired mitochondrial function |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, R.S.; Ibrahim, S.S.; El-Naas, A.; Koklesová, L.; Kubatka, P.; Büsselberg, D. Could Metformin and Resveratrol Support Glioblastoma Treatment? A Mechanistic View at the Cellular Level. Cancers 2023, 15, 3368. https://doi.org/10.3390/cancers15133368
Ibrahim RS, Ibrahim SS, El-Naas A, Koklesová L, Kubatka P, Büsselberg D. Could Metformin and Resveratrol Support Glioblastoma Treatment? A Mechanistic View at the Cellular Level. Cancers. 2023; 15(13):3368. https://doi.org/10.3390/cancers15133368
Chicago/Turabian StyleIbrahim, Raghad Sabaawi, Shahad Sabaawi Ibrahim, Ahmed El-Naas, Lenka Koklesová, Peter Kubatka, and Dietrich Büsselberg. 2023. "Could Metformin and Resveratrol Support Glioblastoma Treatment? A Mechanistic View at the Cellular Level" Cancers 15, no. 13: 3368. https://doi.org/10.3390/cancers15133368
APA StyleIbrahim, R. S., Ibrahim, S. S., El-Naas, A., Koklesová, L., Kubatka, P., & Büsselberg, D. (2023). Could Metformin and Resveratrol Support Glioblastoma Treatment? A Mechanistic View at the Cellular Level. Cancers, 15(13), 3368. https://doi.org/10.3390/cancers15133368








