The Role of Anesthetic Drugs and Statins in Prostate Cancer Recurrence: Starting at the Actual Knowledge and Walking through a New Paradigm
Abstract
Simple Summary
Abstract
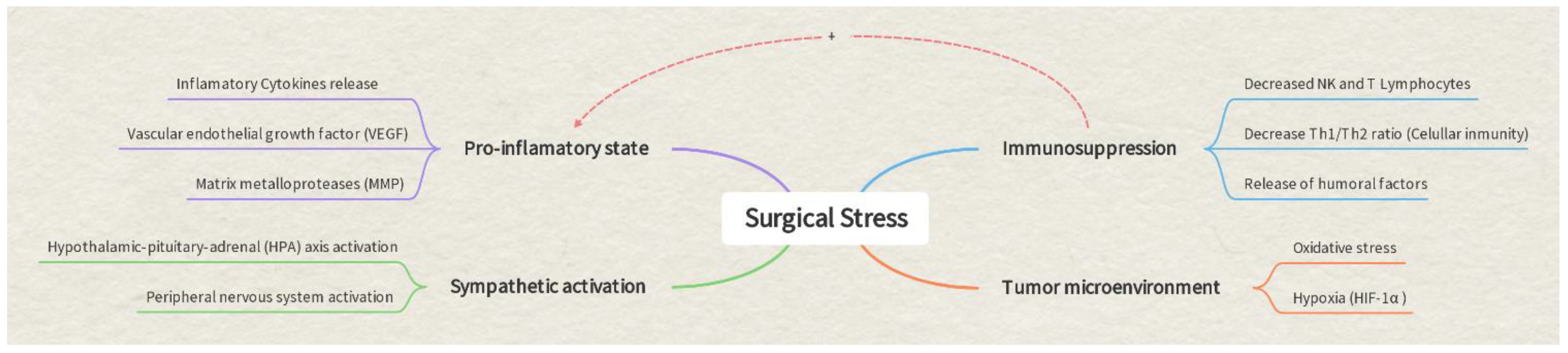
1. Introduction
2. Anesthesia and Prostate Cancer
2.1. Hypnotic Agents: Propofol and Halogenated Agents
2.2. Opioids
2.3. Regional Anesthesia
3. Statins
4. Discussion: Towards a New Paradigm
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cassinello, F.; Prieto, I.; del Olmo, M.; Rivas, S.; Strichartz, G.R. Cáncer surgery: How may anesthesia influence outcome? J. Clin. Anesth. 2017, 265, 22–72. [Google Scholar] [CrossRef] [PubMed]
- Coffey, J.C.; Wang, J.H.; Smith, M.J.; Bouchier-Hayes, D.; Cotter, T.G.; Redmond, H.P. Excisional surgery for cancer cure: Therapy at a cost. Lancet Oncol. 2003, 4, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Wu, M.-Y.; Chien, Y.-J.; Su, I.-M.; Wang, S.-C.; Kao, M.-C. Anesthesia and Long-term Oncological Outcomes: A Systematic Review and Meta-analysis. Anesth. Analg. 2021, 132, 623–634. [Google Scholar] [CrossRef]
- Kinoshita, T.; Goto, T. Links between Inflammation and Postoperative Cancer Recurrence. J. Clin. Med. 2021, 10, 228. [Google Scholar] [CrossRef]
- Onuma, A.E.; Zhang, H.; Gil, L.; Huang, H.; Tsung, A. Surgical Stress Promotes Tumor Progression: A Focus on the Impact of the Immune Response. J. Clin. Med. 2020, 9, 4096. [Google Scholar] [CrossRef]
- Zhang, H.; Kong, Q.; Wang, J.; Jiang, Y.; Hua, H. Complex roles of cAMP–PKA–CREB signaling in cancer. Exp. Hematol. Oncol. 2020, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Wall, T.; Sherwin, A.; Ma, D.; Buggy, D. Influence of perioperative anaesthetic and analgesic interventions on oncological outcomes: A narrative review. Br. J. Anaesth. 2019, 123, 135–150. [Google Scholar] [CrossRef]
- Murdoch, C.; Muthana, M.; Coffelt, S.B.; Lewis, C.E. The role of myeloid cells in the promotion of tumour angiogenesis. Nat. Rev. Cancer 2008, 8, 618–631. [Google Scholar] [CrossRef]
- Chang, S.-H.; Liu, C.H.; Conway, R.; Han, D.K.; Nithipatikom, K.; Trifan, O.C.; Lane, T.F.; Hla, T. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc. Natl. Acad. Sci. USA 2003, 101, 591–596. [Google Scholar] [CrossRef]
- Choi, H.; Hwang, W. Perioperative Inflammatory Response and Cancer Recurrence in Lung Cancer Surgery: A Narrative Review. Front. Surg. 2022, 9, 888630. [Google Scholar] [CrossRef]
- Müller-Edenborn, B.; Roth-Z’Graggen, B.; Bartnicka, K.; Borgeat, A.; Hoos, A.; Borsig, L.; Beck-Schimmer, B. Volatile anesthetics reduce invasion of colorectal cancer cells through down-regulation of matrix metalloproteinase-9. Anesthesiology 2012, 117, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Lebret, T.; Compérat, E.M.; Cowan, N.C.; De Santis, M.; Bruins, H.M.; Hernández, V.; Espinós, E.L.; Dunn, J.; Rouanne, M.; et al. Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur. Urol. 2016, 71, 462–475. [Google Scholar] [CrossRef]
- Ploussard, G.; Shariat, S.F.; Dragomir, A.; Kluth, L.A.; Xylinas, E.; Masson-Lecomte, A.; Rieken, M.; Rink, M.; Matsumoto, K.; Kikuchi, E.; et al. Conditional Survival After Radical Cystectomy for Bladder Cancer: Evidence for a Patient Changing Risk Profile over Time. Eur. Urol. 2013, 66, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Enlund, M.; Berglund, A.; Andreasson, K.; Cicek, C.; Enlund, A.; Bergkvist, L. The choice of anaesthetic—Sevoflurane or propofol—And outcome from cancer surgery: A retrospective analysis. Upsala J. Med. Sci. 2014, 119, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Whittington, R.; Malkowicz, S.B.; Schultz, D.; Blank, K.; Broderick, G.A.; Tomaszewski, J.E.; Renshaw, A.A.; Kaplan, I.; Beard, C.J.; et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998, 280, 969–974. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, B.; Zha, Z.; Zhao, H.; Jiang, Y.; Yuan, J. Positive surgical margin is associated with biochemical recurrence risk following radical prostatectomy: A meta-analysis from high-quality retrospective cohort studies. World J. Surg. Oncol. 2018, 16, 124. [Google Scholar] [CrossRef]
- Rangel, F.P.; Auler, J.O.; Carmona, M.J.; Cordeiro, M.D.; Nahas, W.C.; Coelho, R.F.; Simões, C.M. Opioids and premature biochemical recurrence of prostate cancer: A randomised prospective clinical trial. Br. J. Anaesth. 2021, 126, 931–939. [Google Scholar] [CrossRef]
- Kim, N.Y.; Jang, W.S.; Choi, Y.D.; Hong, J.H.; Noh, S.; Yoo, Y.C. Comparison of Biochemical Recurrence After Robot-assisted Laparoscopic Radical Prostatectomy with Volatile and Total Intravenous Anesthesia. Int. J. Med. Sci. 2020, 17, 449–456. [Google Scholar] [CrossRef]
- Orriach, J.L.G.; Ponferrada, A.R.; Manso, A.M.; Imbroda, B.H.; Belmonte, J.J.E.; Aliaga, M.R.; Fernandez, A.R.; Crespo, J.D.; Perez, A.M.S.; Heredia, A.F.; et al. Anesthesia in Combination with Propofol Increases Disease-Free Survival in Bladder Cancer Patients Who Undergo Radical Tumor Cystectomy as Compared to Inhalational Anesthetics and Opiate-Based Analgesia. Oncology 2020, 98, 161–167. [Google Scholar] [CrossRef]
- Choi, W.-J.; Baek, S.; Joo, E.-Y.; Yoon, S.-H.; Kim, E.; Hong, B.; Hwang, J.-H.; Kim, Y.-K. Comparison of the effect of spinal anesthesia and general anesthesia on 5-year tumor recurrence rates after transurethral resection of bladder tumors. Oncotarget 2017, 8, 87667–87674. [Google Scholar] [CrossRef] [PubMed]
- Exadaktylos, A.K.; Buggy, D.J.; Moriarty, D.C.; Mascha, E.; Sessler, D.I. Can Anesthetic Technique for Primary Breast Cancer Surgery Affect Recurrence or Metastasis? Anesthesiology 2006, 105, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Hasselager, R.P.; Hallas, J.; Gögenur, I. Inhalation or total intravenous anaesthesia and recurrence after colorectal cancer surgery: A propensity score matched Danish registry-based study. Br. J. Anaesth. 2020, 126, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Benzonana, L.L.; Perry, N.J.; Watts, H.R.; Yang, B.; Perry, I.A.; Coombes, C.; Takata, M.; Ma, D. Faculty Opinions recommendation of Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology 2013, 119, 593–605. [Google Scholar] [CrossRef]
- Reis, S.T.; Leite, K.R.M.; Piovesan, L.F.; Pontes-Junior, J.; Viana, N.I.; Abe, D.K.; Crippa, A.; Moura, C.M.; Adonias, S.P.; Srougi, M.; et al. Increased expression of MMP-9 and IL-8 are correlated with poor prognosis of Bladder Cancer. BMC Urol. 2012, 12, 18. [Google Scholar] [CrossRef]
- Chen, X.; Du, Y.; Lin, X.; Qian, Y.; Zhou, T.; Huang, Z. CD4 + CD25 + regulatory T cells in tumor immunity. Int. Immunopharmacol. 2016, 34, 244–249. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Geessink, F.J.; Ritskes-Hoitinga, M.; Scheffer, G.J. A systematic reviewand meta-analysis of the ability of analgesic drugs to reduce metastasis in experimental cancer models. Pain 2015, 156, 1835–1844. [Google Scholar]
- Zhang, X.; Li, F.; Zheng, Y.; Wang, X.; Wang, K.; Yu, Y.; Zhao, H. Propofol Reduced Mammosphere Formation of Breast Cancer Stem Cells via PD-L1/Nanog In Vitro. Oxidative Med. Cell. Longev. 2019, 2019, 9078209. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Chien, Y.-J.; Wu, M.-Y.; Kao, M.-C.; Wang, S.-C. Comment on “Anesthetic technique and cancer outcomes: A meta-analysis of total intravenous versus volatile anesthesia”. Can. J. Anaesth. 2019, 67, 150–151. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, Y.; Huang, L.; Zhang, F.; Kang, R. Effects of propofol on cancer development and chemotherapy: Potential mechanisms. Eur. J. Pharmacol. 2018, 831, 46–51. [Google Scholar] [CrossRef]
- Tatsumi, K.; Hirotsu, A.; Daijo, H.; Matsuyama, T.; Terada, N.; Tanaka, T. Effect of propofol on androgen receptor activity in prostate cancer cells. Eur. J. Pharmacol. 2017, 809, 242–252. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Zhu, Z.; Zheng, Y.; Song, B. Retracted: Propofol inhibits proliferation, migration and invasion of gastric cancer cells by up-regulating microRNA-195. Int. J. Biol. Macromol. 2018, 120, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Shen, S.; Chen, W.; Chen, N. Propofol Reversed Hypoxia-Induced Docetaxel Resistance in Prostate Cancer Cells by Preventing Epithelial–Mesenchymal Transition by Inhibiting Hypoxia-Inducible Factor 1α. BioMed Res. Int. 2018, 2018, 4174232. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Benzonana, L.L.; Zhao, H.; Watts, H.R.; Perry, N.J.S.; Bevan, C.; Brown, R.; Ma, D. Prostate cancer cell malignancy via modulation of HIF-1αpathway with isoflurane and propofol alone and in combination. Br. J. Cancer 2014, 111, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qin, J.; Gong, C.; Yang, J. Propofol enhanced the cell sensitivity to paclitaxel (PTX) in prostatic cancer (PC) through modulation of HOTAIR. Genes Genom. 2021, 43, 807–814. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Derise, O.C.; Sheppard, A.J.; Miriyala, S.; Virgen, C.G.; Kaye, A.J.; Niakan, M.; Cornett, E.M.; Kaye, A.D. The Influence of Analgesic Modalities on Postoperative Cancer Recurrence. Anesthesiol. Pain Med. 2022, 12, 123463. [Google Scholar] [CrossRef]
- McDonald, J.; Lambert, D. Opioid receptors. BJA Educ. 2015, 15, 219. [Google Scholar] [CrossRef]
- Yamamizu, K.; Hamada, Y.; Narita, M. κ Opioid receptor ligands regulate angiogenesis indevelopment and in tumours. Br. J. Pharmacol. 2015, 172, 268. [Google Scholar] [CrossRef]
- Lennon, F.E.; Mirzapoiazova, T.; Mambetsariev, B.; Salgia, R.; Moss, J.; Singleton, P.A. Overexpressión of the μ-opioid receptor in human non-small cell lung cancer promotes Akt and mTOR activation, tumor growth, and metastasis. J. Am. Soc. Anesthesiol. 2012, 116, 857–867. [Google Scholar] [CrossRef]
- Singleton, P.; Mirzapoiazova, T.; Hasina, R.; Salgia, R.; Moss, J. Increased μ-opioid receptor expression in metastatic lung cancer. Br. J. Anaesth. 2014, 113, i103–i108. [Google Scholar] [CrossRef]
- Lec, P.M.; Lenis, A.T.; Golla, V.; Brisbane, W.; Shuch, B.; Garraway, I.P.; Reiter, R.E.; Chamie, K. The Role of Opioids and Their Receptors in Urological Malignancy: A Review. J. Urol. 2020, 204, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Biki, B.; Mascha, E.; Moriarty, D.C.; Fitzpatrick, J.M.; Sessler, D.I.; Buggy, D. Anesthetic Technique for Radical Prostatectomy Surgery Affects Cancer Recurrence. Anesthesiology 2008, 109, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Scavonetto, F.; Yeoh, T.Y.; Umbreit, E.C.; Weingarten, T.N.; Gettman, M.T.; Frank, I.; Boorjian, S.A.; Karnes, R.J.; Schroeder, D.R.; Rangel, L.J.; et al. Association between neuraxial analgesia, cancer progression, and mortality after radical prostatectomy: A large, retrospective matched cohort study. Br. J. Anaesth. 2013, 113, i95–i102. [Google Scholar] [CrossRef] [PubMed]
- Tsui, B.C.H.; Rashiq, S.; Schopflocher, D.; Murtha, A.; Broemling, S.; Pillay, J.; Finucane, B.T. Epidural anesthesia and cancer recurrence rates after radical prostatectomy. Can. J. Anaesth. 2009, 57, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Bakogeorgou, E.; Hatzoglou, A.; Damianaki, A.; Martin, P.-M.; Castanas, E. Opioid alkaloids and casomorphin peptides decrease the proliferation of prostatic cancer cell lines (LNCaP, PC3 and DU145) through a partial interaction with opioid receptors. Eur. J. Pharmacol. 1997, 335, 255–265. [Google Scholar] [CrossRef]
- Roiss, M.; Schiffmann, J.; Tennstedt, P.; Kessler, T.; Blanc, I.; Goetz, A.; Schlomm, T.; Graefen, M.; Reuter, D.A. Oncological long-term outcome of 4772 patients with prostate cancer undergoing radical prostatectomy: Does the anaesthetic technique matter? Eur. J. Surg. Oncol. 2014, 40, 1686–1692. [Google Scholar] [CrossRef]
- Wuethrich, P.Y.; Thalmann, G.N.; Studer, U.E.; Burkhard, F.C. Epidural Analgesia during Open Radical Prostatectomy Does Not Improve Long-Term Cancer-Related Outcome: A Retrospective Study in Patients with Advanced Prostate Cancer. PLoS ONE 2013, 8, e72873. [Google Scholar] [CrossRef]
- Ehdaie, B.; Sjoberg, D.D.; Dalecki, P.H.; Scardino, P.T.; Eastham, J.A.; Amar, D. Association of anesthesia technique for radical prostatectomy with biochemical recurrence: A retrospective cohort study. Can. J. Anaesth. 2014, 61, 1068–1074. [Google Scholar] [CrossRef]
- Xuan, W.; Zhao, H.; Hankin, J.; Chen, L.; Yao, S.; Ma, D. Local anesthetic bupivacaine induced ovarian and prostate cancer apoptotic cell death and underlying mechanisms in vitro. Sci. Rep. 2016, 6, 26277. [Google Scholar] [CrossRef] [PubMed]
- Lusty, A.J.; Hosier, G.W.; Koti, M.; Chenard, S.; Mizubuti, G.B.; Jaeger, M.; Siemens, D.R. Anesthetic technique and oncological outcomes in urology: A clinical practice review. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.M.; Ghotra, V.S.; Karam, J.A.; Hernandez, M.; Pratt, G.; Cata, J.P. Regional anesthesia/analgesia and the risk of cancer recurrence and mortality after prostatectomy: A meta-analysis. Pain Manag. 2015, 5, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Wuethrich, P.Y.; Hsu Schmitz, S.F.; Kessler, T.M.; Thalmann, G.N.; Studer, U.E.; Stueber, F.; Burkhard, F.C. Potential influence of the anesthetic technique used during open radical prostatectomy on prostate cancer-related outcome: A retrospective study. Anesthesiology 2010, 113, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Sessler, D.I.; Pei, L.; Huang, Y.; Fleischmann, E.; Marhofer, P.; Kurz, A.; Mayers, D.B.; Meyer-Treschan, T.A.; Grady, M.; Tan, E.Y.; et al. Recurrence of breast cancer after regional or general anaesthesia: A randomised controlled trial. Lancet 2019, 394, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, C.P. Prostatic cholesterol metabolism: Regulation and alteration. Prog. Clin. Biol. Res. 1981, 279–324. [Google Scholar]
- Hea, Y.O.; Eun, J.L.; Yoon, S.; Byung, H.C.; Kang, S.C.; Sung, J.H. Cholesterol level of lipid raft microdomains regulates apoptotic cell death in prostate cancer cells through EGFR-mediated Akt and ERK signal transduction. Prostate 2007, 67, 1061–1069. [Google Scholar]
- Yue, S.; Li, J.; Lee, S.Y.; Lee, H.J.; Shao, T.; Song, B.; Cheng, L.; Masterson, T.A.; Liu, X.; Ratliff, T.L.; et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014, 19, 393–406. [Google Scholar] [CrossRef]
- Clendening, J.W.; Pandyra, A.; Boutros, P.C.; El Ghamrasni, S.; Khosravi, F.; Trentin, G.A.; Martirosyan, A.; Hakem, A.; Hakem, R.; Jurisica, I.; et al. Dysregulation of the mevalonate pathway promotes transformation. Proc. Natl. Acad. Sci. USA 2010, 107, 15051–15056. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Ko, J.; Um, J.; Chinnathambi, A.; Alharbi, S.A.; Sethi, G.; Ahn, K.S. LDL cholesterol promotes the proliferation of prostate and pancreatic cancer cells by activating the STAT3 pathway. J. Cell. Physiol. 2021, 236, 5253–5264. [Google Scholar] [CrossRef]
- Ponferrada, A.R.; Orriach, J.L.G.; Ruiz, J.C.M.; Molina, S.R.; Luque, A.G.; Mañas, J.C. Breast Cancer and Anaesthesia: Genetic Influence. Int. J. Mol. Sci. 2021, 22, 7653. [Google Scholar] [CrossRef]
- Poynter, J.N.; Gruber, S.B.; Higgins, P.D.; Almog, R.; Bonner, J.D.; Rennert, H.S.; Low, M.; Greenson, J.K.; Rennert, G. Statins and the Risk of Colorectal Cancer. N. Engl. J. Med. 2005, 352, 2184–2192. [Google Scholar] [CrossRef]
- Kumar, A.; Riviere, P.; Luterstein, E.; Nalawade, V.; Vitzthum, L.; Sarkar, R.R.; Bryant, A.K.; Einck, J.P.; Mundt, A.J.; Murphy, J.D.; et al. Associations among statins, preventive care, and prostate cancer mortality. Prostate Cancer Prostatic Dis. 2020, 23, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.W.L.; Dimitroulakos, J.; Minden, M.D.; Penn, L.Z. HMG-CoA reductase inhibitors and the malignant cell: The statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 2002, 16, 508–519. [Google Scholar] [CrossRef]
- Murtola, T.J.; Tammela, T.L.; Lahtela, J.; Auvinen, A. Cholesterol-Lowering Drugs and Prostate Cancer Risk: A Population-based Case-Control Study. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2226–2232. [Google Scholar] [CrossRef] [PubMed]
- Murtola, T.J. Statins and biochemical recurrence after radical prostatectomy—Who benefits? BJU Int. Engl. 2014, 114, 634–635. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.; Eberg, M.; Benayoun, S.; Aprikian, A.; Batist, G.; Suissa, S.; Azoulay, L. Use of Statins and the Risk of Death in Patients with Prostate Cancer. J. Clin. Oncol. 2014, 32, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Allott, E.H.; Howard, L.E.; Cooperberg, M.R.; Kane, C.J.; Aronson, W.J.; Terris, M.K.; Amling, C.L.; Freedland, S.J. Postoperative statin use and risk of biochemical recurrence following radical prostatectomy: Results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. BJU Int. 2014, 114, 661–666. [Google Scholar] [CrossRef]
- Jeong, I.G.; Lim, B.; Yun, S.C.; Lim, J.H.; Hong, J.H.; Kim, C.S. Adjuvant low-dose statin use after radical prostatectomy: The PRO-STAT randomized clinical trial. Clin. Cancer Res. 2021, 27, 5004–5011. [Google Scholar] [CrossRef]
- Raval, A.D.; Thakker, D.; Negi, H.; Vyas, A.; Salkini, M.W. Association between statins and clinical outcomes among men with prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2016, 19, 151–162. [Google Scholar] [CrossRef]
- Jarimba, R.; Lima, J.P.; Eliseu, M.; Carvalho, J.; Antunes, H.; da Silva, E.T.; Moreira, P.; Figueiredo, A. Statins Prevent Biochemical Recurrence of Prostate Cancer After Radical Prostatectomy: A Single-center Retrospective Study with a Median Follow-up of 51.20 Months. Res. Rep. Urol. 2020, 12, 439–446. [Google Scholar] [CrossRef]
- Yin, P.; Han, S.; Hu, Q.; Tong, S. The association of statin use and biochemical recurrence after curative treatment for prostate cancer A systematic review and meta-analysis. Medicine 2022, 101, E28513. [Google Scholar] [CrossRef]
- Sun, J.-X.; Liu, C.-Q.; Zhong, X.-Y.; Xu, J.-Z.; An, Y.; Xu, M.-Y.; Hu, J.; Zhang, Z.-B.; Xia, Q.-D.; Wang, S.-G. Statin Use and the Risk of Prostate Cancer Biochemical Recurrence Following Definitive Therapy: A Systematic Review and Meta-Analysis of Cohort Studies. Front. Oncol. 2022, 12, 887854. [Google Scholar] [CrossRef] [PubMed]
- Sekhoacha, M.; Riet, K.; Motloung, P.; Gumenku, L.; Adegoke, A.; Mashele, S. Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules 2022, 27, 5730. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef] [PubMed]
- Palicelli, A.; Croci, S.; Bisagni, A.; Zanetti, E.; De Biase, D.; Melli, B.; Sanguedolce, F.; Ragazzi, M.; Zanelli, M.; Chaux, A.; et al. What Do We Have to Know about PD-L1 Expression in Prostate Cancer? A Systematic Literature Review. Part 3: PD-L1, Intracellular Signaling Pathways and Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 12330. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.X.; Ng, K.T.; Ang, E.; Wang, C.Y.; Shariffuddin, I.I.B. Effect of perioperative regional anesthesia on cancer recurrence: A meta-analysis of randomized controlled trials. Int. J. Surg. 2020, 82, 192–199. [Google Scholar] [CrossRef]
- Tseng, K.S.; Kulkarni, S.; Humphreys, E.B.; Carter, H.B.; Mostwin, J.L.; Partin, A.W.; Han, M.; Wu, C.L. Spinal anesthesia does not impact prostate cancer recurrence in a cohort of men undergoing radical prostatectomy: An observational study. Reg. Anesth. Pain Med. 2018, 39, 284–288. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24918335 (accessed on 21 November 2018). [CrossRef]
- Sprung, J.; Scavonetto, F.; Yeoh, T.Y.; Kramer, J.M.; Karnes, R.J.; Eisenach, J.H.; Weingarten, T.N.; Schroeder, D.R.; Eisenach, J.H.; Karnes, R.J.; et al. Outcomes after radical prostatectomy for cancer: A comparison between general anesthesia and epidural anesthesia with fentanyl analgesia: A matched cohort study. Anesth. Analg. 2019, 119, 859–866. [Google Scholar] [CrossRef]
- Filipów, S.; Laczmanski, L. Blood Circulating miRNAs as Cancer Biomarkers for Diagnosis and Surgical Treatment Response. Front. Genet. 2019, 10, 169. [Google Scholar] [CrossRef]
- Mirzaei, S.; Paskeh, M.D.A.; Okina, E.; Gholami, M.H.; Hushmandi, K.; Hashemi, M.; Kalu, A.; Zarrabi, A.; Nabavi, N.; Rabiee, N.; et al. Molecular Landscape of LncRNAs in Prostate Cancer: A focus on pathways and therapeutic targets for intervention. J. Exp. Clin. Cancer Res. 2022, 41, 214. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, F.; He, Q.; Li, G.; Ding, G. lncRNA UCA1 Functions as a ceRNA to Promote Prostate Cancer Progression via Sponging miR143. Mol. Ther. Nucleic Acids 2019, 19, 751–758. [Google Scholar] [CrossRef]
- Xing, P.; Wang, Y.; Zhang, L.; Ma, C.; Lu, J. Knockdown of lncRNA MIR44352HG and ST8SIA1 expression inhibits the proliferation, invasion and migration of prostate cancer cells in vitro and in vivo by blocking the activation of the FAK/AKT/β-catenin signaling pathway. Int. J. Mol. Med. 2021, 47, 93. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Iwasaki, M.; Sakamoto, A.; Ma, D. Anesthetics may modulate cancer surgical outcome: A possible role of miRNAs regulation. BMC Anesthesiol. 2021, 21, 71. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.J.S.; Buggy, D.; Ma, D. Can anesthesia influence Cancer outcomes after surgery? JAMA Surg. 2019, 154, 279–280. [Google Scholar] [CrossRef] [PubMed]
| Drug | Effect on Cancer | Mechanism of Action | Pathway Described |
|---|---|---|---|
| Propofol | Anti-Inflammatory Effect | COX-2 Inhibition | COX Pathway |
| Decreases the progression and development of metastases | Decreases HIF-1α expression (angiogenesis, glycolysis, proliferation) | HIF-1α | |
| Promotes apoptosis | MMP expression STAT 3 | STAT 3 | |
| Halogenated Agents | Decrease androgen receptor activation | Decrease the occurrence of castration-resistant prostate cancer | HOTAIR |
| Increase the expression of prometastatic and protumorigenic factors | Increase HIF-1α expression (angiogenesis, glycolysis, proliferation) | HIF-1α | |
| – | Increase HIF-2α expression | P13K/Akt/mTOR pathway | |
| Opioids | Promote angiogenesis and neovascularization | Src-mediated phosphorylation VEGF | MAPK/ERK pathway activation |
| Immunosuppressive effect | Suppress NK cell-mediated cytotoxicity | mU opioid receptor (MOR) | |
| Regional Anesthesia | Decrease surgical stress and sympathetic activation | Attenuates the cytokine changes caused by surgical stress | Increases IL-12 and IFNγ Decreases IL-6 and IL-10 |
| Statins | Decrease the expression of PTEN tumor suppressor gene | Upregulation of apoptotic pathways | PI3K-AKT-mTOR |
| Suppress the growth of tumor cells | Decrease EGFR and androgen receptor | AKT pathways JAK-SATA 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raigon Ponferrada, A.; Molina Ruiz, J.C.; Romero Molina, S.; Rodriguez Garcia, V.; Guerrero Orriach, J.L. The Role of Anesthetic Drugs and Statins in Prostate Cancer Recurrence: Starting at the Actual Knowledge and Walking through a New Paradigm. Cancers 2023, 15, 3059. https://doi.org/10.3390/cancers15113059
Raigon Ponferrada A, Molina Ruiz JC, Romero Molina S, Rodriguez Garcia V, Guerrero Orriach JL. The Role of Anesthetic Drugs and Statins in Prostate Cancer Recurrence: Starting at the Actual Knowledge and Walking through a New Paradigm. Cancers. 2023; 15(11):3059. https://doi.org/10.3390/cancers15113059
Chicago/Turabian StyleRaigon Ponferrada, Aida, Juan Carlos Molina Ruiz, Salvador Romero Molina, Verónica Rodriguez Garcia, and Jose Luis Guerrero Orriach. 2023. "The Role of Anesthetic Drugs and Statins in Prostate Cancer Recurrence: Starting at the Actual Knowledge and Walking through a New Paradigm" Cancers 15, no. 11: 3059. https://doi.org/10.3390/cancers15113059
APA StyleRaigon Ponferrada, A., Molina Ruiz, J. C., Romero Molina, S., Rodriguez Garcia, V., & Guerrero Orriach, J. L. (2023). The Role of Anesthetic Drugs and Statins in Prostate Cancer Recurrence: Starting at the Actual Knowledge and Walking through a New Paradigm. Cancers, 15(11), 3059. https://doi.org/10.3390/cancers15113059







