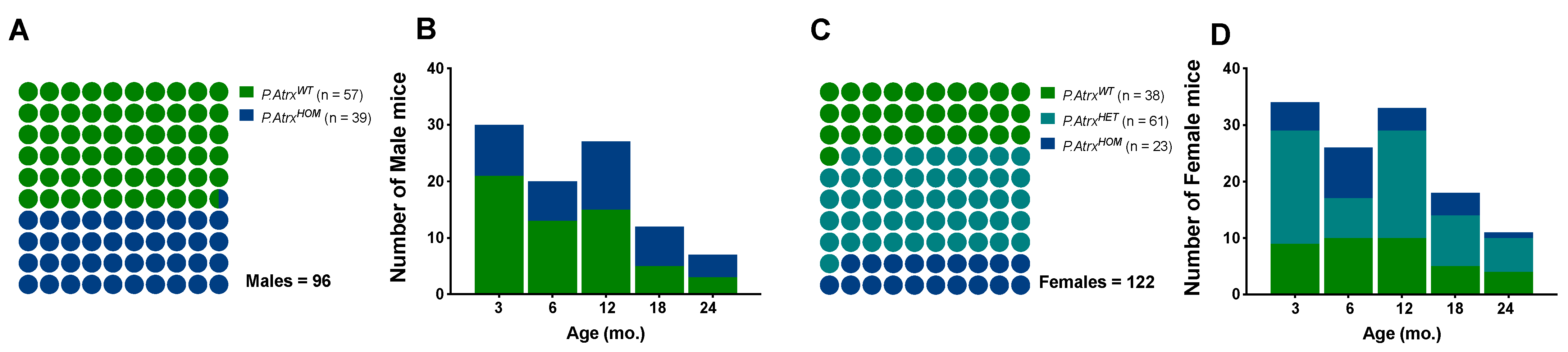Simple Summary
ATRX mutations occur in up to 17% of human pancreatic neuroendocrine tumours (PanNETs), and recent evidence points towards its inability to drive PanNET formation in mouse pancreas while predisposing individuals to inflammageing. Aiming to explore the additional non-tumourigenic consequences of Atrx deletion, we characterised an aged series of Atrx conditional disruption in β cells using the Pdx1 promoter. Homozygous mice (P.AtrxHOM) exhibited obesity, diabetes, glucose intolerance, and pancreatic adiposity at a higher extent than age- and sex-matched controls (P.AtrxWT).
Abstract
Atrx loss was recently ascertained as insufficient to drive pancreatic neuroendocrine tumour (PanNET) formation in mice islets. We have identified a preponderant role of Atrx in the endocrine dysfunction in a Rip-Cre;AtrxKO genetically engineered mouse model (GEMM). To validate the impact of a different Cre-driver line, we used similar methodologies and characterised the Pdx1-Cre;AtrxKO (P.AtrxKO) GEMM to search for PanNET formation and endocrine fitness disruption for a period of up to 24 months. Male and female mice presented different phenotypes. Compared to P.AtrxWT, P.AtrxHOM males were heavier during the entire study period, hyperglycaemic between 3 and 12 mo., and glucose intolerant only from 6 mo.; in contrast, P.AtrxHOM females started exhibiting increased weight gains later (after 6 mo.), but diabetes or glucose intolerance was detected by 3 mo. Overall, all studied mice were overweight or obese from early ages, which challenged the histopathological evaluation of the pancreas and liver, especially after 12 mo. Noteworthily, losing Atrx predisposed mice to an increase in intrapancreatic fatty infiltration (FI), peripancreatic fat deposition, and macrovesicular steatosis. As expected, no animal developed PanNETs. An obese diabetic GEMM of disrupted Atrx is presented as potentially useful for metabolic studies and as a putative candidate for inserting additional tumourigenic genetic events.
1. Introduction
The ATRX chromatin remodeller (ATRX) is an ATP-dependent helicase that belongs to the switch 2/sucrose non-fermentable 2 (SWI2/SNF2) family of chromatin remodelling proteins [1,2]. SWI2/SNF2 proteins participate in DNA recombination and repair mechanisms, nucleosome remodelling, and transcriptional regulation, amid other biological processes [2]. ATRX is a nuclear protein with a ubiquitous distribution, with the highest levels reported in the foetal brain implying a relevant role in brain development [3]. ATRX is also highly conserved between mice and humans (85% homology) [2]. The ATRX protein essentially contains three functional and highly conserved domains: (1) an ATPase/helicase C-terminal domain that provides DNA-dependent ATPase activity [4,5]; (2) an ATRX-DNMT3-DNMT3L (ADD) domain at the N-terminal [4,6], which is a plant homeodomain (PHD)-like zinc finger, structurally similar to DNA methyltransferases (DNMTs), that is involved in heterochromatin organisation, comprising three subdomains: a GATA-like, PHD-like, and a C-terminal α helix [4,7,8]; and (3) a centrally located binding domain for the death domain-associated protein (DAXX), essential for H3.3 deposition [9,10]. The multiple functions of ATRX have been recently reviewed in detail by Valenzuela et al. [5]. ATRX plays essential functions toward heterochromatin homeostasis [11] and recruits DAXX, forming a nuclear chaperone complex that facilitates the recruitment, deposition, and integration of the histone variant H3.3 at heterochromatic regions [12,13,14]. This H3.3 deposition ensures that the chromatin and DNA’s B-form conformation remain stable, which prevents the collapse of a stalled replication fork [15]. ATRX also has essential cellular functions independent of DAXX (e.g., genomic stability, regulation of gene expression, and the binding and resolution of quadruplex G- and C-rich repeats (G4 and i-Motifs, respectively) that could compromise genomic stability [4,5,16]. In addition, ATRX binds and regulates regions enriched for H3K9me3 via its ADD domain and HP1α [11].
Importantly, ATRX, DAXX, H3.3, and promyelocytic leukaemia (PML) nuclear bodies (PML-NBs) are responsible for the epigenetic silencing of transposable elements such as the endogenous retroviral elements (ERVs) in the mouse genome [10,17,18], as well as in the defence against viral infection [19,20,21]. It is plausible that the derepression of ERV silencing may occur upon loss of either of these genetic players, activating transposons and leading to the expression of endogenous genes with an impact on carcinogenesis [18].
In recent years, as we have witnessed the emergence of high-throughput sequencing techniques, ATRX and DAXX mutations have been reported in pancreatic neuroendocrine tumours (PanNETs) (10% and 20%, respectively) [22,23,24,25]. The prevalence of these mutations stresses their importance as putative tumour driver events. A correlation between mutations and the telomerase-independent mechanism of the alternative lengthening of telomeres (ALT) was also established [15,26], with particular importance to PanNETs [27,28,29] that rely on this telomere maintenance mechanism (TMM) twice as often (30%) as other overall tumour types (10–15%, on average) [30]. Losing ATRX or DAXX in addition to ALT positivity correlates with chromosome instability, higher tumour grading, and unfavourable prognosis in PanNET patients [27,28,29,31,32].
Genetically engineered mouse models (GEMMs) are the most powerful tool to study the PanNETs’ multistep tumourigenic pathway, regarding tumour initiation and progression, under an immune-competent phenotype [33]. Despite the increasing acceptance of the chromatin remodellers’ utility as prognostic markers in the context of human PanNETs, their specific contribution to PanNET tumourigenesis is still underexplored. The first GEMMs tackling the pancreas-specific functions of chromatin remodellers have appeared recently in the literature. Atrx and Daxx could not drive neuroendocrine tumour progression nor intensify the effect of Pten or Men1 homozygous deletion [18,34,35]. However, Atrx was relevant to the tumourigenesis of the exocrine pancreas, as the Mist1-Cre;KrasHOM;AtrxHOM (M.K.AtrxHOM) double-mutant mice developed precursor lesions sooner than if only losing Kras [36]. Atrx and Daxx also safeguard pancreatic tissue from inflammation [18,36]—as their loss resulted in an inability to restore tissue homeostasis following caerulein-induced pancreatitis—and endocrine fitness [34]—as Atrx loss caused increased weight gain and endocrine dysfunction. However, the mechanisms leading to the metabolic dysfunction are unknown and these very interesting and novel findings prompted us to validate our results with a distinctive approach.
During the last four years, we have been working on a GEMM of β cell-specific knockout (KO) of Atrx using the Pdx1-Cre system (P.Atrx). Rip-Cre lines, although broadly used, have been solidly linked to endocrine dysfunction [37]. Recently, aristaless-related homeobox (ARX) and pancreatic and duodenal homeobox 1 (PDX1), drivers of α and β cell differentiation, respectively, have been emerging as novel genetic signatures of human PanNET classification. We now know that the most altered endocrine cell population in ATRX/DAXX-mutated PanNETs harbours a typical landscape signature of α cell (ARX+/PDX1-) [38,39]. We and others have confirmed that Atrx is not a robust tumour suppressor in mouse endocrine pancreas by using Rip-Cre [34] and Pdx1-Cre [35]. Following our report on the involvement of Atrx in the homeostasis of the endocrine function using the Rip-Cre system, we decided to study for the first time, in a Pdx1-driven context, the impact of the Atrx disruption in the putative endocrine function impairment, as well as readdress its role in PanNET formation. By using a Cre-driver that is harmless to endocrine fitness, we are also circumventing the inherent phenotype of Rip-Cre in this context [37]. We verified that by using a different promoter, the impact of Atrx loss in endocrine dysfunction and weight gain became more pronounced. In this study, we present evidence of Atrx playing a role in endocrine homeostasis, with mice showing a diabetic state and pancreatic fat infiltration.
2. Materials and Methods
2.1. GEMM Generation
The Atrxy/f male and Atrxf/f female (floxed) mice were a kind donation from Douglas R. Higgs and backcrossed with C57BL/6 (B6). These mice carry a flanked Atrx gene with a floxed neor cassette inserted within intron 17, and loxP sites flanking exon 18 [40,41,42]. B6.FVB-Tg(Pdx1-Cre)6Tuv/J mice were obtained from the Jackson Laboratory [43] and crossed with the Atrx floxed mice to generate Pdx1-Cre+/−;Atrxy/wt and Pdx1-Cre+/−;Atrxwt/wt male and female controls (P.AtrxWT), Pdx1-Cre+/−;Atrxy/f and Pdx1-Cre+/−;Atrxf/f male and female homozygous individuals (P.AtrxHOM), and Pdx1-Cre+/−;Atrxf/wt heterozygous females (P.AtrxHET). In the absence of statistical differences, the P.AtrxHOM and P.AtrxHET genotypes were sometimes analysed together in the same genotype group (“P.AtrxKO”). Graphical representations of the P.AtrxWT, P.AtrxHET, P.AtrxHOM, and P.AtrxKO genotype groups are given by a different colour scheme (green, teal, ocean blue, and light blue). Animal genotyping was performed as previously reported (Supplementary File S1: Table S1) [34]. Mice were rederived once during the project to ensure the absence of disease-causing pathogens.
The in vivo studies were performed following the Portuguese National Regulation established by Decreto-Lei n.° 113/2013, the national transposition of the European Directive 2010/63/EU for the Care and Use of Laboratory Animals. Procedures were submitted for evaluation and approved by the i3S Animal Welfare and Ethics Review Body and the Portuguese National Authority for Animal Health (DGAV)-project license code 13020/2017-05-08. Procedures were carried out by FELASA C-certified operators (TBG and SM). Mice were bred and maintained at the animal facility of i3S, accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), under a standard 12 h light/dark cycle, with water and ad libitum chow diet (Tecklad Global Diet Rodent 2014S, Envigo, Indianapolis, IN, USA).
2.2. Animal Husbandry and Longitudinal Follow-Up
A cohort with ageing mice was followed for up to 24 months (mo.). During the project, animals were subjected to different and complementary in vivo manipulations; procedures, animal sampling, and the sex and genotype data acquired are presented in Supplementary File S1: Table S2, following the ARRIVE 2.0 checklist [44].
An equal strategy, as previously reported [34], at predetermined time points included data acquisitions for the following parameters: monthly longitudinal weighing, glycaemia evaluation, intraperitoneal glucose tolerance tests (ipGTTs), and hemograms. A longitudinal fashion of data collection was, when possible, favoured; for data analysis, both longitudinal and unpaired data were used at all times. Regarding the histopathological (HP) evaluation, euthanasia dates were distributed to represent of all age groups by genotypes, being composed of eight age groups (3, 6, 9, 12, 15, 18, 21, and 24 mo.) For data analyses, age formula and age range (calculated from dates of birth (DOB) and dates of procedure (DOP) or death (DOD)) were considered, as previously described [34]. No data were collected from animals that reached a humane endpoint (HEP). The HEP was defined as a gradual weight loss over three consecutive weighings, evidence of dehydration and lethargy, and reluctance to move when stimulated. The clinical evaluation of mice was performed by animal facility staff and the researcher T.B.G. In all the above-stated analyses, a single animal was our experimental unit. Sample size estimations were calculated in G*Power 3.1.9.2.
2.3. Euthanasia and Organ Collection
Euthanasia was conducted for organ collection and HP evaluations of all age groups. Generally, animals were euthanised via an IP injection of ketamine (150 mg/kg) and medetomidine (2 mg/kg), followed by death confirmation with cervical displacement. Exsanguination allowed blood collection for hemogram analysis and ELISA assay. The pancreas and liver were collected from all necropsied animals; pancreata were collected in the block with duodenum, stomach, spleen, and abdominal fat to preserve their physiological diffuse mesenteric distribution [45]. For each animals’ necropsy, a complete macroscopic form was created and disclosed other organs that exhibited pathological alteration.
2.4. Histopathological Evaluation and Immunohistochemistry Assays
Tissue fixation was prepared in 4% paraformaldehyde (pH 7.4) for 24 h and routinely processed in an automatic tissue processor. Fixed and paraffin-embedded tissue sections with a 4 µm thickness were obtained and stained with haematoxylin and eosin (H&E).
HP evaluation was conducted by human and veterinarian pathologists (T.B.G., I.B., and S.C) using a previously determined score [34]. The scoring system of inflammatory lesions in the pancreas included the following parameters: (1) oedema, (2) fibrosis, (3) loss of lobular pattern, (4) duct/vessel dilation, (5) focal acinar atrophy, (6) peripancreatic chronic inflammation (CI), (7) acinar CI, (8) periductal/perivascular (Pd/Pv) CI, (9) intra-/peri-islet CI, (10) intrapancreatic fatty infiltration (FI), and (11) peripancreatic fat deposition. Except for FI, the parameters mentioned above were evaluated in a four-level scoring system, from 0 (<5% altered) to 1 (low-grade lesion, 5–33% altered), 2 (moderate-grade lesion, 33–66% altered), or 3 (high-grade lesion, >66% altered); FI was classified as 0 (<5% altered), 1 (low-grade lesion, 5–10% altered), 2 (moderate-grade lesion, 10–20% altered), or 3 (high-grade lesion, >20% altered). The sum of the first nine parameters obtained the HP score, and the sums of the most prevalent parameters were evaluated separately, namely Pd/Pv CI, as previously described [34]. The parameters FI and FR were evaluated individually and summed. The presence of ductal dysplasia was also qualitatively determined; the sum of duct/vessel dilation, focal acinar atrophy, and ductal dysplasia obtained the ageing score.
Liver tissue was evaluated using the non-alcoholic fatty liver disease (NAFLD) activity score (NAS) as previously reported [46,47]. The following parameters were considered: (1) microvesicular steatosis, (2) macrovesicular steatosis, (3) hypertrophy, (4) lobular inflammatory foci (all lymphocytic foci except at portal location), (5) portal inflammation, (6) microgranulomas, and (7) oedema. All parameters were also evaluated as previously described [34], and macrovesicular steatosis was evaluated separately.
The differential diagnosis of tumour sections was evaluated using a panel of pan-cytokeratins, vimentin, and CD45 antibodies to discriminate the most probable phenotype of undifferentiated lesions. A list of antibodies and specifications of the respective IHC assays is presented in Supplementary File S1: Table S3.
2.5. Blood Collection and Hemogram Analyses
For the genotypes and age groups (3, 6, and 12 mo.), the hemograms analyses were performed from longitudinal blood collections and as previously described [34].
2.6. Glycaemia Assessment and Glucose Tolerance Tests
Glycaemia assessments were assessed by a unique operator (T.B.G.) according to the current guidelines [48]. Unpaired and longitudinal single glycaemia measurements and ipGTTs were performed in all genotype groups at 3, 6, and 12 mo., as previously described [34]. Glycaemia cut-off values were considered prediabetic when above 150 mg/dL and diabetic when above 240 mg/dL.
2.7. Endocrine Fraction Evaluation
H&E-stained slides scanned at 40× magnification (226 nm/pixel resolution) in NanoZoomer S60 (Hamamatsu, Hamamatsu, Japan) were evaluated by a deep learning algorithm trained in HALO® Image Analysis Platform version 3.5.3577 (Indica Labs Inc., Albuquerque, NM, USA). The algorithm was trained to segment pancreatic islets, the exocrine portion, and the lymphocytic infiltration. Representative images of the markups containing the ‘endocrine’, ‘exocrine’, and ‘lymphocyte’ classes are available in Supplementary File S1: Figure S1. The segmentation output was post-processed in Fiji [49], and a set of measurements was extracted for the morphological characterisation of pancreatic islets, as previously reported [34]. Male and female mice were analysed together, and the results were organised by four age groups (3, 6, 12, and 18–24 mo.).
2.8. ELISA Immunoassay
Serum insulin quantification was performed using the Mouse Insulin ELISA Kit (RAB0817, Millipore, Burlington, MA, USA), following the manufacturer’s recommendations.
2.9. Statistical Analysis
Statistical analyses were conducted on Prism 9 for macOS (Version 9.1.1) or IBM SPSS Statistics (Version 28.0). Significancy between groups was considered at a p-value < 0.05. The outliers’ ROUT (1%) method was applied in the weight and glycaemia analyses. In all graphs *, **, ***, and **** correspond to p-values < 0.05, <0.01, <0.001, and <0.0001, respectively. For inter-parameter comparisons, Pearson’s was used when n ≥ 15 or Kendall’s correlations if n < 15 in at least one comparison group (Supplementary File S2: Table S3); interpretation of the correlation coefficients was presented as Schober et al. [50] (0.10–0.39 negligible, 0.40–0.69 moderate, 0.70–0.89 strong, and 0.90–1.00 very strong correlation).
3. Results
3.1. Study Population
In this study, a population of 218 mice (96 males and 122 females) was euthanised at different time points between 2018 and 2022. At the time of euthanasia (date of death, DOD) the mice’ age was similar between sex groups (mean ± standard deviation (SD)): 9.0 ± 6.2 mo. in males and 9.6 ± 6.3 mo. in females; age variation was also balanced among genotypes: minimum age from 1.7 to 1.9 mo., and maximum ages from 24.4 to 24.3 mo. in males and females, respectively. The P.AtrxHOM genotype was represented in a proportion of 1.0:1.5 and 1.0:1.7 to P.AtrxWT controls in males and females, respectively; the P.AtrxHET genotype was restricted to females and at a higher proportion to allow more robust comparisons with P.AtrxHOM individuals (Figure 1).
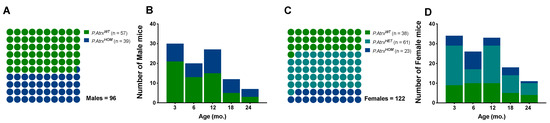
Figure 1.
Study population. The proportion of genotypes between male (A,B) and female mice (C,D), with the respective distribution by the five age groups. Green means P.AtrxWT, teal means P.AtrxHET, and ocean blue means P.AtrxHOM (A–D). Mice’ ages were calculated by the time of death.
3.2. Atrx Disruption at β Cells Enhanced Pancreatic Fat Accumulation and Did Not Cause Pancreatic Neuroendocrine Tumours
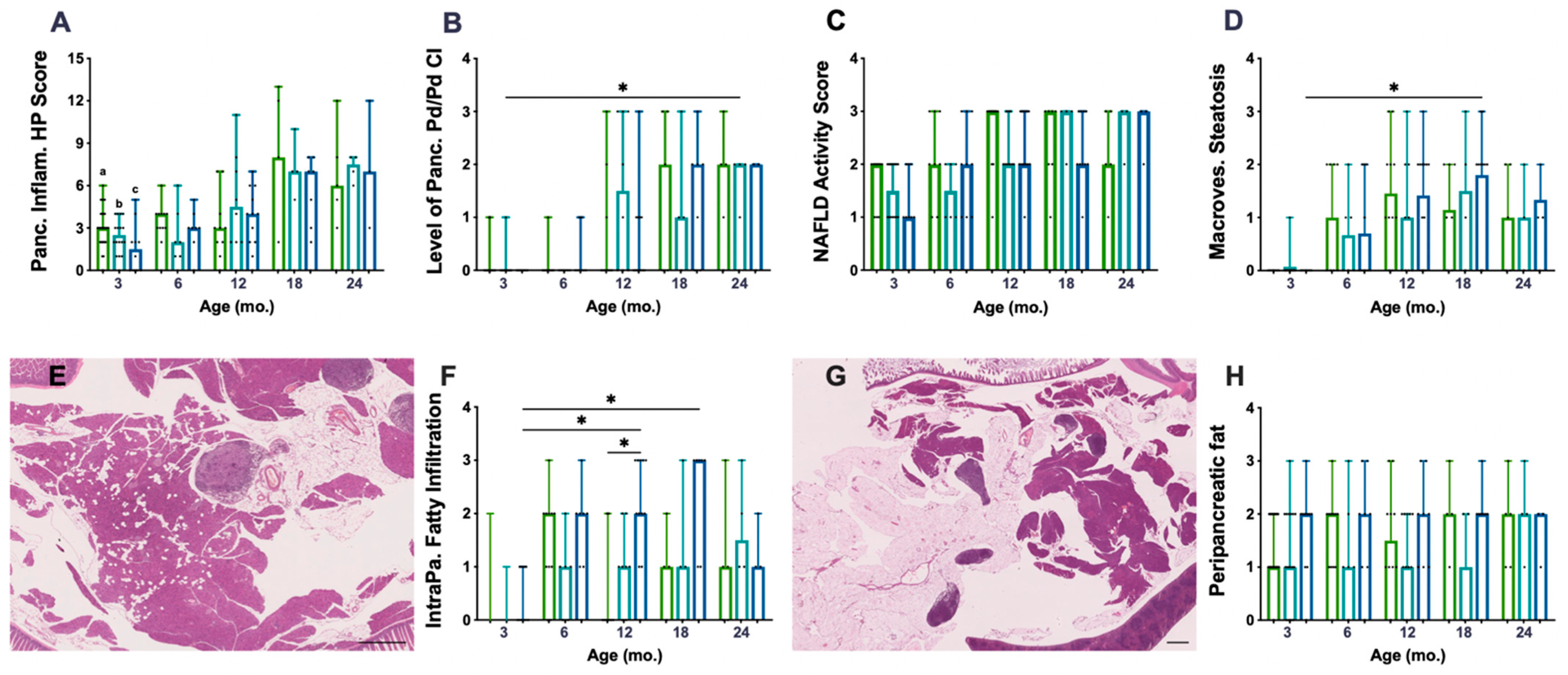
The histopathological (HP) scoring system used to characterise chronic inflammation (CI) lesions was performed on 136 mice pancreata. P.AtrxKO mice did not present an increased HP score compared to age-matched controls (Figure 2A). The Pd/Pv CI, previously described as responsible for the HP score increase, was similar among genotypes (Figure 2B), despite the augmented Pd/Pv CI level in P.AtrxHET females by the age of 12 mo. All genotypes’ pancreatic inflammation increased with ageing (Figure 2A,B), but statistical significance was not reached. When stratifying the different lesions by the three age groups encompassing all animals (3 mo., 6–12 mo., and 15–24 mo.), it was noticeable that, from 6 mo., P.AtrxKO presented a slightly increased percentage of moderate- and marked-grade pancreatic CI lesions in comparison to age-matched controls (Supplementary File S2: Figure S2). Additionally, 15–24-mo.-old P.AtrxKO mice presented an increased ageing score than age-matched P.AtrxWT (Supplementary File S2: Figure S2).
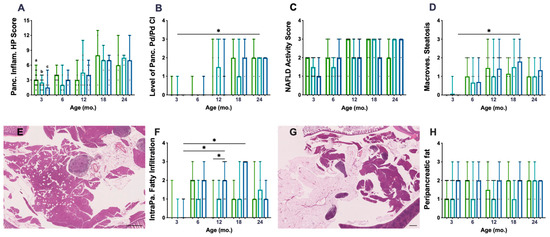
Figure 2.
Inflammatory and fat accumulation in the pancreas and hepatic steatosis. There are no differences among genotypes concerning pancreatic inflammation score (A) nor in periductal/perivascular (Pd/Pv) chronic inflammation (CI) specifically (B). However, P.AtrxHET females present a higher inflammation score, mostly due to Pd/Pv CI, by 12 mo. (A,B). There are no differences among the genotypes in alcoholic fatty liver disease (NAFLD) activity score (NAS) (C) nor in macrovesicular steatosis specifically (D); however, P.AtrxHOM presents higher macrovesicular steatosis compared with age-matched controls by 12 and 18 mo. Intrapancreatic fatty infiltration (FI), defined by scattered vacuoles within the exocrine pancreas (E), is significantly higher in 12- and 18-mo.-old P.AtrxHOM than age-matched controls (F). The extent of peripancreatic fatty tissue around pancreatic lobes (G) is constant (and high) in P.AtrxHOM of all ages, only increasing in comparison to age-matched controls by 3 and 12 mo. Ageing significantly enhances FI in P.AtrxHOM but does not allow discrimination of genotypes concerning peripancreatic fat deposition (H). Results are shown as the median ± range values (A–D,F,H). * p < 0.05, ** p < 0.01. a * P.AtrxWT (3 mo. vs. 18 mo. and 24 mo.) (A), b * P.AtrxHET (3 mo. vs. 18 mo. and 24 mo.) (A), c ** P.AtrxHOM (3 mo. vs. 18 mo. and 24 mo) (A). Green means P.AtrxWT, teal means P.AtrxHET, and ocean blue means P.AtrxHOM (A–D,F,H).
To determine if increased weight profiles could contribute to increased pancreatic CI lesions, we matched weights and pancreatic HP and found a positive and moderate correlation (r = 0.651) between P.AtrxHOM weights and the pancreatic HP score at 18 mo. (Supplementary File S2: Table S3).
When evaluating the non-alcoholic fatty liver disease (NAFLD) activity score (NAS) as an essential feature of ageing, P.AtrxKO mice did not show any significant increase in NAS when compared to age-matched controls (Figure 2C). However, when specifically analysing the macrovesicular steatosis, it was observed that P.AtrxHOM mice between 12 and 18 mo. exhibited an increased score than age-matched controls (Figure 2D). Moreover, unlike with NAS, the ageing-increased macrovesicular steatosis was more easily perceived and statistically significant between 3- and 18-mo.-old P.AtrxHOM individuals. Positive and moderate correlations were found between NAS and the weights of 12-mo.-old P.AtrxWT (τ = 0.584) and 12- and 18-mo.-old P.AtrxHOM (τ = 0.520 and τ = 0.569, respectively). More detailed information about the evolution of NAS over time and its correlations with weight gains can be found in Supplementary File S2: Table S3.
The histopathological evaluation of pancreata showed a preponderant presence of fat vacuoles, either scattered throughout the exocrine pancreas (Figure 2E)—defining intrapancreatic fatty infiltration (FI)—or surrounding pancreatic tissues mainly in the form of mesenteric fat (Figure 2G). Concerning FI, it was observed that P.AtrxHOM mice presented an increased score by 12 and 18 mo., statistically significant by 12 mo. (Figure 2F); ageing significantly contributed to the increase in this parameter. Concerning FR, only by the age of 3 and 12 mo., P.AtrxHOM mice presented an increased score compared to age-matched controls; of note, the FR levels were constant in P.AtrxHOM throughout all age groups, as ageing did not seem to influence this parameter (Figure 2H); by 24 mo., all genotypes presented the same score. Summarising FI and FR, it was observed that P.AtrxKO mice belonging to the 6–12-mo. and 15–24-mo. age groups had higher levels than age-matched controls (Supplementary File S2: Figure S2). Representative images of the pancreas not infiltrated with fat (FI score 0 and FR score 0) are shown in Supplementary File S2: Figure S3.
By the time of necropsy, macroscopic evaluation denoted a slight increase in the prevalence of hepatomegaly and splenomegaly (17% and 11% increase, respectively) when comparing P.AtrxKO mice aged 6–12 mo. with age-matched P.AtrxWT mice (Supplementary File S2: Figure S3).
In all genotypes, 29 tumours were detected, with 22 being malignant, composed of primary or secondary tumours, and then evaluated to determine the most likely phenotype. Lymphomas were the most common phenotype found in P.AtrxWT mice (75%), whereas, in the P.AtrxKO genotype, most tumours were mesenchymal (40%), followed by lymphomas (30%). Tumour incidence was higher in P.AtrxKO female mice (P.AtrxWT vs. P.AtrxKO; 50% vs. 50%, respectively) but decreased in male mice (P.AtrxWT vs. P.AtrxHOM; 64% vs. 36%, respectively) (Supplementary File S2: Figure S4). Pancreatic tumours (n = 4) were found in P.AtrxKO and P.AtrxWT mice with equally distributed phenotypes (50% mesenchymal, 50% lymphoma); representative images are presented in Supplementary File S2: Figure S5. Liver tumours (benign and malignant) presented equal occurrence and were equal between P.AtrxWT and P.AtrxKO.
Like in our previous model [34], we questioned if the exacerbated pancreatic inflammatory lesions would be detected systemically in a longitudinal hemogram evaluation: (a) the total white blood cell (WBC) count, P.AtrxKO mice did not disclose different levels compared to age-matched controls; (b) the lymphocyte percentage, which was not different in P.AtrxKO individuals compared to age-matched controls; and (c) the neutrophil-to-lymphocyte ratio (NLR), assessed due to its potential prognostic relevance in anticipating malignancy, did not differ between genotypes (Supplementary File S2: Figure S6). The complete haematological reports of P.Atrx mice are in Supplementary File S2: Table S4.
3.3. P.AtrxKO Mice Exhibited Increased Weight Gains and Glycaemia Levels since 3 mo.
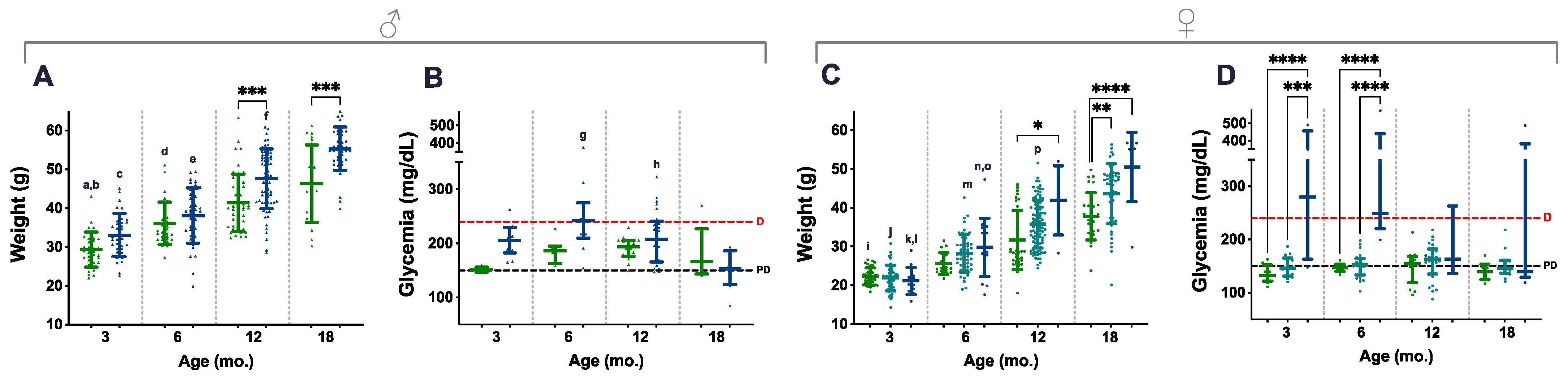
A total of 772 weighings, from 217 animals, were considered and distributed along four age groups (3, 6, 12, and 18 mo.); all the available non-longitudinal weights obtained during DOD were also included. Blood glucose levels were obtained at the same time points, and 207 measurements (from 107 animals) were used for data analysis. Ageing contributed to increasing weights in mice of all genotypes and the difference was statistically significant, especially during the first 12 mo. (Figure 3A,C, letters). In contrast, genotype-related differences were already perceived in 3- and 6-mo. male mice, but statistical significance was only present in the 12- and 18-mo. age groups of both males and females, as P.AtrxHOM individuals consistently presented higher weights than age-matched controls (Figure 3A,C, asterisks); during this period, most P.AtrxHOM presented a median weight corresponding to overweight or obesity.
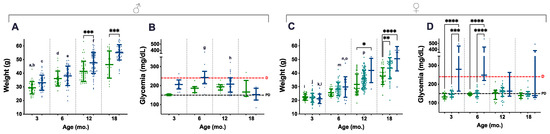
Figure 3.
Weight and glycaemia. Weight and glycaemia evaluations demonstrate that P.AtrxKO male (A,B) and female (C,D) mice present increased weight gains and glycaemia levels than age- and sex-matched controls. P.AtrxHOM males and females are significantly heavier than age- and sex-matched controls by 12 mo. and 18 mo. (A,C, respectively). P.AtrxHET females also present a significantly higher weight than age-matched controls by 18 mo. (C). P.AtrxHOM males and females show increased glycaemia than age- and sex-matched controls up to 12 mo.; while males present median levels above diabetic level only by the age of 6 mo. (p = 0.07) (B), females show such a trend by 3 and 6 mo. old, with statistical significance (D). Results are shown as the mean ± SD (A,C) and median ± interquartile range (B,D). Age groups of 9 and 15 mo. were included in the 12 and 18 mo. age groups, respectively (A–D). Inter-genotype differences are shown by: * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001. Ageing-related comparisons are given by small letters: a ** P.AtrxWT (3 mo. vs. 6 mo.), b **** P.AtrxWT (3 mo. vs. 12 mo. and 18 mo.), c **** P.AtrxHOM (3 mo. vs. 12 mo. and 18 mo.), d **** P.AtrxWT (6 mo. vs. 18 mo.), e **** P.AtrxHOM (6 mo. vs. 12 mo. and 18 mo.), f **** P.AtrxHOM (12 mo. vs. 18 mo.); g **** P.AtrxHOM (6 mo. vs. 18 mo.), h * P.AtrxHOM (12 mo. vs. 18 mo.), i **** P.AtrxWT (3 mo. vs. 12 mo. and 18 mo.), j **** P.AtrxHET (3 mo. vs. 6 mo., 12 mo., and 18 mo.), k * P.AtrxHOM (3 mo. vs. 6 mo.), l **** P.AtrxHOM (3 mo. vs. 12 mo. and 18 mo.), m **** P.AtrxHET (6 mo. vs. 12 mo. and 18 mo.), n ** P.AtrxHOM (6 mo. vs. 12 mo.), o **** P.AtrxHOM (6 mo. vs. 18 mo.), and p **** P.AtrxHET (12 mo. vs. 18 mo.). Green means P.AtrxWT, ocean blue means P.AtrxHOM (A–D), and teal means P.AtrxHET (C,D). D diabetic, PD prediabetic.
In parallel, P.AtrxKO mice of both sexes also tended to present increased glycaemic levels. However, such endocrine dysfunction was only marked in the 3- and 6-mo. age groups, higher in P.AtrxHOM than age-matched controls (Figure 3B,D). Nevertheless, statistical significance was only obtained in females, as the P.AtrxHOM genotype developed significantly higher glycaemia levels, at 3 mo. and 6 mo. of age, within levels compatible with diabetes (Figure 3D). In the correlation analysis, weights positively correlated with glycaemia in the P.AtrxWT genotype by 6 and 12 mo. (r = 0.718 and r = 0.397, respectively) and with NAS in P.AtrxWT and P.AtrxHOM mice (τ = 0.584 and τ = 0.520) by the age of 12 mo. Moreover, by 18 mo., the weights positively correlated with pancreatic HP and hepatic NAS scores in P.AtrxHOM groups (τ = 0.651 and τ = 0.569) (Supplementary File S2: Table S3).
3.4. P.AtrxKO Mice Show Improper Ageing-Related Growth of the Endocrine Fraction and Similar Fasted Insulinaemia
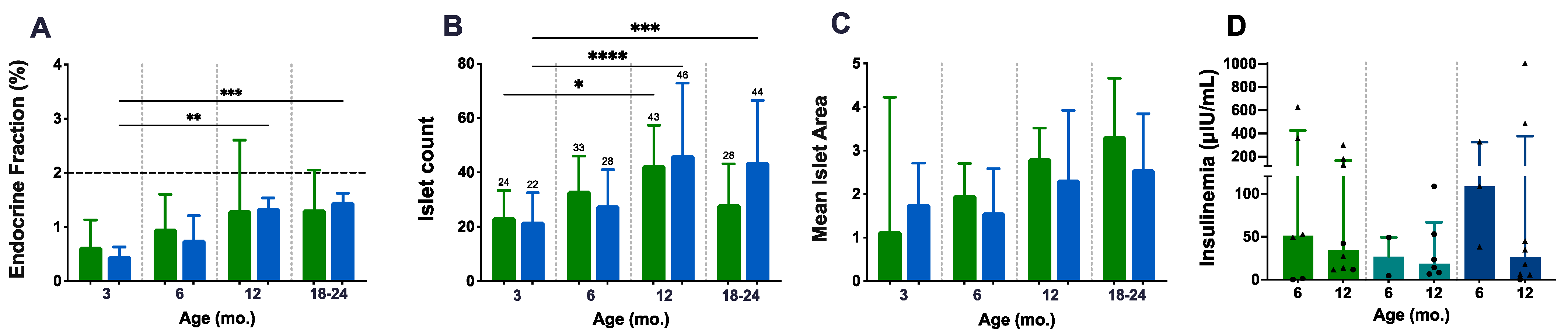
A detailed morphological characterisation of pancreatic islets was performed to complement the weight and glycaemia analysis and as a readout of in situ endocrine fitness (Figure 4A–C). The endocrine fraction (EF) was quantified in HE-stained slides following segmentation of the endocrine and exocrine portions using HALO® software. As age progressed, the EF increased slightly and at a constant rate in both P.AtrxWT and P.AtrxKO mice, being statistically significant in P.AtrxKO individuals (Figure 4A); however, no differences in the EF between P.AtrxKO and P.AtrxWT mice were detected. Comparing the ageing-related increase of EF between 3 and 24 mo., compared to 24-mo.-old P.AtrxWT (1.71%, median value), age-matched P.AtrxKO presented a similar but diminished fraction (1.49, 6% less). Islet numbers in both P.AtrxWT and P.AtrxKO mice equally increased with ageing, being statistically significant in both genotypes (Figure 4B); however, islet numbers at 18–24 mo. tended to be lower than 12-mo.-old counterparts of both genotype groups. The mean islet area (given by EF/islet count) was tendentially higher in P.AtrxWT than in P.AtrxKO mice (Figure 4C).
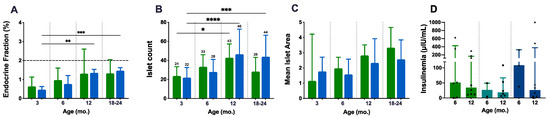
Figure 4.
Endocrine fitness: endocrine fraction, islet count, mean islet area, and insulinaemia. Both genotype groups present an age-related increase in endocrine fraction (EF), islet count, and mean islet area (A–C). The mean islet area shows a preponderance of P.AtrxWT mice over P.AtrxKO (C). Insulinaemia assessments do not retrieve any genotype-related difference; in all genotype groups, the insulinaemia decreases from 6 mo. to 12 mo. Of note, P.AtrxHOM presents higher basal levels by 6 mo. (D). Age groups of 9, 15, and 21 mo. were included in 12 and 18–24 mo., respectively (A–D). Results are shown as the median ± IQR (A,C,D) and mean ± SD (B) * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001. Green means P.AtrxWT (A–D), which was compared with the P.AtrxKO genotype (A–C), represented with a light blue, and with the P.AtrxHET and P.AtrxHOM genotypes coloured with teal and ocean blue, respectively (D). Triangles and circles are used to identify males and females, respectively.
Aiming to further characterise the endocrine metabolism disturbance in P.AtrxKO mice, we used ELISA assays to quantify the insulinaemia in non-fasted and fasted mice of all genotypes (Figure 4D). Although a tendency was noted in all genotype groups, with insulinaemia decreasing from 6 mo. to 12 mo., P.AtrxHOM mice presented higher basal levels by 6 mo. than the age-matched controls and P.AtrxHET; the assay results in Figure 4D analyses both genders, while in Supplementary File S2: Figure S7, the evaluation of non-fasted and fasted insulinaemia values analysed separately are available for consultation. By pooling the results of each genotype regardless of mice age, the level of insulin was higher in the AtrxHOM group (mean ± S.E., 188.80 ± 94.40 µIU/mL, n = 11 male and female mice), followed by AtrxWT male and female controls (128.90 ± 49.05 µIU/mL, n = 14), and by AtrxHET females (33.45 ± 12.62 µIU/mL, n = 8). The insulin resistance (IR), inferred by glycaemia/insulinaemia ratios, were similar, although slightly higher, in 12 mo. AtrxHOM male and AtrxHET female mice than age-matched controls (Supplementary File S2: Figure S7).
3.5. P.AtrxHOM Mice Exhibit Frank Glucose Intolerance by 3 mo.
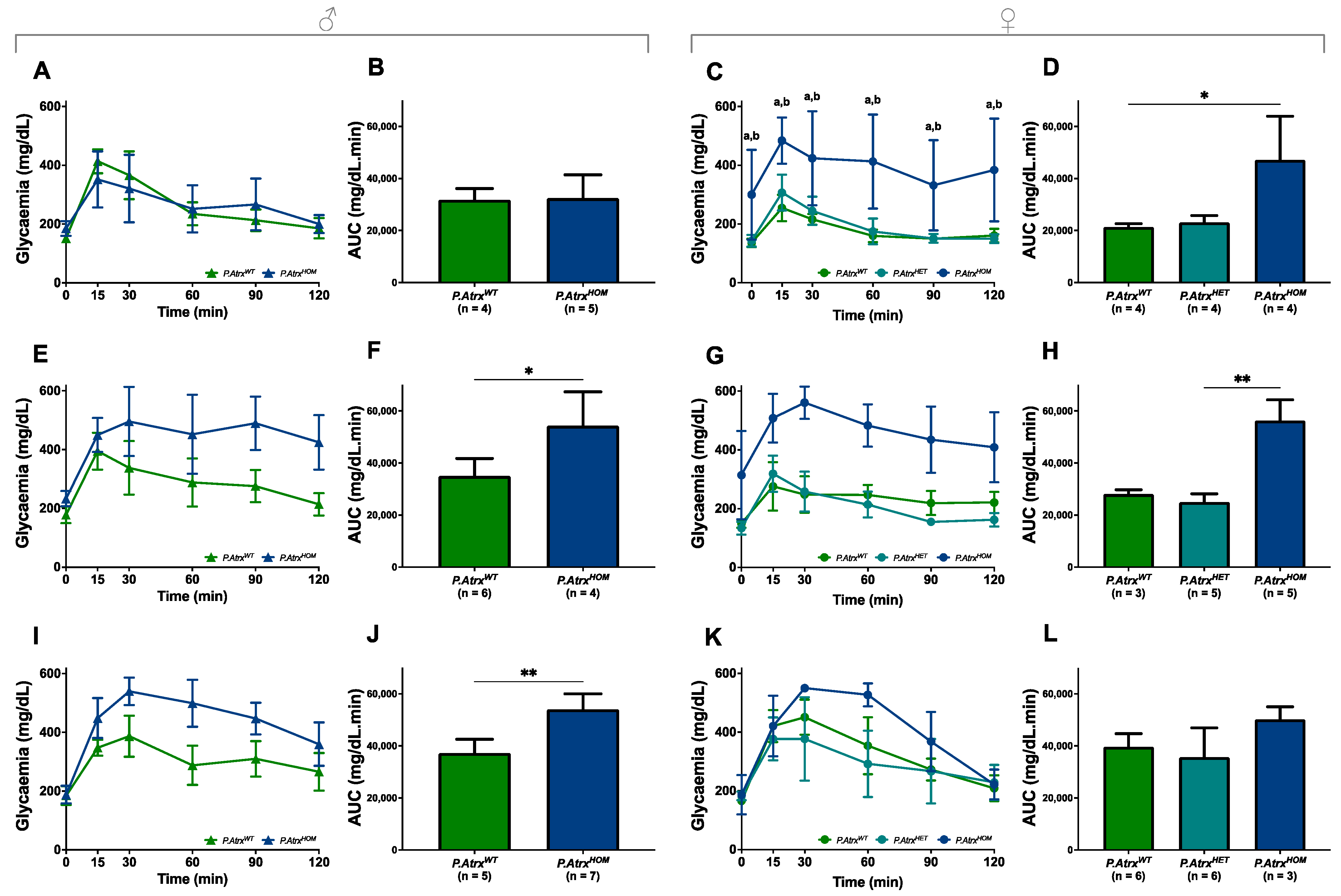
ipGTTs were performed at 3, 6, and 12 mo. for both genders and for all genotypes to evaluate the role of Atrx loss in endocrine dysfunction. By 3 mo., male mice of all genotypes presented a comparable response to glucose administration, as they could gradually restore normoglycaemia within 60 min (Figure 5A,B). In contrast, by the same age, P.AtrxHOM female mice already exhibited significantly increased glucose intolerance (Figure 5C,D). By 6 mo., both P.AtrxHOM male and female mice exhibited a significantly higher glucose intolerance than age-matched controls (Figure 5E–H); by 12 mo., such imbalance is maintained in P.AtrxHOM of both sexes (Figure 5I–L), although the significance was lost in females due to the increased GTT-AUC of P.AtrxWT; the evolution of GTT-AUC over time in all genotypes is available in Supplementary File S2: Figure S8.
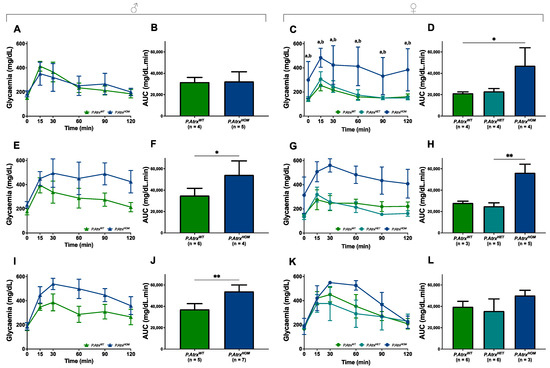
Figure 5.
Evidence of glucose intolerance. Intraperitoneal glucose tolerance tests (ipGTTs) performed in 3-mo.- (A–D), 6-mo.- (E–H), and 12-mo.-old mice (I–L). By 3 mo., P.AtrxHOM females have significantly increased glucose intolerance, whereas males do not (A–D). By 6 mo., P.AtrxHOM males already have significantly increased glucose intolerance (E,F), while females show a similar tendency (G,H); by 12 mo., AtrxHOM males preserve the significant deterioration of glucose tolerance (I,J), whereas females of all genotypes exhibit equivalent GTT-AUC values (K,L). * p < 0.05, ** p < 0.01, **** p < 0.0001. Two-way ANOVA was used for comparisons in each time point: a **** p < 0.0001 (P.AtrxWT vs. P.AtrxHOM) (C), b **** p < 0.0001 (P.AtrxHET vs. P.AtrxHOM) (C).
Moreover, in the correlation analysis, GTT-AUC positively correlated with weight by 12 mo., but solely in the P.AtrxWT genotype (τ = 0.477) (Supplementary File S2: Table S3).
4. Discussion
PanNETs are rare and clinically challenging entities. During the last 15 years, their genetic profile has been progressively unveiled as high-throughput sequencing techniques have emerged, and ATRX and DAXX mutations were reported in PanNETs (11–17% and 14–25%, respectively) [22,23,24]. In addition to MEN1 inherited and acquired mutations, the loss of ATRX or DAXX was indicated to be the primary driver of somatic events in PanNET biology through epigenetic changes [51,52,53]. Evidence from extensive PanNET cohorts has ascertained the prognostic value of ATRX/DAXX mutations [27,28,29,31,32]. In particular, the apparent correlation between chromatin remodellers’ loss of function and ALT activation has emerged as a reliable indicator of poor prognosis, helpful in identifying “high-risk” PanNET patients [54].
We still have limited knowledge concerning the contribution of the newly identified genes to tumour initiation and progression. Knowing the mechanisms involved in ATRX/DAXX inactivation and the initiation of ALT will likely clarify the pertinence of their clinical implications [55]. We believe that suitable GEMMs addressing these issues will help down the road.
Recently, we developed and studied a model generated using the Rip-Cre system to conditionally target Atrx disruption to pancreatic β cells (R.Atrx) and reported for the first time that the conditional disruption of Atrx in mice pancreas is not a sufficient event to drive PanNET formation [34]; other authors have reported similar findings afterwards [35]. Instead, the Atrx disruption at endocrine islets seems to play a role in anticipating and aggravating inflammageing and ageing-related deterioration of endocrine functions [34]. R.AtrxHOM mice exhibited higher glycaemia levels (prediabetic) and glucose intolerance with sexual dimorphism (detected earlier in males) [34]. In 2019, it had already been reported that the loss of Atrx enhanced pancreatic injury and susceptibility to KRAS-mediated (M.K.Atrx) pancreatic damage in female mice, pioneering the attribution of anti-inflammatory and tumour-suppressive roles to Atrx [36] (Table 1).

Table 1.
Summary of the contributions of the chromatin remodellers Atrx and Daxx to pancreatic tumourigenesis and inflammageing. ADM, acinar-to-duct cell metaplasia; EF, endocrine fraction; FI, intrapancreatic fatty infiltration; M, Mist1-Cre; P, Pdx1-Cre; PanIN, pancreatic intraepithelial neoplasia; and R, Rip-Cre. Notes: a, All the mutations were analysed at homozygosity, except for our previous study [34]; b, Atrx/Daxx KO as tumourigenic event or accelerator; and c, All studies evaluated male and female mice. Models: 1, Mist1-Cre;AtrxHOM; 2, Mist1-Cre;KrasHOM; 3, Mist1-Cre;KrasHOM;AtrxHOM; 4, Pdx1-Cre.DaxxHOM; 5, Pdx1-Cre;Men1HOM; 6, Pdx1-Cre;Men1HOM;DaxxHOM; 7, Rip-Cre;AtrxHOM and HET; 8, Pdx1-Cre;AtrxHOM; 9, Pdx1-Cre;Men1HOM; 10, Pdx1-Cre;Men1HOM;AtrxHOM; 11, Pdx1-Cre;PtenHOM; 12, Pdx1-Cre;PtenHOM;AtrxHOM; 13, Rip-Cre;Men1HOM; 14, Rip-Cre;Men1HOM;DaxxHOM; 15, DaxxHOM; and 16, Rip-Cre;DaxxHOM; all the promoters were expressed at heterozygosity.
Considering the relevance of ATRX in sporadic human PanNETs and the scarcity of GEMMs addressing its role in endocrine function, we studied a mouse model that conditionally targets Atrx at pancreatic β cells expressing Cre under the pancreatic and duodenal homeobox 1 (Pdx1) promoter (P.Atrx). By applying the same methodology as before [34], we primarily aimed to assess the putative role of Atrx heterozygous and homozygous loss (P.AtrxKO) as a disruptor of endocrine fitness and putative PanNET driver event.
We started analysing the histopathological (HP) alterations of the pancreas and liver. We observed that no apparent genotype-related differences in the overall pancreatic HP score were perceived. However, by 12 mo., P.AtrxHET females presented a higher HP score and a higher periductal/perivascular (Pd/Pv) score. These results are, in part, comparable to those obtained from R.AtrxHET, which presented the highest Pd/Pv levels by 9 mo., contributing to the rising of the pancreatic HP score [34]. Like before, as ageing progressed, the differences among genotypes seemed to be harder to detect. When looking at NAS, as the liver is an organ sensitive to inflammageing, we observed that losing Atrx did not aggravate the overall hepatic steatosis levels. Still, when only analysing NAS parameters, we detected that macrovesicular steatosis alone could discriminate mice by genotype; we observed that, although not significant, P.AtrxHOM mice presented increased levels of macrovesicular steatosis than age-matched controls by 12 and 18 mo. Most mice presented pancreatic scattered fat accumulation, an alteration we termed intrapancreatic fatty infiltration (FI), also designated by fatty pancreas [56]; we decided to quantify this alteration and observed that FI significantly increased with ageing and that P.AtrxHOM mice significantly presented more adipocyte deposition within pancreatic tissue than age-matched controls by 12 and 18 mo. In many slides, it was noted that the pancreatic lobes were surrounded by peripancreatic fatty tissue, which was also quantified. It remains to be disclosed if, to some extent, this fatty tissue is replacing pancreatic lobes, a situation termed pancreatic fatty replacement (FR). Contrary to FI, the peripancreatic fat extension did not alter much with ageing; P.AtrxHOM mice exhibited the same median levels over time, higher than age-matched controls by 3 and 12 mo.
Weight profiles help explain all these results. As expected, mice weights constantly increased over time in all genotypes; however, genotype-related differences were noticed with a mild sexual dimorphism. Both males and females with Atrx homozygous deletion presented increased weights than age-matched controls, such difference was statistically significant by 12 and 18 mo. Nevertheless, while the mean weight of the male mice was already higher than controls by 3 mo., more time is required to confirm such genotype-related differences in females. P.AtrxHET seem to have mean weights between P.AtrxWT and P.AtrxHOM. Male and female mice (controls and KO) presented weights compatible with overweight or obese measurements by 3 mo. or 6 mo., respectively, and kept progressing with ageing, especially in males; such augmented weights and the subsequent fat accumulation likely hampered discriminating the genotype-related differences in pancreatic HP and NAS scores.
Evaluating longitudinally glycaemias revealed that P.AtrxHOM males and females presented increased glycaemias than age-matched controls starting at 3 mo.; such hyperglycaemic values were within diabetic values (i.e., above 240 mg/dL) in females (3 mo.) sooner than in males (6 mo.). ipGTT tests were subsequently performed and reinsured the sexual dimorphism of endocrine dysfunction onset: P.AtrxHOM females presented obvious glucose intolerance by 3 mo., which was maintained until 6 mo., but less pronounced by 12 mo.; in contrast, P.AtrxHOM males were tolerant to glucose administration by 3 mo., but intolerant in the 6- and 12-mo. age groups.
As a readout of endocrine fitness, an endocrine fraction measurement and insulinaemia quantification using ELISA assay were performed. Neither evaluation highlighted marked differences between P.AtrxWT and P.AtrxKO. The main motive behind the choice of another promoter rather than the rat insulin promoter (Rip) was the reports of the prudent use of Rip-Cre driver lines, whose main side effects are a leaky expression in the hypothalamus [57,58] and the spontaneous early development of glucose intolerance and impaired insulin secretion (before two months) [59,60]. By using the Pdx1-Cre system, we could study the effects of losing Atrx in the endocrine pancreas regardless of the putative influence of the Rip-Cre system. Unexpectedly, besides the early-onset of overweight and obese measurements, P.AtrxWT and P.AtrxKO mice did not double their EF as their age progressed as expected [61]; their EF remained constant from 12 mo., consistently below 2%, the described value for most mammalian species [62]. Since the quantification method is the same as the one used in our previous study [34], we believe that, contrarily to the non-obese R.Atrx mice, the increased peripancreatic fat deposition and (consequent) putative FR in the P.Atrx model may be deflating this parameter in the context of overweight and obese weight measurements. Moreover, we cannot exclude a putative contribution of Atrx loss in exocrine tissue that could, in turn, predispose an acinar counterpart to FI/FR.
Concerning the insulinaemia results, we find them comparable to the R.Atrx mice, as the P.AtrxWT and P.AtrxKO age groups presented decreased insulinaemia values by 12 mo. compared to genotype-related 6-mo.-old individuals; peripheral insulin resistance (IR), given by the glycaemia to insulinaemia ratio, was also higher at 12 mo. than 6 mo. in both genotype groups. P.AtrxHOM mice presented the highest insulinaemias regardless of mice age and developed the highest IR by 12 mo. Of note, the IR values were over two-fold the R.AtrxKO, probably due to their substantially higher weights.
We developed an obese diabetic GEMM. Obesity is a preponderant factor in inflammageing onset and progression. Lipid accumulation, accentuated by ageing, leads to obesity, type 2 diabetes (T2D), and dyslipidaemia; these have been set as direct contributors to the fatty pancreas, i.e., intrapancreatic fatty infiltration [56]. FI causes a release of cytokines, adipokines, and pro-inflammatory factors responsible for the progression of pancreatic intraepithelial neoplasia (PanIN) lesions to PDAC [63].
Daxx has been implicated in endogenous retroviral element (ERV) silencing by safeguarding chromatin through epigenetic mechanisms. Its loss is accompanied by installing a more permissive transcriptional landscape that puts cells at a higher risk of additional tumourigenic events at pancreatic islets [18]. Intriguingly, some HIV-1-infected patients develop lipodystrophy syndrome (HALS), characterised by alterations in fatty tissue distribution (lipodystrophy) and systemic metabolic complications. HALS patients typically present a peripheral lipoatrophy of subcutaneous adipose tissue, visceral fat accumulation, lipomatosis, dyslipidemia, and insulin resistance [64]. We wonder if the molecular mechanisms downstream of Atrx loss in vivo include ERV derepression with equivalent consequences on adipose tissue.
We verified that the loss of Atrx does not induce PanNET in the mouse pancreas [34]. Considering the increasingly explored epigenomes and transcriptomes of human PanNETs, based on the expression of aristaless-related homeobox (ARX) and PDX1, the drivers of α and β cell differentiation, respectively, the alterations induced by us in our two β cell-conditional GEMMs (R.Atrx and P.Atrx) may have been mistargeted. It was described that the most altered endocrine cell population in ATRX/DAXX-mutated PanNETs harbours a typical landscape signature of α cells (ARX+/PDX1−) [38,39]. Therefore, the conditional targeting of β cells may not be ideal. Notwithstanding, we should bear in mind that Pdx1-driven genetic alterations target not only β but also α cells, as recently confirmed [35]. This also reinforces the importance of using Pdx1-Cre (instead of Rip-Cre) to study chromatin remodellers.
The results obtained with our Atrx models interestingly suggest the potential role of ATRX in the homeostasis of human pancreatic endocrine function. T2D is a significant risk factor for PanNET development [65,66], but these patients are also at risk for T2D development [67,68]. An association between metabolic syndrome and GEP-NET patients has also been found [69]. In PanNET patients, the prevalence of longstanding T2D, new-onset T2D, and impaired fasting glucose (IFG) was approximately 12–17%, 8–9%, and 7–26%, respectively [67,68]. Moreover, older PanNET patients (≥60 years) presented longstanding T2D at a higher risk (26%) than the overall population of the same age (below 20%) [67,70]. Specific parameters, such as age and tumour size, are risk factors for T2D in PanNET patients [67]. The prognostic value of preoperative T2D in patients with non-functioning (NF) PanNETs has also been determined: the preoperative new onset of T2D and IFG were associated with aggressive tumour behaviour and poor DFS in patients with NF tumours [67]. A glucose profile assessment is already part of PanNET patient management. As far as we know, no works report a correlation or association between genetic events (such as gene mutations) and HbA1C levels or T2D development. It would be interesting to evaluate the HbA1C levels in PanNET patients presenting the ATRX (or DAXX) mutation to ascertain its potential involvement in endocrine function and contribution to PanNET disease.
Soon, novel models will likely explore the actual contribution of chromatin remodellers to PanNETs. The new generation of GEMMs could help clarify whether the mutations found in human PanNETs are aetiologically relevant or passenger events [22,24,71,72] and even explore the putative synergistic effects that could arise from multi-targeting approaches. Nevertheless, our works and others have alerted us that tumourigenesis upon ATRX or DAXX loss may probably only occur in human genomes [34,35], and such potential species-specificity should be considered.
5. Conclusions
Using the Pdx1-Cre;AtrxKO GEMM, we reinforced the preponderant role of Atrx disruption in the β cells of adult mice with endocrine dysfunction. Unlike Rip-Cre;AtrxKO, in which a mild compromise of pancreatic homeostasis was perceived in younger ages, Atrx disruption in this different driver line had major metabolic implications, leading to obesity and diabetes at young ages. Adiposity led to intrapancreatic fatty infiltration that progressively increased with ageing and resulted in enhanced pancreatic fatty infiltration and peripancratic fat deposits, hampering the interpretation of pancreatic histopathological and hepatic steatosis scores.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15113018/s1, Supplementary File S1 (Materials and Methods): Figure S1: Markups of endocrine fraction classifier; Table S1: Primers and genotyping conditions; Table S2: Study population and procedures; Table S3: List of antibodies used in immunohistochemistry; Supplementary File S2 (Results): Figure S2: Pancreatic inflammageing lesions, hepatic steatosis, intrapancreatic fatty infiltration, and macroscopic lesions by three age groups; Figure S3: Normal pancreas; Figure S4: Tumour incidence analysis; Figure S5: Pancreatic tumours; Figure S6: Hemograms; Figure S7: Non-fasted and fasted insulinaemias; Figure S8: GTT-AUC over time; Table S4: Overview of main results by age and genotype; Table S5: Hemograms, all parameters.
Author Contributions
Conceptualisation, J.V. and T.B.G.; methodology, T.B.G., J.V. and P.S.; software, T.B.G. and D.F.R.; validation, T.B.G., T.T.J., M.T.A., L.C., J.M.L., P.S. and J.V.; formal analysis, T.B.G., T.T.J., M.T.A. and S.M.; investigation, T.B.G., T.T.J., M.T.A., S.M., M.A.S., R.S.M., R.L., D.F.R., L.C., I.B., S.C., F.G., J.M.L. and J.V.; resources, J.V. and P.S.; data curation, T.B.G.; writing—original draft preparation, T.B.G., T.T.J., M.T.A. and J.V.; writing—review and editing, all authors; visualisation, T.B.G. and J.V.; supervision, J.V., J.M.L., P.S. and L.M.-A.; project administration, T.B.G., J.V. and P.S.; funding acquisition, T.B.G., J.V. and P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by national funds by FCT—Fundação para a Ciência e Tecnologia, I.P., through a Ph.D. grant to T.B.G. (SFRH/BD/129431/2017), a research contract to J.V. (CEECIND/00201/2017 and 2022.00276.CEECIND), and to the project PTDC/MED-ONC/0531/2021—CTRL+ALT+CEL: how ATRX controls an alternative program in the β cell. This study is also part of the project “Institute for Research and Innovation in Health Sciences” (UID/BIM/04293/2019); the project “Cancer Research on Therapy Resistance: From Basic Mechanisms to Novel Targets”-NORTE-01-0145-FEDER-000051, supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF); and the project “The Porto Comprehensive Cancer Center” with the reference NORTE-01-0145-FEDER-072678-Consórcio PORTO.CCC–Porto.Comprehensive Cancer Center Raquel Seruca. S.M. was funded by an FCT Ph.D. grant (SFRH/BD/137802/2018); M.A.S. was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brazil (CAPES)—88881.361674/2019-01; and L.M.-A. by CNE-FAPERJ E-26/200.798/2021 and the Programa de Redes de Pesquisa em Saúde do Estado do Rio de Janeiro—2019 E-26/010.002429/2019).
Institutional Review Board Statement
The animal study protocol was approved by the Portuguese National Regulation established by Decreto-Lei n.° 113/2013, which is the national transposition of the European Directive 2010/63/EU for the Care and Use of Laboratory Animals. Procedures were evaluated and approved by the i3S Animal Welfare and Ethics Review Body and the Portuguese National Authority for Animal Health (DGAV)—project license code 13020/2017-05-08.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
We thank Douglas Roland Higgs for providing us with the Atrx floxed mice, Sónia Melo for the Pdx1 mice, and Ghislaine Lioux for providing us access to the HALO® Image Analysis Platform. We thank Sofia Lamas and the animal facility technical staff for assistance with the animal model. The authors acknowledge the support of the i3S Scientific Platforms Histology and Electron Microscopy (HEMS) and Advanced Light Microscopy (ALM), both members of the national infrastructure Portuguese Platform of Bioimaging (PPBI-POCI-01-0145-FEDER-022122) and Cell Culture and Genotyping (CCGen).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stayton, C.L.; Dabovic, B.; Gulisano, M.; Gecz, J.; Broccoli, V.; Giovanazzi, S.; Bossolasco, M.; Monaco, L.; Rastan, S.; Boncinelli, E.; et al. Cloning and characterization of a new human Xq13 gene, encoding a putative helicase. Hum. Mol. Genet. 1994, 3, 1957–1964. [Google Scholar] [CrossRef] [PubMed]
- Picketts, D.J.; Higgs, D.R.; Bachoo, S.; Blake, D.J.; Quarrell, O.W.; Gibbons, R.J. ATRX encodes a novel member of the SNF2 family of proteins: Mutations point to a common mechanism underlying the ATR-X syndrome. Hum. Mol. Genet. 1996, 5, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Gecz, J.; Pollard, H.; Consalez, G.; Villard, L.; Stayton, C.; Millasseau, P.; Khrestchatisky, M.; Fontes, M. Cloning and expression of the murine homologue of a putative human X-linked nuclear protein gene closely linked to PGK1 in Xq13.3. Hum. Mol. Genet. 1994, 3, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Haase, S.; Garcia-Fabiani, M.B.; Carney, S.; Altshuler, D.; Nunez, F.J.; Mendez, F.M.; Nunez, F.; Lowenstein, P.R.; Castro, M.G. Mutant ATRX: Uncovering a new therapeutic target for glioma. Expert Opin. Ther. Targets 2018, 22, 599–613. [Google Scholar] [CrossRef]
- Valenzuela, M.; Amato, R.; Sgura, A.; Antoccia, A.; Berardinelli, F. The Multiple Facets of ATRX Protein. Cancers 2021, 13, 2211. [Google Scholar] [CrossRef]
- Gibbons, R.J.; Wada, T.; Fisher, C.A.; Malik, N.; Mitson, M.J.; Steensma, D.P.; Fryer, A.; Goudie, D.R.; Krantz, I.D.; Traeger-Synodinos, J. Mutations in the chromatin-associated protein ATRX. Hum. Mutat. 2008, 29, 796–802. [Google Scholar] [CrossRef]
- Iwase, S.; Xiang, B.; Ghosh, S.; Ren, T.; Lewis, P.W.; Cochrane, J.C.; Allis, C.D.; Picketts, D.J.; Patel, D.J.; Li, H.; et al. ATRX ADD domain links an atypical histone methylation recognition mechanism to human mental-retardation syndrome. Nat. Struct. Mol. Biol. 2011, 18, 769–776. [Google Scholar] [CrossRef]
- Dhayalan, A.; Tamas, R.; Bock, I.; Tattermusch, A.; Dimitrova, E.; Kudithipudi, S.; Ragozin, S.; Jeltsch, A. The ATRX-ADD domain binds to H3 tail peptides and reads the combined methylation state of K4 and K9. Hum. Mol. Genet. 2011, 20, 2195–2203. [Google Scholar] [CrossRef]
- Tang, J.; Wu, S.; Liu, H.; Stratt, R.; Barak, O.G.; Shiekhattar, R.; Picketts, D.J.; Yang, X. A novel transcription regulatory complex containing death domain-associated protein and the ATR-X syndrome protein. J. Biol. Chem. 2004, 279, 20369–20377. [Google Scholar] [CrossRef]
- Hoelper, D.; Huang, H.; Jain, A.Y.; Patel, D.J.; Lewis, P.W. Structural and mechanistic insights into ATRX-dependent and -independent functions of the histone chaperone DAXX. Nat. Commun. 2017, 8, 1193. [Google Scholar] [CrossRef]
- Dyer, M.A.; Qadeer, Z.A.; Valle-Garcia, D.; Bernstein, E. ATRX and DAXX: Mechanisms and Mutations. Cold Spring Harb. Perspect. Med. 2017, 7, a026567. [Google Scholar] [CrossRef]
- Goldberg, A.D.; Banaszynski, L.A.; Noh, K.M.; Lewis, P.W.; Elsaesser, S.J.; Stadler, S.; Dewell, S.; Law, M.; Guo, X.; Li, X.; et al. Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell 2010, 140, 678–691. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Gibbons, R.; Yan, Z.; Yang, D.; McDowell, T.L.; Sechi, S.; Qin, J.; Zhou, S.; Higgs, D.; Wang, W. The ATRX syndrome protein forms a chromatin-remodeling complex with Daxx and localizes in promyelocytic leukemia nuclear bodies. Proc. Natl. Acad. Sci. USA 2003, 100, 10635–10640. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.P.; Santos, G.; Vinagre, J.; Soares, P. The Role of ATRX in the Alternative Lengthening of Telomeres (ALT) Phenotype. Genes 2016, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Clynes, D.; Jelinska, C.; Xella, B.; Ayyub, H.; Scott, C.; Mitson, M.; Taylor, S.; Higgs, D.R.; Gibbons, R.J. Suppression of the alternative lengthening of telomere pathway by the chromatin remodelling factor ATRX. Nat. Commun. 2015, 6, 7538. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Martin, V.; Soriano, M.; Garcia-Salcedo, J.A. Quadruplex Ligands in Cancer Therapy. Cancers 2021, 13, 3156. [Google Scholar] [CrossRef] [PubMed]
- Elsasser, S.J.; Noh, K.M.; Diaz, N.; Allis, C.D.; Banaszynski, L.A. Histone H3.3 is required for endogenous retroviral element silencing in embryonic stem cells. Nature 2015, 522, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Wasylishen, A.R.; Sun, C.; Moyer, S.M.; Qi, Y.; Chau, G.P.; Aryal, N.K.; McAllister, F.; Kim, M.P.; Barton, M.C.; Estrella, J.S.; et al. Daxx maintains endogenous retroviral silencing and restricts cellular plasticity in vivo. Sci. Adv. 2020, 6, eaba8415. [Google Scholar] [CrossRef]
- Tsai, K.; Thikmyanova, N.; Wojcechowskyj, J.A.; Delecluse, H.J.; Lieberman, P.M. EBV tegument protein BNRF1 disrupts DAXX-ATRX to activate viral early gene transcription. PLoS Pathog. 2011, 7, e1002376. [Google Scholar] [CrossRef]
- Everett, R.D.; Parada, C.; Gripon, P.; Sirma, H.; Orr, A. Replication of ICP0-null mutant herpes simplex virus type 1 is restricted by both PML and Sp100. J. Virol. 2008, 82, 2661–2672. [Google Scholar] [CrossRef]
- Glass, M.; Everett, R.D. Components of promyelocytic leukemia nuclear bodies (ND10) act cooperatively to repress herpesvirus infection. J. Virol. 2013, 87, 2174–2185. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Shi, C.; Edil, B.H.; de Wilde, R.F.; Klimstra, D.S.; Maitra, A.; Schulick, R.D.; Tang, L.H.; Wolfgang, C.L.; Choti, M.A.; et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011, 331, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gao, Z.; Li, L.; Jiang, X.; Shan, A.; Cai, J.; Peng, Y.; Li, Y.; Jiang, X.; Huang, X.; et al. Whole exome sequencing of insulinoma reveals recurrent T372R mutations in YY1. Nat. Commun. 2013, 4, 2810. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, A.; Chang, D.K.; Nones, K.; Corbo, V.; Patch, A.M.; Bailey, P.; Lawlor, R.T.; Johns, A.L.; Miller, D.K.; Mafficini, A.; et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature 2017, 543, 65–71. [Google Scholar] [CrossRef] [PubMed]
- cBioPortal for Cancer Genomics. Gene Query, 118 Samples, 2 Genes. Available online: https://bit.ly/3eKEkoi (accessed on 1 January 2022).
- Lovejoy, C.A.; Li, W.; Reisenweber, S.; Thongthip, S.; Bruno, J.; de Lange, T.; De, S.; Petrini, J.H.; Sung, P.A.; Jasin, M.; et al. Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway. PLoS Genet. 2012, 8, e1002772. [Google Scholar] [CrossRef]
- Marinoni, I.; Kurrer, A.S.; Vassella, E.; Dettmer, M.; Rudolph, T.; Banz, V.; Hunger, F.; Pasquinelli, S.; Speel, E.J.; Perren, A. Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology 2014, 146, 453–460 e455. [Google Scholar] [CrossRef]
- Singhi, A.D.; Liu, T.C.; Roncaioli, J.L.; Cao, D.; Zeh, H.J.; Zureikat, A.H.; Tsung, A.; Marsh, J.W.; Lee, K.K.; Hogg, M.E.; et al. Alternative Lengthening of Telomeres and Loss of DAXX/ATRX Expression Predicts Metastatic Disease and Poor Survival in Patients with Pancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2017, 23, 600–609. [Google Scholar] [CrossRef]
- Kim, J.Y.; Brosnan-Cashman, J.A.; An, S.; Kim, S.J.; Song, K.B.; Kim, M.S.; Kim, M.J.; Hwang, D.W.; Meeker, A.K.; Yu, E.; et al. Alternative Lengthening of Telomeres in Primary Pancreatic Neuroendocrine Tumors Is Associated with Aggressive Clinical Behavior and Poor Survival. Clin. Cancer Res. 2017, 23, 1598–1606. [Google Scholar] [CrossRef]
- Gaspar, T.B.; Sa, A.; Lopes, J.M.; Sobrinho-Simoes, M.; Soares, P.; Vinagre, J. Telomere Maintenance Mechanisms in Cancer. Genes 2018, 9, 241. [Google Scholar] [CrossRef]
- Chou, A.; Itchins, M.; de Reuver, P.R.; Arena, J.; Clarkson, A.; Sheen, A.; Sioson, L.; Cheung, V.; Perren, A.; Nahm, C.; et al. ATRX loss is an independent predictor of poor survival in pancreatic neuroendocrine tumors. Hum. Pathol. 2018, 82, 249–257. [Google Scholar] [CrossRef]
- Roy, S.; LaFramboise, W.A.; Liu, T.C.; Cao, D.; Luvison, A.; Miller, C.; Lyons, M.A.; O’Sullivan, R.J.; Zureikat, A.H.; Hogg, M.E.; et al. Loss of Chromatin-Remodeling Proteins and/or CDKN2A Associates with Metastasis of Pancreatic Neuroendocrine Tumors and Reduced Patient Survival Times. Gastroenterology 2018, 154, 2060–2063.e2068. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, T.B.; Lopes, J.M.; Soares, P.; Vinagre, J. An update on genetically engineered mouse models of pancreatic neuroendocrine neoplasms. Endocr. Relat. Cancer 2022, 29, R191–R208. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, T.B.; Macedo, S.; Sa, A.; Soares, M.A.; Rodrigues, D.F.; Sousa, M.; Mendes, N.; Martins, R.S.; Cardoso, L.; Borges, I.; et al. Characterisation of an Atrx Conditional Knockout Mouse Model: Atrx Loss Causes Endocrine Dysfunction Rather Than Pancreatic Neuroendocrine Tumour. Cancers 2022, 14, 3865. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Estrella, J.S.; Whitley, E.M.; Chau, G.P.; Lozano, G.; Wasylishen, A.R. Context matters—Daxx and Atrx are not robust tumor suppressors in the murine endocrine pancreas. Dis. Model. Mech. 2022, 15, dmm049552. [Google Scholar] [CrossRef]
- Young, C.C.; Baker, R.M.; Howlett, C.J.; Hryciw, T.; Herman, J.E.; Higgs, D.; Gibbons, R.; Crawford, H.; Brown, A.; Pin, C.L. The Loss of ATRX Increases Susceptibility to Pancreatic Injury and Oncogenic KRAS in Female but Not Male Mice. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 93–113. [Google Scholar] [CrossRef]
- The Jackson Laboratory. B6.Cg-Tg(Ins2-cre)25Mgn/J. Stock No: 003573. RIP-Cre. Available online: https://www.jax.org/strain/003573 (accessed on 1 November 2022).
- Cejas, P.; Drier, Y.; Dreijerink, K.M.A.; Brosens, L.A.A.; Deshpande, V.; Epstein, C.B.; Conemans, E.B.; Morsink, F.H.M.; Graham, M.K.; Valk, G.D.; et al. Enhancer signatures stratify and predict outcomes of non-functional pancreatic neuroendocrine tumors. Nat. Med. 2019, 25, 1260–1265. [Google Scholar] [CrossRef]
- Di Domenico, A.; Pipinikas, C.P.; Maire, R.S.; Brautigam, K.; Simillion, C.; Dettmer, M.S.; Vassella, E.; Thirlwell, C.; Perren, A.; Marinoni, I. Epigenetic landscape of pancreatic neuroendocrine tumours reveals distinct cells of origin and means of tumour progression. Commun. Biol. 2020, 3, 740. [Google Scholar] [CrossRef]
- Bérubé, N.G.; Mangelsdorf, M.; Jagla, M.; Vanderluit, J.; Garrick, D.; Gibbons, R.J.; Higgs, D.R.; Slack, R.S.; Picketts, D.J. The chromatin-remodeling protein ATRX is critical for neuronal survival during corticogenesis. J. Clin. Investig. 2005, 115, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Garrick, D.; Sharpe, J.A.; Arkell, R.; Dobbie, L.; Smith, A.J.; Wood, W.G.; Higgs, D.R.; Gibbons, R.J. Loss of Atrx affects trophoblast development and the pattern of X-inactivation in extraembryonic tissues. PLoS Genet. 2006, 2, e58. [Google Scholar] [CrossRef]
- Mouse Genome Informatics. Atrxtm1Rjg. Available online: http://www.informatics.jax.org/allele/MGI:3528480 (accessed on 1 January 2022).
- Mouse Genome Informatics. B6.FVB-Tg(Pdx1-cre)6Tuv/J. Available online: https://www.informatics.jax.org/strain/MGI:5293639 (accessed on 15 February 2023).
- du Sert, N.P.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Tsuchitani, M.; Sato, J.; Kokoshima, H. A comparison of the anatomical structure of the pancreas in experimental animals. J. Toxicol. Pathol. 2016, 29, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; van den Hoek, A.M. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic-Silva, J.; Beleza, J.; Coxito, P.; Pereira, S.; Rocha, H.; Gaspar, T.B.; Gartner, F.; Correia, R.; Martins, M.J.; Guimaraes, T.; et al. Maternal high-fat high-sucrose diet and gestational exercise modulate hepatic fat accumulation and liver mitochondrial respiratory capacity in mothers and male offspring. Metabolism 2021, 116, 154704. [Google Scholar] [CrossRef] [PubMed]
- Benede-Ubieto, R.; Estevez-Vazquez, O.; Ramadori, P.; Cubero, F.J.; Nevzorova, Y.A. Guidelines and Considerations for Metabolic Tolerance Tests in Mice. Diabetes Metab. Syndr. Obes. 2020, 13, 439–450. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Marinoni, I.; Wiederkeher, A.; Wiedmer, T.; Pantasis, S.; Di Domenico, A.; Frank, R.; Vassella, E.; Schmitt, A.; Perren, A. Hypo-methylation mediates chromosomal instability in pancreatic NET. Endocr. Relat. Cancer 2017, 24, 137–146. [Google Scholar] [CrossRef]
- Kitkumthorn, N.; Mutirangura, A. Long interspersed nuclear element-1 hypomethylation in cancer: Biology and clinical applications. Clin. Epigenetics 2011, 2, 315–330. [Google Scholar] [CrossRef]
- Di Domenico, A.; Wiedmer, T.; Marinoni, I.; Perren, A. Genetic and epigenetic drivers of neuroendocrine tumours (NET). Endocr. Relat. Cancer 2017, 24, R315–R334. [Google Scholar] [CrossRef]
- Luchini, C.; Lawlor, R.T.; Bersani, S.; Vicentini, C.; Paolino, G.; Mattiolo, P.; Pea, A.; Cingarlini, S.; Milella, M.; Scarpa, A. Alternative Lengthening of Telomeres (ALT) in Pancreatic Neuroendocrine Tumors: Ready for Prime-Time in Clinical Practice? Curr. Oncol. Rep. 2021, 23, 106. [Google Scholar] [CrossRef]
- Marinoni, I. Prognostic value of DAXX/ATRX loss of expression and ALT activation in PanNETs: Is it time for clinical implementation? Gut 2022, 71, 847–848. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Hori, M.; Ishigamori, R.; Mutoh, M.; Imai, T.; Nakagama, H. Fatty pancreas: A possible risk factor for pancreatic cancer in animals and humans. Cancer Sci. 2018, 109, 3013–3023. [Google Scholar] [CrossRef] [PubMed]
- Gannon, M.; Shiota, C.; Postic, C.; Wright, C.V.; Magnuson, M. Analysis of the Cre-mediated recombination driven by rat insulin promoter in embryonic and adult mouse pancreas. Genesis 2000, 26, 139–142. [Google Scholar] [CrossRef]
- Magnuson, M.A.; Osipovich, A.B. Pancreas-specific Cre driver lines and considerations for their prudent use. Cell Metab. 2013, 18, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Ristow, M.; Lin, X.; White, M.F.; Magnuson, M.A.; Hennighausen, L. RIP-Cre revisited, evidence for impairments of pancreatic beta-cell function. J. Biol. Chem. 2006, 281, 2649–2653. [Google Scholar] [CrossRef] [PubMed]
- Pomplun, D.; Florian, S.; Schulz, T.; Pfeiffer, A.F.; Ristow, M. Alterations of pancreatic beta-cell mass and islet number due to Ins2-controlled expression of Cre recombinase: RIP-Cre revisited; part 2. Horm. Metab. Res. 2007, 39, 336–340. [Google Scholar] [CrossRef]
- Kehm, R.; Konig, J.; Nowotny, K.; Jung, T.; Deubel, S.; Gohlke, S.; Schulz, T.J.; Hohn, A. Age-related oxidative changes in pancreatic islets are predominantly located in the vascular system. Redox Biol. 2018, 15, 387–393. [Google Scholar] [CrossRef]
- Liggit, D.; Dintzis, S.M. Pancreas. In Comparative Anatomy and Histology. A Mouse, Rat, and Human Atlas, 2 ed.; Treutin, P.M., Dintzis, S.M., Montine, K.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 241–250. [Google Scholar]
- Desai, V.; Patel, K.; Sheth, R.; Barlass, U.; Chan, Y.M.; Sclamberg, J.; Bishehsari, F. Pancreatic Fat Infiltration Is Associated with a Higher Risk of Pancreatic Ductal Adenocarcinoma. Visc. Med. 2020, 36, 220–226. [Google Scholar] [CrossRef]
- Villarroya, F.; Domingo, P.; Giralt, M. Drug-induced lipotoxicity: Lipodystrophy associated with HIV-1 infection and antiretroviral treatment. Biochim. Biophys. Acta 2010, 1801, 392–399. [Google Scholar] [CrossRef]
- Capurso, G.; Falconi, M.; Panzuto, F.; Rinzivillo, M.; Boninsegna, L.; Bettini, R.; Corleto, V.; Borgia, P.; Pederzoli, P.; Scarpa, A.; et al. Risk factors for sporadic pancreatic endocrine tumors: A case-control study of prospectively evaluated patients. Am. J. Gastroenterol. 2009, 104, 3034–3041. [Google Scholar] [CrossRef]
- Ben, Q.; Zhong, J.; Fei, J.; Chen, H.; Yv, L.; Tan, J.; Yuan, Y. Risk Factors for Sporadic Pancreatic Neuroendocrine Tumors: A Case-Control Study. Sci. Rep. 2016, 6, 36073. [Google Scholar] [CrossRef] [PubMed]
- Zhuge, X.; Wang, Y.; Chen, X.; Guo, C. Diabetes in Patients with Pancreatic Neuroendocrine Neoplasms. Front. Endocrinol. 2020, 11, 615082. [Google Scholar] [CrossRef]
- Tan, Q.; Wang, X.; Chen, C.; Liu, X.; Chen, Y.; Tan, C. Prognostic value of preoperative diabetes mellitus in patients with non-functional pancreatic neuroendocrine neoplasms. Am. J. Surg. 2022, 224, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.P.; Santos, A.C.; Castro, C.; Raposo, L.; Pereira, S.S.; Torres, I.; Henrique, R.; Cardoso, H.; Monteiro, M.P. Visceral Obesity and Metabolic Syndrome Are Associated with Well-Differentiated Gastroenteropancreatic Neuroendocrine Tumors. Cancers 2018, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Diabetesatlas.org. IDF Diabetes Atlas. Available online: https://diabetesatlas.org/ (accessed on 4 September 2022).
- Cives, M.; Partelli, S.; Palmirotta, R.; Lovero, D.; Mandriani, B.; Quaresmini, D.; Pelle, E.; Andreasi, V.; Castelli, P.; Strosberg, J.; et al. DAXX mutations as potential genomic markers of malignant evolution in small nonfunctioning pancreatic neuroendocrine tumors. Sci. Rep. 2019, 9, 18614. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Qiao, S.; Li, F.; Wang, W.; Jiang, R.; Wu, H.; Chen, H.; Liu, L.; Peng, J.; Wang, J.; et al. Whole-genome sequencing reveals distinct genetic bases for insulinomas and non-functional pancreatic neuroendocrine tumours: Leading to a new classification system. Gut 2020, 69, 877–887. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).