The Relationship between Telomere Length and Nucleoplasmic Bridges and Severity of Disease in Prostate Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Blood Collection and CBMN Cytome Assay
2.3. DNA Isolation and Real-Time qPCR Assay for Measuring TL
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
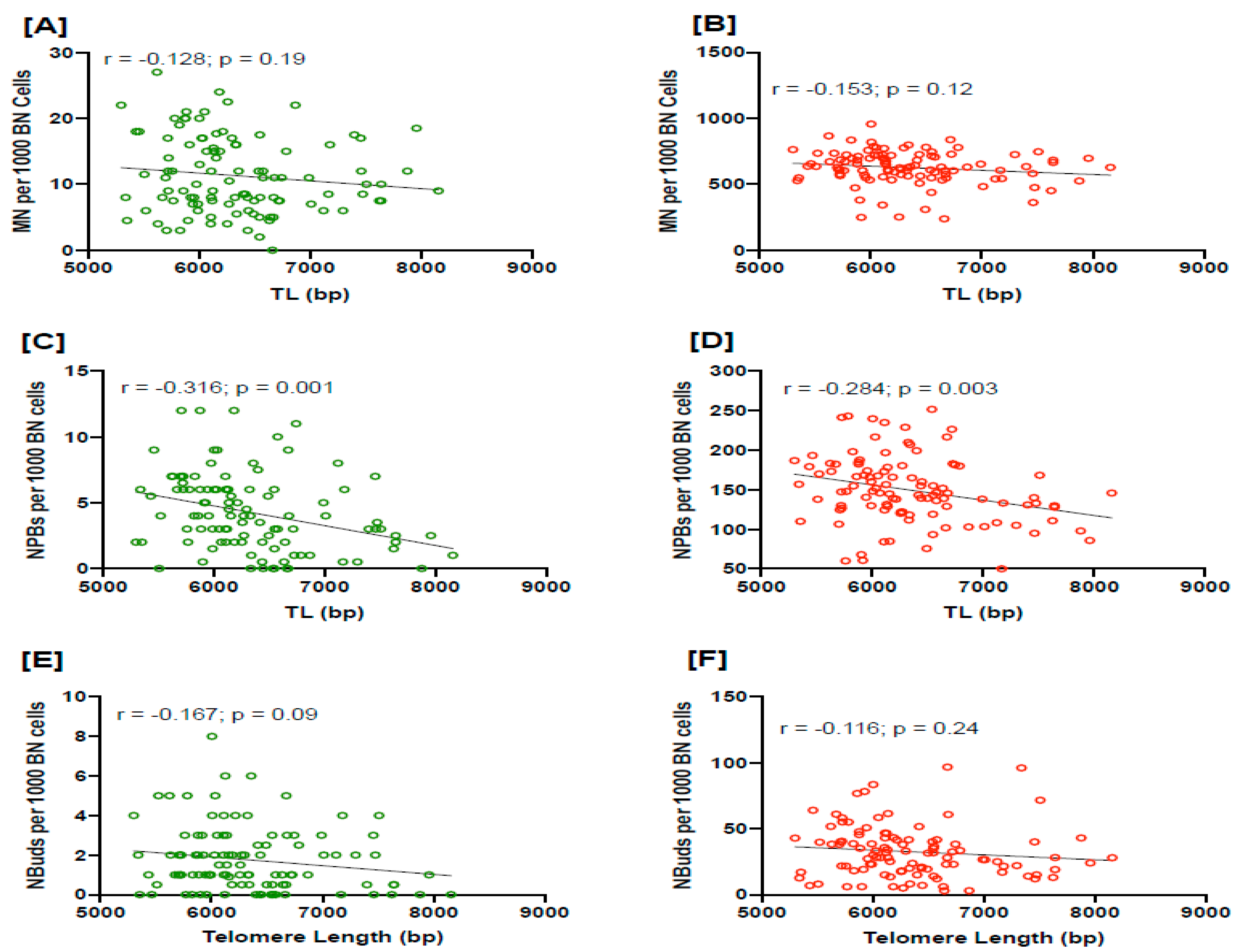
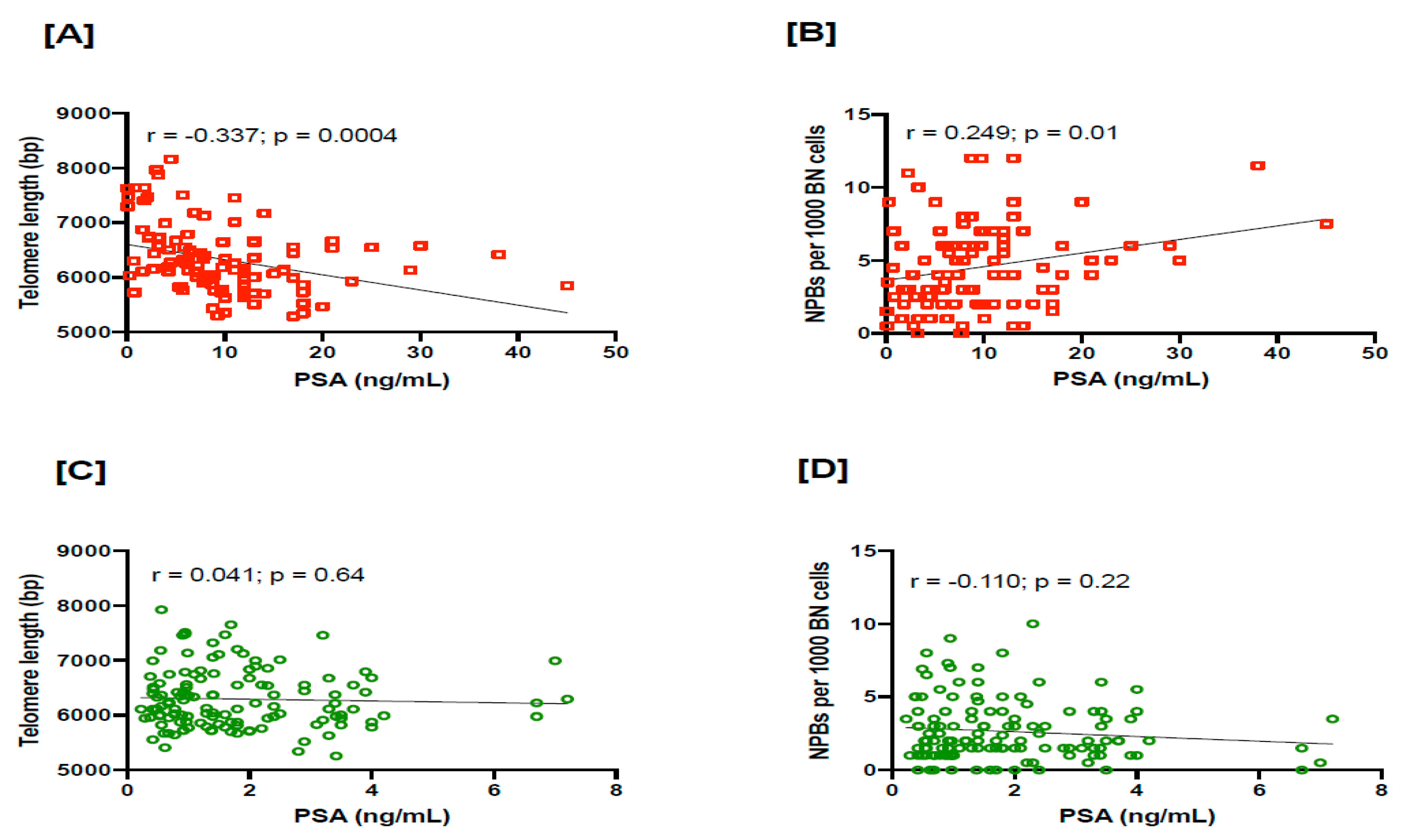
3.2. Relationship between TL and NPBs in PC Patients
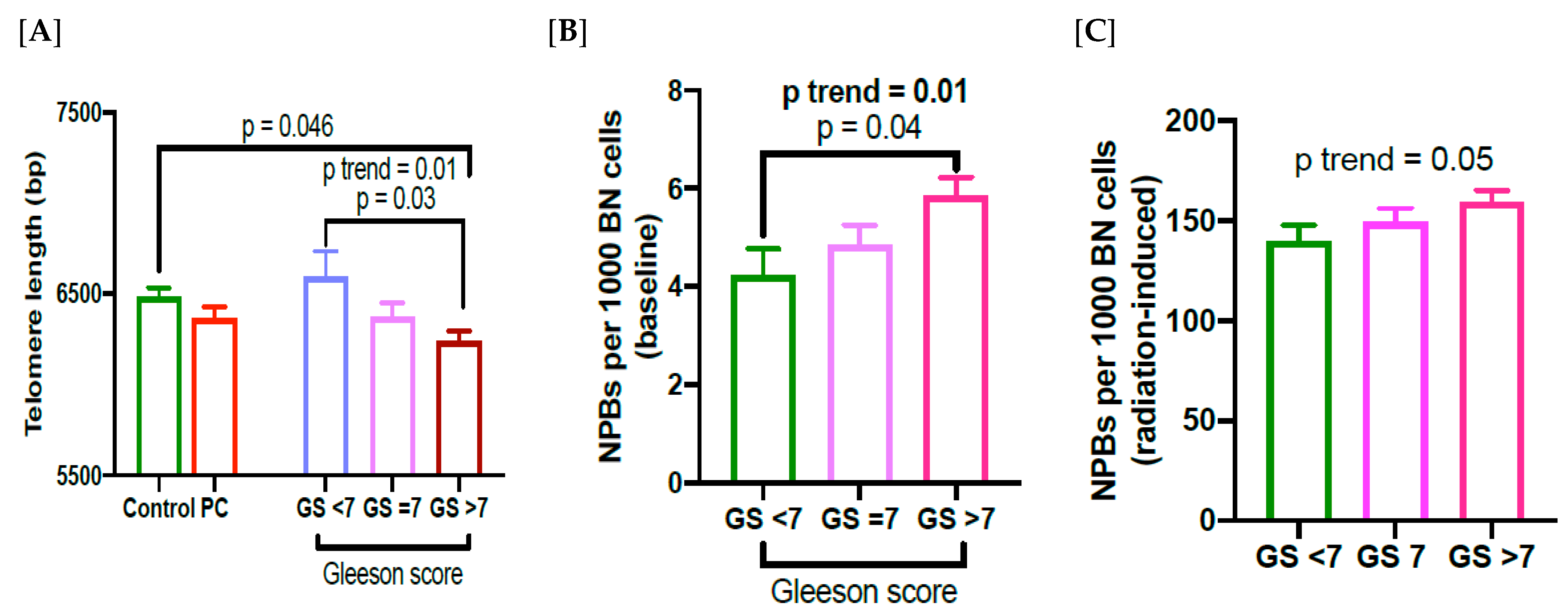
3.3. Relationship between TL, NPBs and Gleason Score in PC Patients
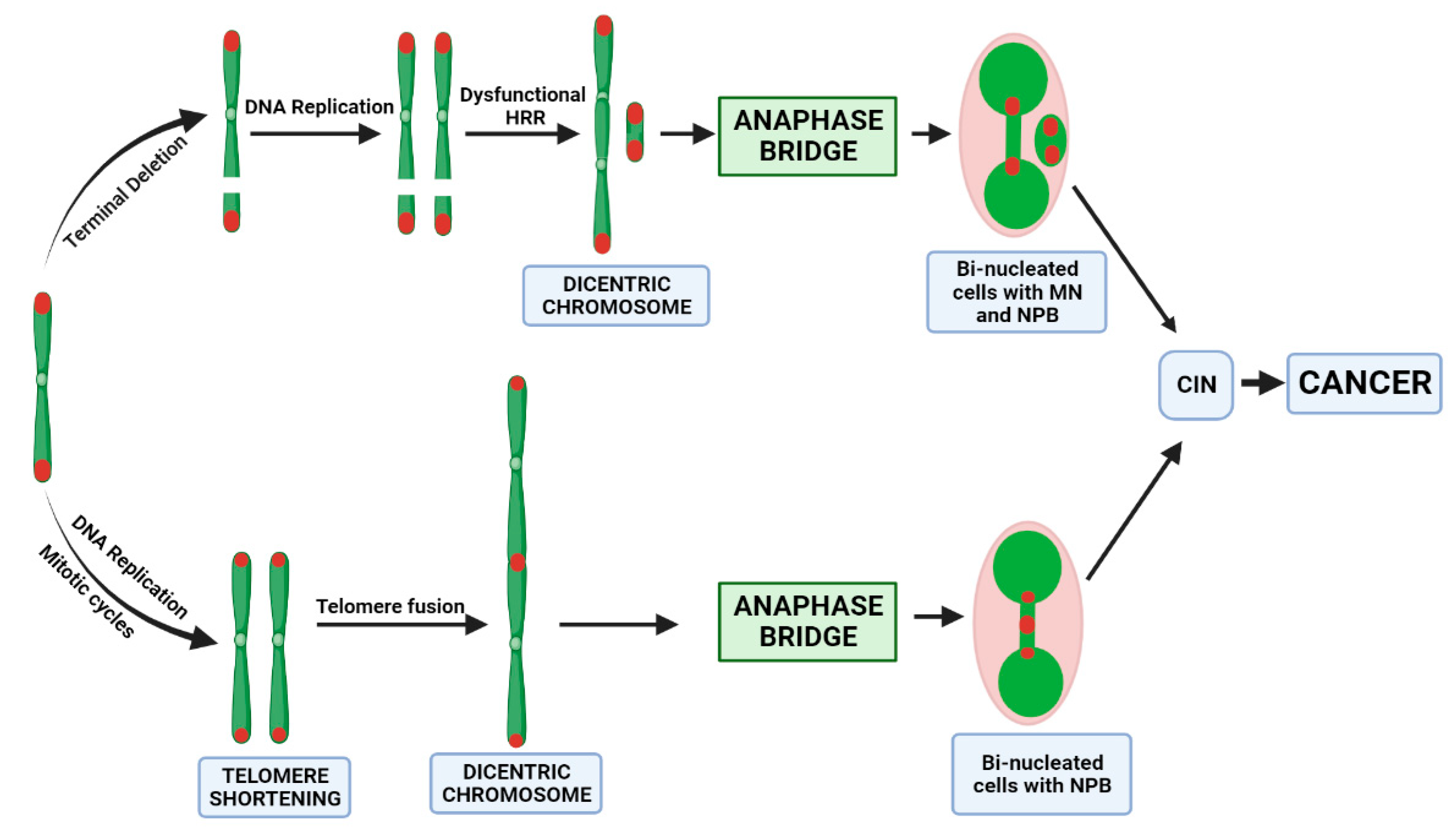
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Ge, R.; Wang, Z.; Montironi, R.; Jiang, Z.; Cheng, M.; Santoni, M.; Huang, K.; Massari, F.; Lu, X.; Cimadamore, A.; et al. Epigenetic modulations and lineage plasticity in advanced prostate cancer. Ann. Oncol. 2020, 31, 470–479. [Google Scholar] [CrossRef]
- Rebbeck, T.R. Prostate Cancer Genetics: Variation by Race, Ethnicity, and Geography. Semin. Radiat. Oncol. 2016, 27, 3–10. [Google Scholar] [CrossRef]
- Viegas, S.; Ladeira, C.; Costa-Veiga, A.; Perelman, J.; Gajski, G. Forgotten public health impacts of cancer—An overview. Arch. Ind. Hyg. Toxicol. 2017, 68, 287–297. [Google Scholar] [CrossRef]
- de Lange, T. Shelterin-Mediated Telomere Protection. Annu. Rev. Genet. 2018, 52, 223–247. [Google Scholar] [CrossRef]
- Cicconi, A.; Chang, S. Shelterin and the replisome: At the intersection of telomere repair and replication. Curr. Opin. Genet. Dev. 2020, 60, 77–84. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Greider, C.W.; Szostak, J.W. Telomeres and telomerase: The path from maize, Tetrahymena and yeast to human cancer and aging. Nat. Med. 2006, 12, 1133–1138. [Google Scholar] [CrossRef]
- Arnoult, N.; Karlseder, J. Complex interactions between the DNA-damage response and mammalian telomeres. Nat. Struct. Mol. Biol. 2015, 22, 859–866. [Google Scholar] [CrossRef]
- Livingstone, J.; Shiah, Y.-J.; Yamaguchi, T.N.; Heisler, L.E.; Huang, V.; Lesurf, R.; Gebo, T.; Carlin, B.; Eng, S.; Drysdale, E.; et al. The telomere length landscape of prostate cancer. Nat. Commun. 2021, 12, 6893. [Google Scholar] [CrossRef]
- Gilley, D.; Herbert, B.-S.; Huda, N.; Tanaka, H.; Reed, T. Factors impacting human telomere homeostasis and age-related disease. Mech. Ageing Dev. 2008, 129, 27–34. [Google Scholar] [CrossRef]
- Joshua, A.M.; Shen, E.; Yoshimoto, M.; Marrano, P.; Zielenska, M.; Evans, A.J.; Van der Kwast, T.; Squire, J.A. Topographical analysis of telomere length and correlation with genomic instability in whole mount prostatectomies. Prostate 2011, 71, 778–790. [Google Scholar] [CrossRef]
- Cai, T.; Santi, R.; Tamanini, I.; Galli, I.C.; Perletti, G.; Bjerklund Johansen, T.E.; Nesi, G. Current Knowledge of the Potential Links between Inflammation and Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 3833. [Google Scholar] [CrossRef]
- De Marzo, A.M.; Platz, E.A.; Sutcliffe, S.; Xu, J.; Grönberg, H.; Drake, C.G.; Nakai, Y.; Isaacs, W.B.; Nelson, W.G. Inflammation in prostate carcinogenesis. Nat. Rev. Cancer 2007, 7, 256–269. [Google Scholar] [CrossRef]
- Squire, J.A.; Park, P.C.; Yoshimoto, M.; Alami, J.; Williams, J.L.; Evans, A.; Joshua, A.M. Prostate Cancer as a Model System for Genetic Diversity in Tumors. Adv. Cancer Res. 2011, 112, 183–216. [Google Scholar] [CrossRef]
- Hu, R.; Hua, X.; Jiang, Q. Associations of telomere length in risk and recurrence of prostate cancer: A meta-analysis. Andrologia 2019, 51, e13304. [Google Scholar] [CrossRef]
- Hou, L.; Joyce, B.; Gao, T.; Liu, L.; Zheng, Y.; Penedo, F.J.; Liu, S.; Zhang, W.; Bergan, R.; Dai, Q.; et al. Blood Telomere Length Attrition and Cancer Development in the Normative Aging Study Cohort. eBioMedicine 2015, 2, 591–596. [Google Scholar] [CrossRef]
- Weischer, M.; Nordestgaard, B.G.; Cawthon, R.M.; Freiberg, J.J.; Tybjærg-Hansen, A.; Bojesen, S.E. Short Telomere Length, Cancer Survival, and Cancer Risk in 47102 Individuals. Gynecol. Oncol. 2013, 105, 459–468. [Google Scholar] [CrossRef]
- Lan, Q.; Cawthon, R.; Gao, Y.; Hu, W.; Hosgood, H.D., 3rd; Barone-Adesi, F.; Ji, B.T.; Bassig, B.; Chow, W.H.; Shu, X.; et al. Longer telomere length in peripheral white blood cells is associated with risk of lung cancer and the rs2736100 (CLPTM1L-TERT) polymorphism in a prospective cohort study among women in China. PLoS ONE 2013, 8, e59230. [Google Scholar] [CrossRef]
- Julin, B.; Shui, I.; Heaphy, C.M.; Joshu, C.E.; Meeker, A.K.; Giovannucci, E.; De Vivo, I.; Platz, E.A. Circulating leukocyte telomere length and risk of overall and aggressive prostate cancer. Br. J. Cancer 2015, 112, 769–776. [Google Scholar] [CrossRef]
- Xu, J.; Chang, W.-S.; Tsai, C.-W.; Bau, D.-T.; Xu, Y.; Davis, J.W.; Thompson, T.C.; Logothetis, C.J.; Gu, J. Leukocyte telomere length is associated with aggressive prostate cancer in localized prostate cancer patients. eBioMedicine 2020, 52, 102616. [Google Scholar] [CrossRef]
- Rybicki, B.A.; Sadasivan, S.M.; Chen, Y.; Loveless, I.; Gupta, N.S.; Chitale, D.A.; Williamson, S.R.; Rundle, A.G.; Tang, D.L. Race Differences in Telomere Length in Benign Prostate Biopsies and Subsequent Risk of Prostate Cancer. Cancer Epidemiol. Biomark. Prev. 2022, 31, 991–998. [Google Scholar] [CrossRef]
- Renner, W.; Krenn-Pilko, S.; Gruber, H.-J.; Herrmann, M.; Langsenlehner, T. Relative telomere length and prostate cancer mortality. Prostate Cancer Prostatic. Dis. 2018, 21, 579–583. [Google Scholar] [CrossRef]
- Loeb, L.A.; Bielas, J.H.; Beckman, R.A. Cancers Exhibit a Mutator Phenotype: Clinical Implications. Cancer Res. 2008, 68, 3551–3557. [Google Scholar] [CrossRef]
- Stratton, M.R.; Campbell, P.J.; Futreal, P.A. The cancer genome. Nature 2009, 458, 719–724. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-Block Micronucleus Cytome Assay Evolution into a More Comprehensive Method to Measure Chromosomal Instability. Genes 2020, 11, 1203. [Google Scholar] [CrossRef]
- Fenech, M.; Knasmueller, S.; Knudsen, L.E.; Kirsch-Volders, M.; Deo, P.; Franzke, B.; Stopper, H.; Andreassi, M.G.; Bolognesi, C.; Dhillon, V.S.; et al. “Micronuclei and Disease” special issue: Aims, scope, and synthesis of outcomes. Mutat. Res. Rev. Mutat. Res. 2021, 788, 108384. [Google Scholar] [CrossRef]
- McHugh, M.K.; Lopez, M.S.; Ho, C.-H.; Spitz, M.R.; Etzel, C.J.; El-Zein, R.A. Use of the Cytokinesis-Blocked Micronucleus Assay to Detect Gender Differences and Genetic Instability in a Lung Cancer Case–Control Study. Cancer Epidemiol. Biomark. Prev. 2013, 22, 135–145. [Google Scholar] [CrossRef]
- Podrimaj-Bytyqi, A.; Borovečki, A.; Selimi, Q.; Manxhuka-Kerliu, S.; Gashi, G.; Elezaj, I.R. The frequencies of micronuclei, nucleoplasmic bridges and nuclear buds as biomarkers of genomic instability in patients with urothelial cell carcinoma. Sci. Rep. 2018, 8, 17873. [Google Scholar] [CrossRef]
- Dhillon, V.S.; Yeoh, E.; Salisbury, C.; Butters, J.; Di Matteo, A.; Olver, I.; Fenech, M. Cytokinesis Block Micronucleus Cytome (CBMN Cyt) Assay Biomarkers and Their Association With Radiation Sensitivity Phenotype in Prostate Cancer Cases and DNA Repair Gene hOGG1 (C1245G) Polymorphism. Environ. Mol. Mutagen. 2018, 59, 813–821. [Google Scholar] [CrossRef]
- Yazici, I.; Caglar, O.; Guclu, O.; Cobanoglu, H.; Coskun, M.; Coskun, M.; Kilic, A.; Dereköy, F.S. Micronucleus, nucleoplasmic bridge and nuclear bud frequencies in patients with laryngeal carcinoma. Acta Otorhinolaryngol. Ital. 2020, 40, 410–414. [Google Scholar] [CrossRef]
- Bitgen, N.; Bayram, F.; Hamurcu, Z.; Ozturk, F.; Simsek, Y.; Baskol, G.; Kurtsoy, A.; Donmez-Altuntas, H. Chromosomal and oxidative DNA damage in non-functioning pituitary adenomas. Endokrynol. Pol. 2021, 72, 97–103. [Google Scholar] [CrossRef]
- Norppa, H.; Bonassi, S.; Hansteen, I.-L.; Hagmar, L.; Strömberg, U.; Rössner, P.; Boffetta, P.; Lindholm, C.; Gundy, S.; Lazutka, J.; et al. Chromosomal aberrations and SCEs as biomarkers of cancer risk. Mutat. Res. Mol. Mech. Mutagen. 2006, 600, 37–45. [Google Scholar] [CrossRef]
- Gisselsson, D.; Jonson, T.; Petersén, A.; Strömbeck, B.; Dal Cin, P.; Höglund, M.; Mitelman, F.; Mertens, F.; Mandahl, N. Telomere dysfunction triggers extensive DNA fragmentation and evolution of complex chromosome abnormalities in human malignant tumors. Proc. Natl. Acad. Sci. USA 2001, 98, 12683–12688. [Google Scholar] [CrossRef]
- Pampalona, J.; Soler, D.; Genescà, A.; Tusell, L. Telomere dysfunction and chromosome structure modulate the contribution of individual chromosomes in abnormal nuclear morphologies. Mutat. Res. Mol. Mech. Mutagen. 2010, 683, 16–22. [Google Scholar] [CrossRef]
- Gisselsson, D. Chromosome instability in cancer: How, when, and why? Adv. Cancer Res. 2003, 87, 1–29. [Google Scholar] [CrossRef]
- Dhillon, V.S.; Deo, P.; Fenech, M. Plasma Micronutrient Profile of Prostate Cancer Cases Is Altered Relative to Healthy Controls—Results of a Pilot Study in South Australia. Cancers 2022, 15, 77. [Google Scholar] [CrossRef]
- Dhillon, V.S.; Deo, P.; Fenech, M. Effect of Selenium and Lycopene on Radiation Sensitivity in Prostate Cancer Patients Relative to Controls. Cancers 2023, 15, 979. [Google Scholar] [CrossRef]
- Yeoh, E.K.; Krol, R.; Botten, R.; Di Matteo, A.; Butters, J.; Brock, A.R.; Esterman, A.; Salisbury, C.; Fenech, M. Predictors of radiation-induced gastrointestinal morbidity: A prospective, longitudinal study following radiotherapy for carcinoma of the prostate. Acta Oncol. 2016, 55, 604–610. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- Dhillon, V.S.; Deo, P.; Thomas, P.; Fenech, M. Low Magnesium in Conjunction with High Homocysteine and Less Sleep Accelerates Telomere Attrition in Healthy Elderly Australian. Int. J. Mol. Sci. 2023, 24, 982. [Google Scholar] [CrossRef]
- Tsai, C.-W.; Chang, W.-S.; Xu, J.; Xu, Y.; Huang, M.; Pettaway, C.; Bau, D.-T.; Gu, J. Leukocyte telomere length is associated with aggressive prostate cancer in localized African American prostate cancer patients. Carcinogenesis 2020, 41, 1213–1218. [Google Scholar] [CrossRef]
- Mirabello, L.; Huang, W.-Y.; Wong, J.Y.; Chatterjee, N.; Reding, D.; Crawford, E.D.; De Vivo, I.; Hayes, R.B.; Savage, S.A. The association between leukocyte telomere length and cigarette smoking, dietary and physical variables, and risk of prostate cancer. Aging Cell 2009, 8, 405–413. [Google Scholar] [CrossRef]
- Pooley, K.A.; Bojesen, S.E.; Weischer, M.; Nielsen, S.F.; Thompson, D.; Al Olama, A.A.; Michailidou, K.; Tyrer, J.P.; Benlloch, S.; Brown, J.; et al. A genome-wide association scan (GWAS) for mean telomere length within the COGS project: Identified loci show little association with hormone-related cancer risk. Hum. Mol. Genet. 2013, 22, 5056–5064. [Google Scholar] [CrossRef]
- Hurwitz, L.M.; Heaphy, C.M.; Joshu, C.E.; Isaacs, W.B.; Konishi, Y.; De Marzo, A.M.; Isaacs, S.D.; Wiley, K.E.; Platz, E.A.; Meeker, A.K. Telomere length as a risk factor for hereditary prostate cancer. Prostate 2013, 74, 359–364. [Google Scholar] [CrossRef]
- Rode, L.; Nordestgaard, B.G.; Bojesen, S.E. Long telomeres and cancer risk among 95 568 individuals from the general population. Leuk. Res. 2016, 45, 1634–1643. [Google Scholar] [CrossRef]
- Heaphy, C.M.; Joshu, C.E.; Barber, J.R.; Davis, C.; Lu, J.; Zarinshenas, R.; Giovannucci, E.; Mucci, L.A.; Stampfer, M.J.; Han, M.; et al. The prostate tissue-based telomere biomarker as a prognostic tool for metastasis and death from prostate cancer after prostatectomy. J. Pathol. Clin. Res. 2022, 8, 481–491. [Google Scholar] [CrossRef]
- Meeker, A.K.; Hicks, J.L.; Platz, E.A.; March, G.E.; Bennett, C.J.; Delannoy, M.J.; De Marzo, A.M. Telomere shortening is an early somatic DNA alteration in human prostate tumorigenesis. Cancer Res. 2002, 62, 6405–6409. [Google Scholar]
- Heaphy, C.M.; Yoon, G.S.; Peskoe, S.B.; Joshu, C.E.; Lee, T.K.; Giovannucci, E.; Mucci, L.A.; Kenfield, S.A.; Stampfer, M.J.; Hicks, J.L.; et al. Prostate Cancer Cell Telomere Length Variability and Stromal Cell Telomere Length as Prognostic Markers for Metastasis and Death. Cancer Discov. 2013, 3, 1130–1141. [Google Scholar] [CrossRef]
- Ornish, D.; Lin, J.; Daubenmier, J.; Weidner, G.; Epel, E.; Kemp, C.; Magbanua, M.J.M.; Marlin, R.; Yglecias, L.; Carroll, P.R.; et al. Increased telomerase activity and comprehensive lifestyle changes: A pilot study. Lancet Oncol. 2008, 9, 1048–1057. [Google Scholar] [CrossRef]
- Gashi, G.; Mahovlić, V.; Manxhuka-Kerliu, S.; Podrimaj-Bytyqi, A.; Gashi, L.; Elezaj, I.R. The association between micronucleus, nucleoplasmic bridges, and nuclear buds frequency and the degree of uterine cervical lesions. Biomarkers 2018, 23, 364–372. [Google Scholar] [CrossRef]
- Negrini, S.; Gorgoulis, V.G.; Halazonetis, T.D. Genomic instability--an evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 220–228. [Google Scholar] [CrossRef]
- Andor, N.; Maley, C.C.; Ji, H.P. Genomic Instability in Cancer: Teetering on the Limit of Tolerance. Cancer Res. 2017, 77, 2179–2185. [Google Scholar] [CrossRef]
- Gisselsson, D.; Björk, J.; Höglund, M.; Mertens, F.; Dal Cin, P.; Åkerman, M.; Mandahl, N. Abnormal Nuclear Shape in Solid Tumors Reflects Mitotic Instability. Am. J. Pathol. 2001, 158, 199–206. [Google Scholar] [CrossRef]
- Stroik, S.; Hendrickson, E.A. Telomere fusions and translocations: A bridge too far? Curr. Opin. Genet. Dev. 2020, 60, 85–91. [Google Scholar] [CrossRef]
- Guérin, T.M.; Marcand, S. Breakage in breakage-fusion-bridge cycle: An 80-year-old mystery. Trends Genet. 2022, 38, 641–645. [Google Scholar] [CrossRef]
- Robin, J.D.; Ludlow, A.T.; Batten, K.; Magdinier, F.; Stadler, G.; Wagner, K.R.; Shay, J.W.; Wright, W.E. Telomere position effect: Regulation of gene expression with progressive telomere shortening over long distances. Genes Dev. 2014, 28, 2464–2476. [Google Scholar] [CrossRef]
- Gekara, N.O. DNA damage-induced immune response: Micronuclei provide key platform. J. Cell Biol. 2017, 216, 2999–3001. [Google Scholar] [CrossRef]
- Hoffelder, D.R.; Luo, L.; Burke, N.A.; Watkins, S.; Gollin, S.; Saunders, W.S. Resolution of anaphase bridges in cancer cells. Chromosoma 2004, 112, 389–397. [Google Scholar] [CrossRef]
- Liddiard, K.; Ruis, B.; Kan, Y.; Cleal, K.; Ashelford, K.E.; A Hendrickson, E.; Baird, D.M. DNA Ligase 1 is an essential mediator of sister chromatid telomere fusions in G2 cell cycle phase. Nucleic Acids Res. 2018, 47, 2402–2424. [Google Scholar] [CrossRef]
- Qian, W.; Kumar, N.; Roginskaya, V.; Fouquerel, E.; Opresko, P.L.; Shiva, S.; Watkins, S.C.; Kolodieznyi, D.; Bruchez, M.P.; Van Houten, B. Chemoptogenetic damage to mitochondria causes rapid telomere dysfunction. Proc. Natl. Acad. Sci. USA 2019, 116, 18435–18444. [Google Scholar] [CrossRef]
- Fouquerel, E.; Barnes, R.P.; Uttam, S.; Watkins, S.C.; Bruchez, M.P.; Opresko, P.L. Targeted and Persistent 8-Oxoguanine Base Damage at Telomeres Promotes Telomere Loss and Crisis. Mol. Cell 2019, 75, 117–130.e6. [Google Scholar] [CrossRef]
- Paschos, A.; Pandya, R.; Duivenvoorden, W.C.; Pinthus, J.H. Oxidative stress in prostate cancer: Changing research concepts towards a novel paradigm for prevention and therapeutics. Prostate Cancer Prostatic. Dis. 2013, 16, 217–225. [Google Scholar] [CrossRef]
- Graham, M.K.; Meeker, A. Telomeres and telomerase in prostate cancer development and therapy. Nat. Rev. Urol. 2017, 14, 607–619. [Google Scholar] [CrossRef]
- Gajski, G.; Gerić, M.; Oreščanin, V.; Garaj-Vrhovac, V. Cytokinesis-block micronucleus cytome assay parameters in peripheral blood lymphocytes of the general population: Contribution of age, sex, seasonal variations and lifestyle factors. Ecotoxicol. Environ. Saf. 2018, 148, 561–570. [Google Scholar] [CrossRef]
- McNamee, J.P.; Flegal, F.N.; Greene, H.B.; Marro, L.; Wilkins, R.C. Validation of the cytokinesis-block micronucleus (CBMN) assay for use as a triage biological dosimetry tool. Radiat. Prot. Dosim. 2009, 135, 232–242. [Google Scholar] [CrossRef]
- Vral, A.; Endesfelder, D.; Balázs, J.; Beinke, C.; Petrenci, C.C.; Finot, F.; Garty, G.; Hadjiiska, L.; Hristova, R.; Ivanova, I.; et al. RENEB Inter-Laboratory Comparison 2021: The Cytokinesis-Block Micronucleus Assay. Radiat. Res. 2023, 199, 571–582. [Google Scholar] [CrossRef]

| Characteristics | Cases | Controls | p Value |
|---|---|---|---|
| Age (years; mean ± SD) | 71.24 ± 7.18 | 69.07 ± 7.99 | 0.88 |
| PSA (ng/mL; mean ± SD) | 9.5 ± 8.5 | 2.4 ± 2.45 | 0.0001 |
| Smoking status | |||
| Current smokers | 9 | 3 | 0.0001 |
| Ex-smokers | 60 | 39 | |
| Non-smokers | 25 | 54 | |
| Un-declared | 12 | 36 | |
| Gleason score (GS) | |||
| (<7) | 23 | - | |
| (7) | 41 | - | |
| (>7) | 42 | - | |
| Telomere length (TL; bp) | 6365 ± 648.1 | 6454 ± 569.8 | 0.26 |
| TL and GS | |||
| (<7) | 6595 ± 675.9 | ||
| (7) | 6373 ± 484.7 | ||
| (>7) | 6241 ± 438.2 | 0.03 | |
| MN (baseline) | 11.64 ± 0.62 | 10.70 ± 0.48 | 0.23 |
| MN (radiation-induced) | 641 ± 10.65 | 653.30 ± 8.3 | 0.62 |
| NPBs (baseline) | 4.26 ± 0.28 | 2.72 ± 0.18 | 0.0001 |
| NPBs (radiation-induced) | 151.8 ± 4.27 | 149 ± 2.86 | 0.62 |
| NBuds (baseline) | 1.75 ± 0.16 | 1.35 ± 0.14 | 0.057 |
| NBuds (radiation-induced) | 33.99 ± 2.29 | 23.97 ± 1.75 | 0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhillon, V.S.; Deo, P.; Fenech, M. The Relationship between Telomere Length and Nucleoplasmic Bridges and Severity of Disease in Prostate Cancer Patients. Cancers 2023, 15, 3351. https://doi.org/10.3390/cancers15133351
Dhillon VS, Deo P, Fenech M. The Relationship between Telomere Length and Nucleoplasmic Bridges and Severity of Disease in Prostate Cancer Patients. Cancers. 2023; 15(13):3351. https://doi.org/10.3390/cancers15133351
Chicago/Turabian StyleDhillon, Varinderpal S., Permal Deo, and Michael Fenech. 2023. "The Relationship between Telomere Length and Nucleoplasmic Bridges and Severity of Disease in Prostate Cancer Patients" Cancers 15, no. 13: 3351. https://doi.org/10.3390/cancers15133351
APA StyleDhillon, V. S., Deo, P., & Fenech, M. (2023). The Relationship between Telomere Length and Nucleoplasmic Bridges and Severity of Disease in Prostate Cancer Patients. Cancers, 15(13), 3351. https://doi.org/10.3390/cancers15133351






