Identification of a Twelve-microRNA Signature with Prognostic Value in Stage II Microsatellite Stable Colon Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Samples
2.2. Sample Processing
2.3. Small RNA-seq Analysis of the Discovery Series
2.4. Expression Data of the Discovery Series
2.5. Bioinformatics Analysis
2.6. Statistical Analysis of Prognosis
2.7. Validation Analysis
2.8. Functional Characterization
3. Results
3.1. Study Population Characteristics and Quality Control of Samples
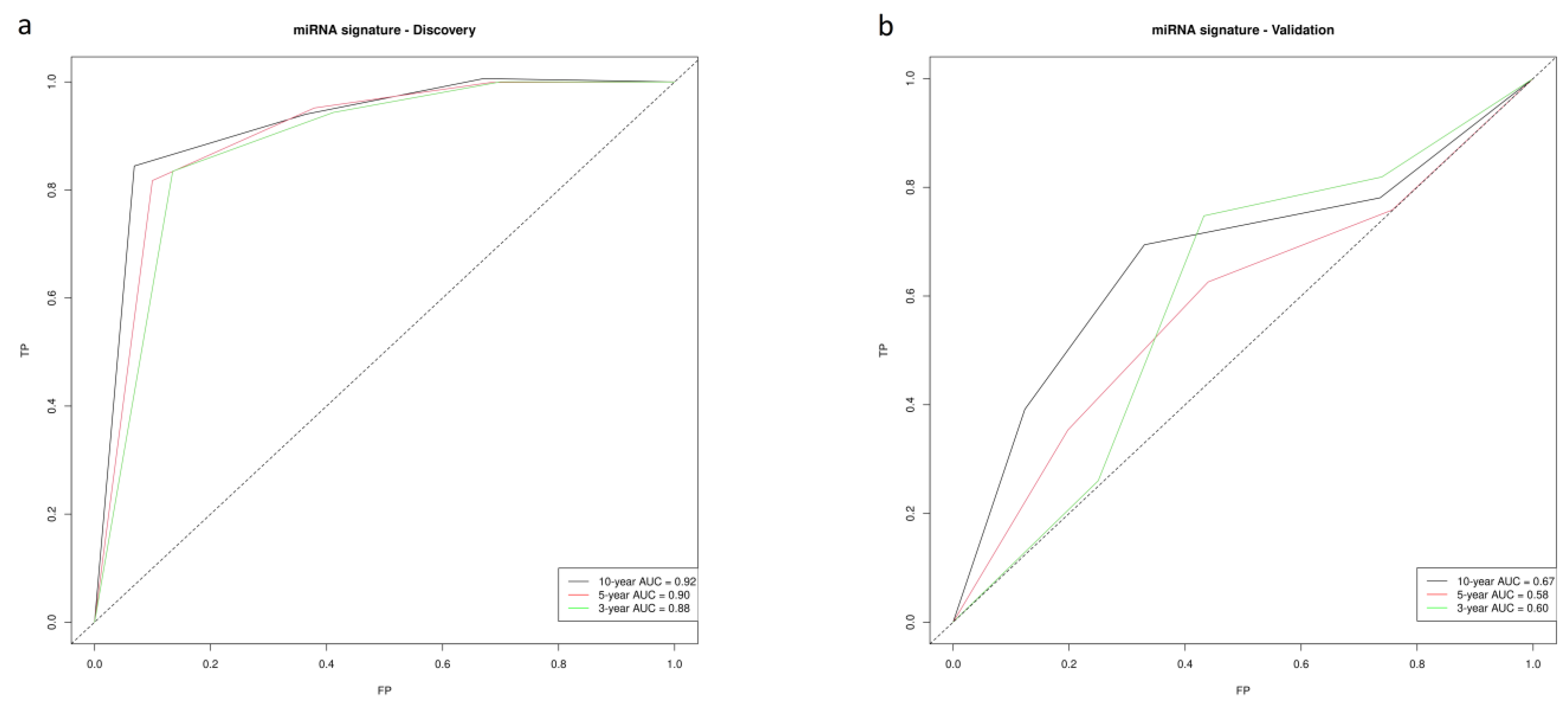
3.2. miRNA Signature and Score
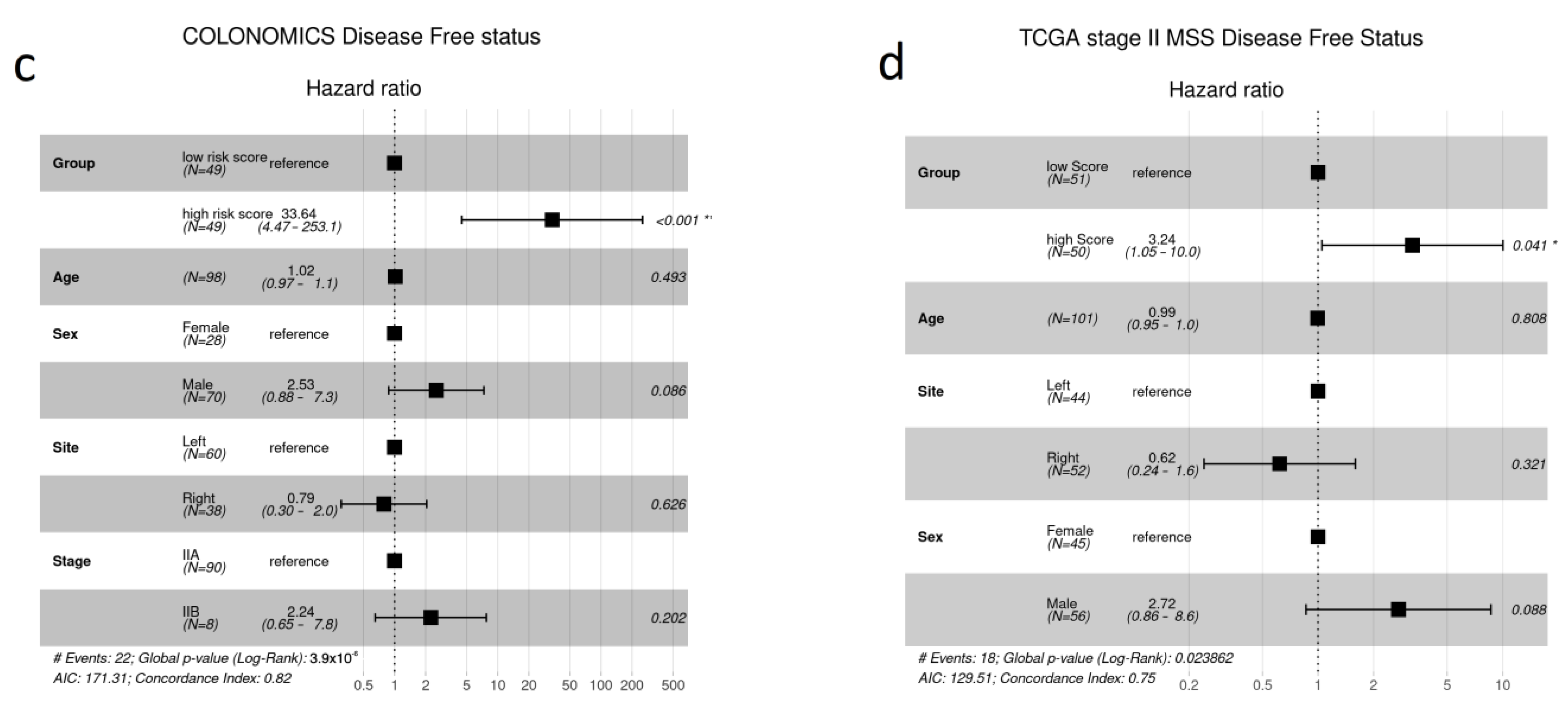
3.3. Validation
3.4. Functional Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Navarro, M.; Nicolas, A.; Ferrandez, A.; Lanas, A. Colorectal Cancer Population Screening Programs Worldwide in 2016: An Update. World J. Gastroenterol. 2017, 23, 3632. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- AJCC Cancer Staging Manual|SpringerLink. Available online: https://link.springer.com/book/9783319406176 (accessed on 4 January 2023).
- Guo, W.; Cai, Y.; Liu, X.; Ji, Y.; Zhang, C.; Wang, L.; Liao, W.; Liu, Y.; Cui, N.; Xiang, J.; et al. Single-Exosome Profiling Identifies ITGB3+ and ITGAM+ Exosome Subpopulations as Promising Early Diagnostic Biomarkers and Therapeutic Targets for Colorectal Cancer. Research 2023, 6, 0041. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.J.; Zavras, J.P.; Veziroglu, E.M.; Nasir-Moin, M.; Kolling, F.W.; Christensen, B.C.; Salas, L.A.; Barney, R.E.; Palisoul, S.M.; Ren, B.; et al. Identification of Spatial Proteomic Signatures of Colon Tumor Metastasis: A Digital Spatial Profiling Approach. Am. J. Pathol. 2023, 193, 778–795. [Google Scholar] [CrossRef]
- Ciocan, A.; Ciocan, R.A.; Al Hajjar, N.; Benea, A.M.; Pandrea, S.L.; Cătană, C.S.; Drugan, C.; Oprea, V.C.; Dîrzu, D.S.; Bolboacă, S.D. Exploratory Evaluation of Neopterin and Chitotriosidase as Potential Circulating Biomarkers for Colorectal Cancer. Biomedicines 2023, 11, 894. [Google Scholar] [CrossRef] [PubMed]
- Linke, C.; Hunger, R.; Reinwald, M.; Deckert, M.; Mantke, R. Quantification of Mitochondrial CfDNA Reveals New Perspectives for Early Diagnosis of Colorectal Cancer. BMC Cancer 2023, 23, 291. [Google Scholar] [CrossRef]
- Hosseini, F.A.; Rejali, L.; Zabihi, M.R.; Salehi, Z.; Daskar-Abkenar, E.; Taraz, T.; Fatemi, N.; Hashemi, M.; Asadzadeh-Aghdaei, H.; Nazemalhosseini-Mojarad, E. Long Non-coding RNA LINC00460 Contributes as a Potential Prognostic Biomarker through Its Oncogenic Role with ANXA2 in Colorectal Polyps. Mol. Biol. Rep. 2023, 50, 4505–4515. [Google Scholar] [CrossRef]
- Cortes-Ciriano, I.; Lee, S.; Park, W.-Y.; Kim, T.-M.; Park, P.J. A Molecular Portrait of Microsatellite Instability across Multiple Cancers. Nat. Commun. 2017, 8, 15180. [Google Scholar] [CrossRef]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive Biomarkers for Checkpoint Inhibitor-Based Immunotherapy. Lancet Oncol. 2016, 17, e542–e551. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- MacFarlane, L.-A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010, 11, 537–561. [Google Scholar] [CrossRef]
- Yu, W.; Liang, X.; Li, X.; Zhang, Y.; Sun, Z.; Liu, Y.; Wang, J. MicroRNA-195: A Review of Its Role in Cancers. OncoTargets Ther. 2018, 11, 7109–7123. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Sun, Y.; Yuan, Y.; Han, Z.; Zhang, P.; Zhang, J.; You, M.J.; Teruya-Feldstein, J.; Wang, M.; Gupta, S.; et al. MiR-100 Induces Epithelial-Mesenchymal Transition but Suppresses Tumorigenesis, Migration and Invasion. PLoS Genet. 2014, 10, e1004177. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Yang, H.; Bai, M.; Ning, T.; Deng, T.; Liu, R.; Fan, Q.; Zhu, K.; Li, J.; et al. Exosome-Delivered CircRNA Promotes Glycolysis to Induce Chemoresistance through the MiR-122-PKM2 Axis in Colorectal Cancer. Mol. Oncol. 2020, 14, 539–555. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, L.; Shan, N.; Ma, H.; Lu, S.; Chen, X.; Long, H. Establishing a Three-MiRNA Signature as a Prognostic Model for Colorectal Cancer through Bioinformatics Analysis. Aging 2021, 13, 19894–19907. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhao, Y.; He, M.; Zhao, H.; Zhang, Y.; Zhou, S.; Gao, M.; Di, D.; Wang, J.; Ding, J.; et al. Identifying a Ten-MicroRNA Signature as a Superior Prognosis Biomarker in Colon Adenocarcinoma. Cancer Cell Int. 2019, 19, 360. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Song, W.; Chen, Z.-H.; Wei, J.-H.; Liao, Y.-J.; Lei, J.; Hu, M.; Chen, G.-Z.; Liao, B.; Lu, J.; et al. Prognostic and Predictive Value of a MicroRNA Signature in Stage II Colon Cancer: A MicroRNA Expression Analysis. Lancet Oncol. 2013, 14, 1295–1306. [Google Scholar] [CrossRef]
- Slattery, M.L.; Herrick, J.S.; Mullany, L.E.; Valeri, N.; Stevens, J.; Caan, B.J.; Samowitz, W.; Wolff, R.K. An Evaluation and Replication of MiRNAs with Disease Stage and Colorectal Cancer-Specific Mortality. Int. J. Cancer 2015, 137, 428–438. [Google Scholar] [CrossRef] [PubMed]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M.; Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics. REporting Recommendations for Tumour MARKer Prognostic Studies (REMARK). Br. J. Cancer 2005, 93, 387–391. [Google Scholar] [CrossRef]
- Díez-Villanueva, A.; Sanz-Pamplona, R.; Solé, X.; Cordero, D.; Crous-Bou, M.; Guinó, E.; Lopez-Doriga, A.; Berenguer, A.; Aussó, S.; Paré-Brunet, L.; et al. COLONOMICS—Integrative Omics Data of One Hundred Paired Normal-Tumoral Samples from Colon Cancer Patients. Sci. Data 2022, 9, 595. [Google Scholar] [CrossRef]
- Sasson, A.; Michael, T.P. Filtering Error from SOLiD Output. Bioinformatics 2010, 26, 849–850. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. MiRBase: From MicroRNA Sequences to Function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- FASTX-Toolkit. Available online: http://hannonlab.cshl.edu/fastx_toolkit/ (accessed on 11 April 2023).
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet. J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve Years of SAMtools and BCFtools. GigaScience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Therneau, T. A Package for Survival Analysis in R. Available online: https://cran.r-project.org/web/packages/survival/vignettes/survival.pdf (accessed on 20 April 2023).
- Simon, N.; Friedman, J.H.; Hastie, T.; Tibshirani, R. Regularization Paths for Cox’s Proportional Hazards Model via Coordinate Descent. J. Stat. Softw. 2011, 39, 1–13. [Google Scholar] [CrossRef] [PubMed]
- R core team. R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 25 April 2023).
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The CBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the CBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. MiRDB: An Online Database for Prediction of Functional MicroRNA Targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, X. Prediction of Functional MicroRNA Targets by Integrative Modeling of MicroRNA Binding and Target Expression Data. Genome Biol. 2019, 20, 18. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Lin, Y.-C.-D.; Cui, S.; Huang, Y.; Tang, Y.; Xu, J.; Bao, J.; Li, Y.; Wen, J.; Zuo, H.; et al. MiRTarBase Update 2022: An Informative Resource for Experimentally Validated MiRNA-Target Interactions. Nucleic Acids Res. 2022, 50, D222–D230. [Google Scholar] [CrossRef]
- Csardi, G.; Nepusz, T. The Igraph Software Package for Complex Network Research. InterJ. Complex Syst. 2005, 1695, 1–9. [Google Scholar]
- Yu, G.; He, Q.-Y. ReactomePA: An R/Bioconductor Package for Reactome Pathway Analysis and Visualization. Mol. Biosyst. 2016, 12, 477–479. [Google Scholar] [CrossRef]
- Becht, E.; Giraldo, N.A.; Lacroix, L.; Buttard, B.; Elarouci, N.; Petitprez, F.; Selves, J.; Laurent-Puig, P.; Sautès-Fridman, C.; Fridman, W.H.; et al. Estimating the Population Abundance of Tissue-Infiltrating Immune and Stromal Cell Populations Using Gene Expression. Genome Biol. 2016, 17, 218. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xie, X.; Jiang, H. Establishment of a 7-MicroRNA Prognostic Signature and Identification of Hub Target Genes in Colorectal Carcinoma. Transl. Cancer Res. 2022, 11, 367. [Google Scholar] [CrossRef]
- Sanz-Pamplona, R.; Berenguer, A.; Cordero, D.; Riccadonna, S.; Solé, X.; Crous-Bou, M.; Guinó, E.; Sanjuan, X.; Biondo, S.; Soriano, A.; et al. Clinical Value of Prognosis Gene Expression Signatures in Colorectal Cancer: A Systematic Review. PLoS ONE 2012, 7, e48877. [Google Scholar] [CrossRef]
- Yang, Z.; Lu, S.; Wang, Y.; Tang, H.; Wang, B.; Sun, X.; Qu, J.; Rao, B. A Novel Defined Necroptosis-Related MiRNAs Signature for Predicting the Prognosis of Colon Cancer. Int. J. Gen. Med. 2022, 15, 555–565. [Google Scholar] [CrossRef]
- Jacob, H.; Stanisavljevic, L.; Storli, K.E.; Hestetun, K.E.; Dahl, O.; Myklebust, M.P. A Four-MicroRNA Classifier as a Novel Prognostic Marker for Tumor Recurrence in Stage II Colon Cancer. Sci. Rep. 2018, 8, 6157. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xu, X.; Ke, H.; Pan, X.; Ai, J.; Xie, R.; Lan, G.; Hu, Y.; Wu, Y. MicroRNA-16-5p Suppresses Cell Proliferation and Angiogenesis in Colorectal Cancer by Negatively Regulating Forkhead Box K1 to Block the PI3K/Akt/MTOR Pathway. Eur. J. Histochem. EJH 2022, 66, 3333. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Lin, Z.; Han, S.; Wei, Y.; Lu, G.; Zhang, Y.; Xiao, W.; Wang, Z.; Jia, X.; Gong, W. Low MiR-16 Expression Induces Regulatory CD4+NKG2D+ T Cells Involved in Colorectal Cancer Progression. Am. J. Cancer Res. 2021, 11, 1540–1556. [Google Scholar] [PubMed]
- Liu, D.; Bi, X.; Yang, Y. Circular RNA Hsa_circ_0011324 Is Involved in Endometrial Cancer Progression and the Evolution of Its Mechanism. Bioengineered 2022, 13, 7485–7499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gao, S.; Bao, Z.; Pan, C.; Tian, Q.; Tang, Q. MicroRNA-656-3p Inhibits Colorectal Cancer Cell Migration, Invasion, and Chemo-Resistance by Targeting Sphingosine-1-Phosphate Phosphatase 1. Bioengineered 2022, 13, 3810–3826. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Yin, Y.; Jin, G.; Li, D.; Li, M.; Hu, Y.; Feng, Y.; Liu, Y.; Bian, Z.; Wang, X.; et al. Exosome-Mediated Delivery of MiR-204-5p Inhibits Tumor Growth and Chemoresistance. Cancer Med. 2020, 9, 5989–5998. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Jin, L.; Zhang, J.; Yin, Y.; Quan, C.; Hu, Y.; Feng, Y.; Liu, H.; Fei, B.; Mao, Y.; et al. LncRNA—UCA1 Enhances Cell Proliferation and 5-Fluorouracil Resistance in Colorectal Cancer by Inhibiting MiR-204-5p. Sci. Rep. 2016, 6, 23892. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, W.; Jiang, X.; Li, H. MicroRNA-935 Acts as a Prognostic Marker and Promotes Cell Proliferation, Migration, and Invasion in Colorectal Cancer. Cancer Biomark. 2019, 26, 229–237. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, S.; Zhang, C.; Li, P.; Mao, B.; Guan, X.; Zhou, W.; Peng, J.; Wang, X.; Li, S.; et al. MicroRNA-935 Directly Targets FZD6 to Inhibit the Proliferation of Human Glioblastoma and Correlate to Glioma Malignancy and Prognosis. Front. Oncol. 2021, 11, 566492. [Google Scholar] [CrossRef]
- Yang, M.; Cui, G.; Ding, M.; Yang, W.; Liu, Y.; Dai, D.; Chen, L. MiR-935 Promotes Gastric Cancer Cell Proliferation by Targeting SOX7. Biomed. Pharmacother. Biomed. Pharmacother. 2016, 79, 153–158. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.R.C.P.; Castanhole-Nunes, M.M.U.; Biselli-Chicote, P.M.; Pavarino, É.C.; da Silva, R.C.M.A.; da Silva, R.F.; Goloni-Bertollo, E.M. Differential Expression of Angiogenesis-Related MiRNAs and VEGFA in Cirrhosis and Hepatocellular Carcinoma. Arch. Med. Sci. AMS 2020, 16, 1150–1157. [Google Scholar] [CrossRef]
- Yerukala Sathipati, S.; Tsai, M.-J.; Shukla, S.K.; Ho, S.-Y.; Liu, Y.; Beheshti, A. MicroRNA Signature for Estimating the Survival Time in Patients with Bladder Urothelial Carcinoma. Sci. Rep. 2022, 12, 4141. [Google Scholar] [CrossRef] [PubMed]
- Plata-Bello, J.; Fariña-Jerónimo, H.; Betancor, I.; Salido, E. High Expression of FOXP2 Is Associated with Worse Prognosis in Glioblastoma. World Neurosurg. 2021, 150, e253–e278. [Google Scholar] [CrossRef]
- Luedemann, C.; Reinersmann, J.-L.; Klinger, C.; Degener, S.; Dreger, N.M.; Roth, S.; Kaufmann, M.; Savelsbergh, A. Prostate Cancer-Associated MiRNAs in Saliva: First Steps to an Easily Accessible and Reliable Screening Tool. Biomolecules 2022, 12, 1366. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xiang, J.; Li, Z.; Lu, S.; Hu, J.; Gao, X.; Yu, L.; Wang, L.; Wang, J.; Wu, Y.; et al. A Plasma MicroRNA Panel for Early Detection of Colorectal Cancer. Int. J. Cancer 2015, 136, 152–161. [Google Scholar] [CrossRef]
- Chen, Z.; Shen, Z.; Zhang, Z.; Zhao, D.; Xu, L.; Zhang, L. RNA-Associated Co-Expression Network Identifies Novel Biomarkers for Digestive System Cancer. Front. Genet. 2021, 12, 659788. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.-J.; Ye, B.-L.; Zheng, Z.-H.; Li, S.-T.; Zheng, X.-F.; Zhang, S.-Z. TGFβ1I1 Suppressed Cell Migration and Invasion in Colorectal Cancer by Inhibiting the TGF-β Pathway and EMT Progress. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7294–7302. [Google Scholar] [CrossRef]
- Amirkhah, R.; Schmitz, U.; Linnebacher, M.; Wolkenhauer, O.; Farazmand, A. MicroRNA-MRNA Interactions in Colorectal Cancer and Their Role in Tumor Progression. Genes Chromosomes Cancer 2015, 54, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, J.; Zhang, Y.; Cheng, Y.; Wu, Z.; Zhan, W.; Deng, Y. Identification of Immune Cell Infiltration Landscape for Predicting Prognosis of Colorectal Cancer. Gastroenterol. Rep. 2023, 11, goad014. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wang, K.; Zhou, H.; Peng, L.; You, W.; Fu, Z. Profiles of Immune Infiltration in Colorectal Cancer and Their Clinical Significant: A Gene Expression-Based Study. Cancer Med. 2018, 7, 4496–4508. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The Immune Contexture in Human Tumours: Impact on Clinical Outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Teng, M.W.L.; Ngiow, S.F.; Ribas, A.; Smyth, M.J. Classifying Cancers Based on T-Cell Infiltration and PD-L1. Cancer Res. 2015, 75, 2139–2145. [Google Scholar] [CrossRef]
- Potocki, P.M.; Wójcik, P.; Chmura, Ł.; Goc, B.; Fedewicz, M.; Bielańska, Z.; Swadźba, J.; Konopka, K.; Kwinta, Ł.; Wysocki, P.J. Clinical Characterization of Targetable Mutations (BRAF V600E and KRAS G12C) in Advanced Colorectal Cancer-A Nation-Wide Study. Int. J. Mol. Sci. 2023, 24, 9073. [Google Scholar] [CrossRef]
- Ma, Z.; Qi, Z.; Gu, C.; Yang, Z.; Ding, Y.; Zhou, Y.; Wang, W.; Zou, Q. BRAFV600E Mutation Promoted the Growth and Chemoresistance of Colorectal Cancer. Am. J. Cancer Res. 2023, 13, 1486–1497. [Google Scholar] [PubMed]
- Mirzapoor Abbasabadi, Z.; Hamedi Asl, D.; Rahmani, B.; Shahbadori, R.; Karami, S.; Peymani, A.; Taghizadeh, S.; Samiee Rad, F. KRAS, NRAS, BRAF, and PIK3CA Mutation Rates, Clinicopathological Association, and Their Prognostic Value in Iranian Colorectal Cancer Patients. J. Clin. Lab. Anal. 2023, 37, e24868. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Ye, L.; Wang, D.; Han, L.; Zhou, S.; Wang, H.; Dai, W.; Wang, Y.; Luo, W.; Wang, R.; et al. Early Detection of Molecular Residual Disease and Risk Stratification for Stage I to III Colorectal Cancer via Circulating Tumor DNA Methylation. JAMA Oncol. 2023, 9, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Debelius, J.W.; Engstrand, L.; Matussek, A.; Brusselaers, N.; Morton, J.T.; Stenmarker, M.; Olsen, R.S. The Local Tumor Microbiome Is Associated with Survival in Late-Stage Colorectal Cancer Patients. Microbiol. Spectr. 2023, 11, e0506622. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, J.; Ma, Y.; Liu, J.; Cui, Y.; Yuan, Y.; Xiang, C.; Ma, D.; Liu, H. The Microbiome Types of Colorectal Tissue Are Potentially Associated with the Prognosis of Patients with Colorectal Cancer. Front. Microbiol. 2023, 14, 1100873. [Google Scholar] [CrossRef]
- Kamal, Y.; Dwan, D.; Hoehn, H.J.; Sanz-Pamplona, R.; Alonso, M.H.; Moreno, V.; Cheng, C.; Schell, M.J.; Kim, Y.; Felder, S.I.; et al. Tumor Immune Infiltration Estimated from Gene Expression Profiles Predicts Colorectal Cancer Relapse. Oncoimmunology 2021, 10, 1862529. [Google Scholar] [CrossRef]


| Colonomics n (%) | TCGA n (%) | |
|---|---|---|
| Number of Patients | 98 | 130 |
| Gender | ||
| Male | 70 (71.43%) | 69 (53.08%) |
| Female | 28 (28.57%) | 61 (46.92%) |
| Median Age (Years) | 71 | 69 |
| Tumor Site | ||
| Right | 38 (38.78%) | 75 (57.69%) |
| Left | 60 (61.22%) | 50 (38.46%) |
| Stage | ||
| II-A | 90 (91.84%) | 99 (76.15%) |
| II-B | 8 (8.16%) | 6 (4.62%) |
| Disease-Free Survival | ||
| No Event | 76 (77.55%) | 104 (80.00%) |
| Event | 22 (22.45%) | 26 (20.00%) |
| Microsatellite Instability | ||
| MSS | 98 (100%) | 101 (77.69%) |
| MSI | 0 (0%) | 20 (15.38%) |
| Median Metastatic Lymph Nodes | 0 (100%) | 0 (100%) |
| Median Isolated Lymph Nodes | 18.5 | 20.0 |
| Lymphatic Invasion | ||
| Yes | 7 (0.07%) | 26 (20.00%) |
| No | 86 (87.76%) | 92 (70.77%) |
| Perineural Invasion | ||
| Yes | 2 (2.04%) | 13 (10.00%) |
| No | 83 (84.69%) | 38 (29.23%) |
| miRNA | Coefficient |
|---|---|
| hsa-miR-1185-5p | −0.185 |
| hsa-miR-16-5p | −0.111 |
| hsa-miR-181a-2-3p | 0.181 |
| hsa-miR-204-5p | 0.003 |
| hsa-miR-2355-3p | 0.242 |
| hsa-miR-29b-2-5p | −0.306 |
| hsa-miR-331-3p | 0.153 |
| hsa-miR-423-3p | −0.355 |
| hsa-miR-432-5p | −0.187 |
| hsa-miR-497-5p | −0.183 |
| hsa-miR-656-3p | −0.526 |
| hsa-miR-935 | −0.136 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moratalla-Navarro, F.; Díez-Villanueva, A.; Garcia-Serrano, A.; Closa, A.; Cordero, D.; Solé, X.; Guinó, E.; Sanz-Pamplona, R.; Sanjuan, X.; Santos, C.; et al. Identification of a Twelve-microRNA Signature with Prognostic Value in Stage II Microsatellite Stable Colon Cancer. Cancers 2023, 15, 3301. https://doi.org/10.3390/cancers15133301
Moratalla-Navarro F, Díez-Villanueva A, Garcia-Serrano A, Closa A, Cordero D, Solé X, Guinó E, Sanz-Pamplona R, Sanjuan X, Santos C, et al. Identification of a Twelve-microRNA Signature with Prognostic Value in Stage II Microsatellite Stable Colon Cancer. Cancers. 2023; 15(13):3301. https://doi.org/10.3390/cancers15133301
Chicago/Turabian StyleMoratalla-Navarro, Ferran, Anna Díez-Villanueva, Ainhoa Garcia-Serrano, Adrià Closa, David Cordero, Xavier Solé, Elisabet Guinó, Rebeca Sanz-Pamplona, Xavier Sanjuan, Cristina Santos, and et al. 2023. "Identification of a Twelve-microRNA Signature with Prognostic Value in Stage II Microsatellite Stable Colon Cancer" Cancers 15, no. 13: 3301. https://doi.org/10.3390/cancers15133301
APA StyleMoratalla-Navarro, F., Díez-Villanueva, A., Garcia-Serrano, A., Closa, A., Cordero, D., Solé, X., Guinó, E., Sanz-Pamplona, R., Sanjuan, X., Santos, C., Biondo, S., Salazar, R., & Moreno, V. (2023). Identification of a Twelve-microRNA Signature with Prognostic Value in Stage II Microsatellite Stable Colon Cancer. Cancers, 15(13), 3301. https://doi.org/10.3390/cancers15133301








