Paradigm Shift in the Management of Acute Myeloid Leukemia—Approved Options in 2023
Abstract
Simple Summary
Abstract
1. Introduction
2. Changes in Disease Classification in 2022
3. Treatment of Newly Diagnosed AML
3.1. Standard Induction: 7 + 3 Chemotherapy
3.2. Continuous vs. Intermittent Cytarabine
3.3. Anthracycline Choice and Dose
3.3.1. Optimizing Daunorubicin Dosing in AML
3.3.2. Which Anthracycline Is Best in AML?
4. Building on the 7 + 3 Backbone
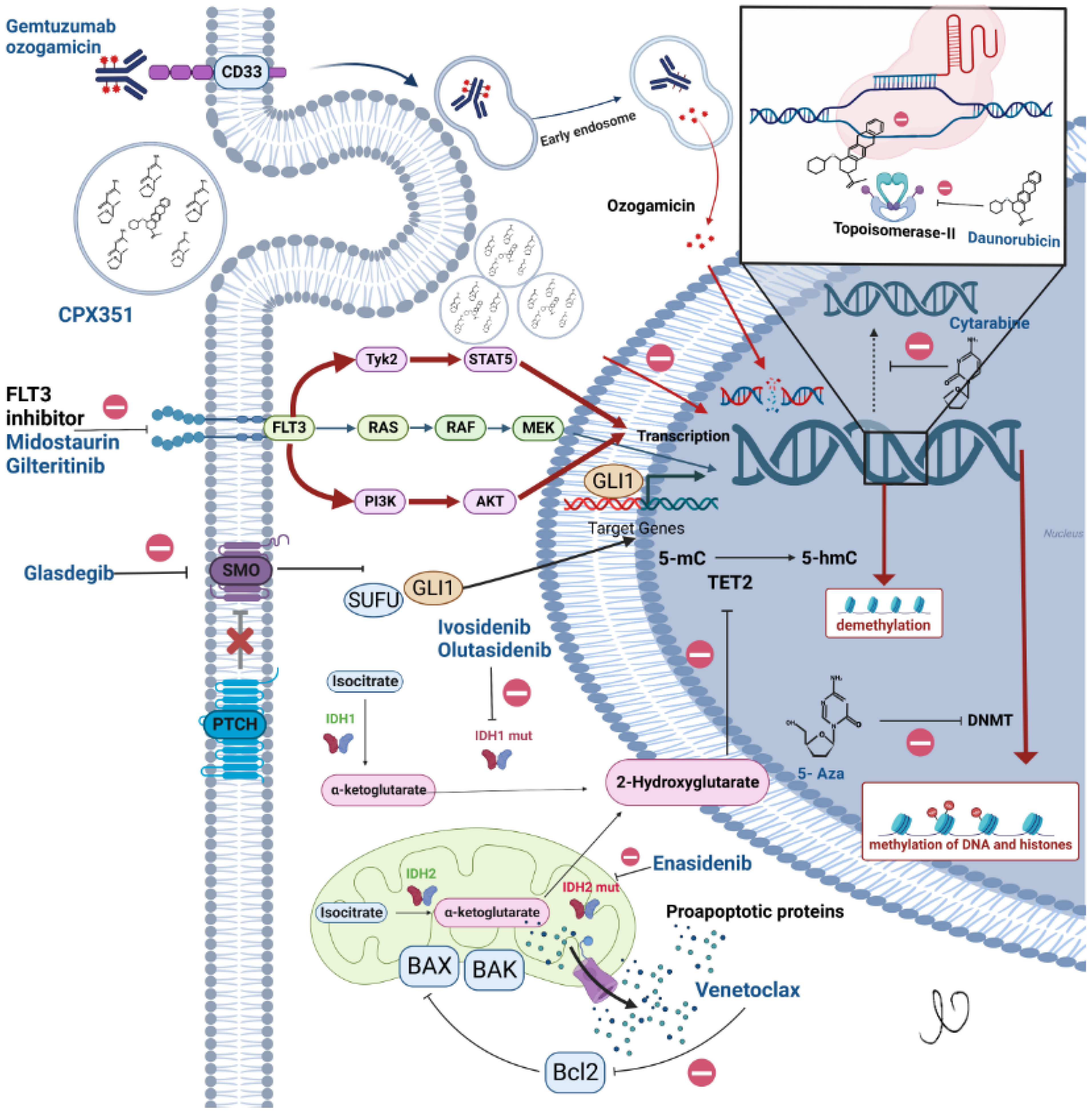
4.1. Gemtuzumab Ozogamicin (GO)
4.2. FLT3 Inhibitors
4.3. Lomustine
5. Liposomal Formulation of Cytarabine and Daunorubicin CPX-351
6. Lower Intensity Induction for Older Patients with AML
6.1. Low Dose Cytarabine
6.2. DNA Methyltransferase (DNMT) Inhibitors
7. FDA Approved Lower-Intensity Upfront Combinations
7.1. Venetoclax
Venetoclax with Low Dose Cytarabine
7.2. Venetoclax with DNMT Inhibitors
7.2.1. Venetoclax with Azacitidine
7.2.2. Venetoclax with Decitabine
7.3. Glasdegib with Low Dose Cytarabine
7.4. Isocitrate Dehydrogenase (IDH) Inhibitors with Azacitidine
8. Relapsed/Refractory AML
8.1. Hematopoietic Stem Cell Transplant (HSCT)
8.2. Targeted Therapy Approved in Relapsed/Refractory AML
8.2.1. Isocitrate Dehydrogenase Inhibitors
8.2.2. Enasidenib-IDH2 Inhibitor
8.2.3. Ivosidenib—IDH1 Inhibitor
8.2.4. Olutasidenib—IDH1 Inhibitor
8.2.5. FLT3 Inhibitor
9. Post Remission Therapy (Aka Consolidation Therapy)
10. Maintenance Therapy
Maintenance Therapy Post Allogenic Transplant
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madanat, Y.; Nazha, A. Novel and Investigational Therapies in Acute Myeloid Leukemia. In Acute Leukemias; Springer: Berlin/Heidelberg, Germany, 2021; pp. 133–144. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Steensma, D.P.; Bejar, R.; Jaiswal, S.; Lindsley, R.C.; Sekeres, M.A.; Hasserjian, R.P.; Ebert, B.L. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015, 126, 9–16. [Google Scholar] [CrossRef]
- Ley, T.J.; Miller, C.; Ding, L.; Raphael, B.J.; Mungall, A.J.; Robertson, A.; Hoadley, K.; Triche, T.J., Jr.; Laird, P.W.; Baty, J.D.; et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013, 368, 2059–2074. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.F.; Matthews, J.P.; Young, G.A.; Szer, J.; Gillett, A.; Joshua, D.; Bradstock, K.; Enno, A.; Wolf, M.M.; Fox, R.; et al. A randomized study of high-dose cytarabine in induction in acute myeloid leukemia. Blood 1996, 87, 1710–1717. [Google Scholar] [CrossRef]
- Weick, J.K.; Kopecky, K.J.; Appelbaum, F.R.; Head, D.R.; Kingsbury, L.L.; Balcerzak, S.P.; Bickers, J.N.; Hynes, H.E.; Welborn, J.L.; Simon, S.R.; et al. A randomized investigation of high-dose versus standard-dose cytosine arabinoside with daunorubicin in patients with previously untreated acute myeloid leukemia: A Southwest Oncology Group study. Blood 1996, 88, 2841–2851. [Google Scholar] [CrossRef]
- Kern, W.; Estey, E.H. High-dose cytosine arabinoside in the treatment of acute myeloid leukemia: Review of three randomized trials. Cancer 2006, 107, 116–124. [Google Scholar] [CrossRef]
- Löwenberg, B.; Pabst, T.; Vellenga, E.; van Putten, W.; Schouten, H.C.; Graux, C.; Ferrant, A.; Sonneveld, P.; Biemond, B.J.; Gratwohl, A.; et al. Cytarabine dose for acute myeloid leukemia. N. Engl. J. Med. 2011, 364, 1027–1036. [Google Scholar] [CrossRef]
- Willemze, R.; Suciu, S.; Meloni, G.; Labar, B.; Marie, J.P.; Halkes, C.J.; Muus, P.; Mistrik, M.; Amadori, S.; Specchia, G.; et al. High-dose cytarabine in induction treatment improves the outcome of adult patients younger than age 46 years with acute myeloid leukemia: Results of the EORTC-GIMEMA AML-12 trial. J. Clin. Oncol. 2014, 32, 219–228. [Google Scholar] [CrossRef]
- Watts, J.M.; Baer, M.R.; Yang, J.; Prebet, T.; Lee, S.; Schiller, G.J.; Dinner, S.N.; Pigneux, A.; Montesinos, P.; Wang, E.S.; et al. Olutasidenib alone or with azacitidine in IDH1-mutated acute myeloid leukaemia and myelodysplastic syndrome: Phase 1 results of a phase 1/2 trial. Lancet Haematol. 2023, 10, e46–e58. [Google Scholar] [CrossRef]
- Löwenberg, B.; Ossenkoppele, G.J.; van Putten, W.; Schouten, H.C.; Graux, C.; Ferrant, A.; Sonneveld, P.; Maertens, J.; Jongen-Lavrencic, M.; von Lilienfeld-Toal, M.; et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N. Engl. J. Med. 2009, 361, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, H.F.; Sun, Z.; Yao, X.; Litzow, M.R.; Luger, S.M.; Paietta, E.M.; Racevskis, J.; Dewald, G.W.; Ketterling, R.P.; Bennett, J.M.; et al. Anthracycline dose intensification in acute myeloid leukemia. N. Engl. J. Med. 2009, 361, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Luskin, M.R.; Lee, J.W.; Fernandez, H.F.; Abdel-Wahab, O.; Bennett, J.M.; Ketterling, R.P.; Lazarus, H.M.; Levine, R.L.; Litzow, M.R.; Paietta, E.M.; et al. Benefit of high-dose daunorubicin in AML induction extends across cytogenetic and molecular groups. Blood 2016, 127, 1551–1558. [Google Scholar] [CrossRef]
- Devillier, R.; Bertoli, S.; Prébet, T.; Huguet, F.; Etienne, A.; Charbonnier, A.; Rey, J.; Delabesse, E.; D’Incan, E.; Huynh, A.; et al. Comparison of 60 or 90 mg/m2 of daunorubicin in induction therapy for acute myeloid leukemia with intermediate or unfavorable cytogenetics. Am. J. Hematol. 2015, 90, E29–E30. [Google Scholar] [CrossRef] [PubMed]
- Prebet, T.; Bertoli, S.; Delaunay, J.; Pigneux, A.; Delabesse, E.; Mozziconacci, M.J.; Bidet, A.; Recher, C.; Vey, N. Anthracycline dose intensification improves molecular response and outcome of patients treated for core binding factor acute myeloid leukemia. Haematologica 2014, 99, e185–e187. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.K.; Russell, N.H.; Hills, R.K.; Kell, J.; Cavenagh, J.; Kjeldsen, L.; McMullin, M.F.; Cahalin, P.; Dennis, M.; Friis, L.; et al. A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: Results from the UK NCRI AML17 trial in 1206 patients. Blood 2015, 125, 3878–3885. [Google Scholar] [CrossRef] [PubMed]
- Röllig, C.; Steffen, B.; Schliemann, C.; Mikesch, J.-H.; Alakel, N.; Herbst, R.; Haenel, M.; Noppeney, R.; Hanoun, M.; Kaufmann, M.; et al. Single Versus Double Induction with “7 + 3” Containing 60 Versus 90 Mg Daunorubicin for Newly Diagnosed AML: Results from the Randomized Controlled SAL Dauno-Double Trial. Blood 2022, 140, 523–525. [Google Scholar] [CrossRef]
- AML Collaborative Group. A systematic collaborative overview of randomized trials comparing idarubicin with daunorubicin (or other anthracyclines) as induction therapy for acute myeloid leukaemia. Br. J. Haematol. 1998, 103, 100–109. [Google Scholar] [CrossRef]
- Pautas, C.; Merabet, F.; Thomas, X.; Raffoux, E.; Gardin, C.; Corm, S.; Bourhis, J.H.; Reman, O.; Turlure, P.; Contentin, N.; et al. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: Results of the ALFA-9801 study. J. Clin. Oncol. 2010, 28, 808–814. [Google Scholar] [CrossRef]
- Gardin, C.; Chevret, S.; Pautas, C.; Turlure, P.; Raffoux, E.; Thomas, X.; Quesnel, B.; de Revel, T.; de Botton, S.; Gachard, N.; et al. Superior long-term outcome with idarubicin compared with high-dose daunorubicin in patients with acute myeloid leukemia age 50 years and older. J. Clin. Oncol. 2013, 31, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, H.; Joo, Y.-D.; Lee, W.-S.; Bae, S.H.; Zang, D.Y.; Kwon, J.; Kim, M.K.; Lee, J.; Lee, G.W.; et al. Prospective Randomized Comparison of Idarubicin and High-Dose Daunorubicin in Induction Chemotherapy for Newly Diagnosed Acute Myeloid Leukemia. J. Clin. Oncol. 2017, 35, 2754–2763. [Google Scholar] [CrossRef] [PubMed]
- Bross, P.F.; Beitz, J.; Chen, G.; Chen, X.H.; Duffy, E.; Kieffer, L.; Roy, S.; Sridhara, R.; Rahman, A.; Williams, G.; et al. Approval summary: Gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin. Cancer Res. 2001, 7, 1490–1496. [Google Scholar] [PubMed]
- Sievers, E.L.; Larson, R.A.; Stadtmauer, E.A.; Estey, E.; Löwenberg, B.; Dombret, H.; Karanes, C.; Theobald, M.; Bennett, J.M.; Sherman, M.L.; et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J. Clin. Oncol. 2001, 19, 3244–3254. [Google Scholar] [CrossRef]
- Petersdorf, S.H.; Kopecky, K.J.; Slovak, M.; Willman, C.; Nevill, T.; Brandwein, J.; Larson, R.A.; Erba, H.P.; Stiff, P.J.; Stuart, R.K.; et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 2013, 121, 4854–4860. [Google Scholar] [CrossRef]
- Wadleigh, M.; Richardson, P.G.; Zahrieh, D.; Lee, S.J.; Cutler, C.; Ho, V.; Alyea, E.P.; Antin, J.H.; Stone, R.M.; Soiffer, R.J.; et al. Prior gemtuzumab ozogamicin exposure significantly increases the risk of veno-occlusive disease in patients who undergo myeloablative allogeneic stem cell transplantation. Blood 2003, 102, 1578–1582. [Google Scholar] [CrossRef]
- Castaigne, S.; Pautas, C.; Terré, C.; Raffoux, E.; Bordessoule, D.; Bastie, J.N.; Legrand, O.; Thomas, X.; Turlure, P.; Reman, O.; et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet 2012, 379, 1508–1516. [Google Scholar] [CrossRef]
- Hills, R.K.; Castaigne, S.; Appelbaum, F.R.; Delaunay, J.; Petersdorf, S.; Othus, M.; Estey, E.H.; Dombret, H.; Chevret, S.; Ifrah, N.; et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: A meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014, 15, 986–996. [Google Scholar] [CrossRef]
- Amadori, S.; Suciu, S.; Selleslag, D.; Aversa, F.; Gaidano, G.; Musso, M.; Annino, L.; Venditti, A.; Voso, M.T.; Mazzone, C.; et al. Gemtuzumab Ozogamicin Versus Best Supportive Care in Older Patients with Newly Diagnosed Acute Myeloid Leukemia Unsuitable for Intensive Chemotherapy: Results of the Randomized Phase III EORTC-GIMEMA AML-19 Trial. J. Clin. Oncol. 2016, 34, 972–979. [Google Scholar] [CrossRef]
- Taksin, A.L.; Legrand, O.; Raffoux, E.; de Revel, T.; Thomas, X.; Contentin, N.; Bouabdallah, R.; Pautas, C.; Turlure, P.; Reman, O.; et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: A prospective study of the alfa group. Leukemia 2007, 21, 66–71. [Google Scholar] [CrossRef]
- Guo, Y.; Deng, L.; Qiao, Y.; Liu, B. Efficacy and safety of adding gemtuzumab ozogamicin to conventional chemotherapy for adult acute myeloid leukemia: A systematic review and meta-analysis. Hematology 2022, 27, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Erba, H.; Montesinos, P.; Vrhovac, R.; Patkowska, E.; Kim, H.J.; Zak, P.; Wang, P.N.; Mitov, T.; Hanyok, J.; Liu, L.; et al. S100: Quizartinib prolonged survival vs placebo plus intensive induction and consolidation therapy followed by single-agent continuation in patients aged 18–75 years with newly diagnosed flt3-itd+ aml. HemaSphere 2022, 6, 1–4130. [Google Scholar] [CrossRef]
- Pigneux, A.; Harousseau, J.L.; Witz, F.; Sauvezie, M.; Bene, M.C.; Luquet, I.; Hunault-Berger, M.; Recher, C.; Lioure, B.; Himberlin, C.; et al. Addition of lomustine to idarubicin and cytarabine improves the outcome of elderly patients with de novo acute myeloid leukemia: A report from the GOELAMS. J. Clin. Oncol. 2010, 28, 3028–3034. [Google Scholar] [CrossRef]
- Largeaud, L.; Cornillet-Lefebvre, P.; Hamel, J.-F.; Dumas, P.-Y.; Prade, N.; Dufrechou, S.; Plenecassagnes, J.; Luquet, I.; Blanchet, O.; Banos, A.; et al. Lomustine is beneficial to older AML with ELN2017 adverse risk profile and intermediate karyotype: A FILO study. Leukemia 2021, 35, 1291–1300. [Google Scholar] [CrossRef]
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; et al. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients with Newly Diagnosed Secondary Acute Myeloid Leukemia. J. Clin. Oncol. 2018, 36, 2684–2692. [Google Scholar] [CrossRef]
- Bewersdorf, J.P.; Patel, K.K.; Goshua, G.; Shallis, R.M.; Podoltsev, N.A.; Huntington, S.F.; Zeidan, A.M. Cost-effectiveness of liposomal cytarabine/daunorubicin in patients with newly diagnosed acute myeloid leukemia. Blood 2022, 139, 1766–1770. [Google Scholar] [CrossRef]
- Sekeres, M.A.; Guyatt, G.; Abel, G.; Alibhai, S.; Altman, J.K.; Buckstein, R.; Choe, H.; Desai, P.; Erba, H.; Hourigan, C.S.; et al. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv. 2020, 4, 3528–3549. [Google Scholar] [CrossRef]
- Tilly, H.; Castaigne, S.; Bordessoule, D.; Casassus, P.; Le Prisé, P.Y.; Tertian, G.; Desablens, B.; Henry-Amar, M.; Degos, L. Low-dose cytarabine versus intensive chemotherapy in the treatment of acute nonlymphocytic leukemia in the elderly. J. Clin. Oncol. 1990, 8, 272–279. [Google Scholar] [CrossRef]
- Burnett, A.K.; Milligan, D.; Prentice, A.G.; Goldstone, A.H.; McMullin, M.F.; Hills, R.K.; Wheatley, K. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer 2007, 109, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; Thomas, X.G.; Dmoszynska, A.; Wierzbowska, A.; Mazur, G.; Mayer, J.; Gau, J.P.; Chou, W.C.; Buckstein, R.; Cermak, J.; et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J. Clin. Oncol. 2012, 30, 2670–2677. [Google Scholar] [CrossRef]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef]
- Lagadinou, E.D.; Sach, A.; Callahan, K.; Rossi, R.M.; Neering, S.J.; Minhajuddin, M.; Ashton, J.M.; Pei, S.; Grose, V.; O’Dwyer, K.M.; et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell 2013, 12, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Teh, T.C.; Nguyen, N.Y.; Moujalled, D.M.; Segal, D.; Pomilio, G.; Rijal, S.; Jabbour, A.; Cummins, K.; Lackovic, K.; Blombery, P.; et al. Enhancing venetoclax activity in acute myeloid leukemia by co-targeting MCL1. Leukemia 2018, 32, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.H.; Montesinos, P.; Ivanov, V.; DiNardo, C.D.; Novak, J.; Laribi, K.; Kim, I.; Stevens, D.A.; Fiedler, W.; Pagoni, M.; et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: A phase 3 randomized placebo-controlled trial. Blood 2020, 135, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef]
- Kwag, D.; Cho, B.-S.; Bang, S.-Y.; Lee, J.H.; Min, G.-J.; Park, S.-S.; Park, S.; Yoon, J.-H.; Lee, S.-E.; Eom, K.-S.; et al. Venetoclax with decitabine versus decitabine monotherapy in elderly acute myeloid leukemia: A propensity score-matched analysis. Blood Cancer J. 2022, 12, 169. [Google Scholar] [CrossRef]
- Queiroz, K.C.; Ruela-de-Sousa, R.R.; Fuhler, G.M.; Aberson, H.L.; Ferreira, C.V.; Peppelenbosch, M.P.; Spek, C.A. Hedgehog signaling maintains chemoresistance in myeloid leukemic cells. Oncogene 2010, 29, 6314–6322. [Google Scholar] [CrossRef]
- Cortes, J.E.; Heidel, F.H.; Hellmann, A.; Fiedler, W.; Smith, B.D.; Robak, T.; Montesinos, P.; Pollyea, D.A.; DesJardins, P.; Ottmann, O.; et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia 2019, 33, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Heuser, M.; Smith, B.D.; Fiedler, W.; Sekeres, M.A.; Montesinos, P.; Leber, B.; Merchant, A.; Papayannidis, C.; Pérez-Simón, J.A.; Hoang, C.J.; et al. Clinical benefit of glasdegib plus low-dose cytarabine in patients with de novo and secondary acute myeloid leukemia: Long-term analysis of a phase II randomized trial. Ann. Hematol. 2021, 100, 1181–1194. [Google Scholar] [CrossRef]
- Montesinos, P.; Recher, C.; Vives, S.; Zarzycka, E.; Wang, J.; Bertani, G.; Heuser, M.; Calado, R.T.; Schuh, A.C.; Yeh, S.P.; et al. Ivosidenib and Azacitidine in IDH1-Mutated Acute Myeloid Leukemia. N. Engl. J. Med. 2022, 386, 1519–1531. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Schuh, A.C.; Stein, E.M.; Montesinos, P.; Wei, A.H.; de Botton, S.; Zeidan, A.M.; Fathi, A.T.; Kantarjian, H.M.; Bennett, J.M.; et al. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia (AG221-AML-005): A single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol. 2021, 22, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Koreth, J.; Schlenk, R.; Kopecky, K.J.; Honda, S.; Sierra, J.; Djulbegovic, B.J.; Wadleigh, M.; DeAngelo, D.J.; Stone, R.M.; Sakamaki, H.; et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: Systematic review and meta-analysis of prospective clinical trials. Jama 2009, 301, 2349–2361. [Google Scholar] [CrossRef] [PubMed]
- Heuser, M.; Freeman, S.D.; Ossenkoppele, G.J.; Buccisano, F.; Hourigan, C.S.; Ngai, L.L.; Tettero, J.M.; Bachas, C.; Baer, C.; Béné, M.-C.; et al. 2021 Update on MRD in acute myeloid leukemia: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2021, 138, 2753–2767. [Google Scholar] [CrossRef]
- Stelljes, M.; Middeke, J.M.; Bug, G.; Wagner, E.-M.; Mueller, L.P.; Christoph, S.; Krause, S.W.; Bethge, W.; Jost, E.; Platzbecker, U.; et al. In Patients with Relapsed/Refractory AML Sequential Conditioning and Immediate Allogeneic Stem Cell Transplantation (allo-HCT) Results in Similar Overall and Leukemia-Free Survival Compared to Intensive Remission Induction Chemotherapy Followed By Allo-HCT: Results from the Randomized Phase III ASAP Trial. Blood 2022, 140, 9–11. [Google Scholar] [CrossRef]
- Stein, E.M.; DiNardo, C.D.; Pollyea, D.A.; Fathi, A.T.; Roboz, G.J.; Altman, J.K.; Stone, R.M.; DeAngelo, D.J.; Levine, R.L.; Flinn, I.W.; et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017, 130, 722–731. [Google Scholar] [CrossRef]
- de Botton, S.; Montesinos, P.; Schuh, A.C.; Papayannidis, C.; Vyas, P.; Wei, A.H.; Ommen, H.; Semochkin, S.; Kim, H.J.; Larson, R.A.; et al. Enasidenib vs conventional care in older patients with late-stage mutant-IDH2 relapsed/refractory AML: A randomized phase 3 trial. Blood 2023, 141, 156–167. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Stein, E.M.; de Botton, S.; Roboz, G.J.; Altman, J.K.; Mims, A.S.; Swords, R.; Collins, R.H.; Mannis, G.N.; Pollyea, D.A.; et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N. Engl. J. Med. 2018, 378, 2386–2398. [Google Scholar] [CrossRef]
- Choe, S.; Wang, H.; DiNardo, C.D.; Stein, E.M.; de Botton, S.; Roboz, G.J.; Altman, J.K.; Mims, A.S.; Watts, J.M.; Pollyea, D.A.; et al. Molecular mechanisms mediating relapse following ivosidenib monotherapy in IDH1-mutant relapsed or refractory AML. Blood Adv. 2020, 4, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Golub, D.; Iyengar, N.; Dogra, S.; Wong, T.; Bready, D.; Tang, K.; Modrek, A.S.; Placantonakis, D.G. Mutant Isocitrate Dehydrogenase Inhibitors as Targeted Cancer Therapeutics. Front. Oncol. 2019, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Schlenk, R.F.; Russell, N.H.; Levis, M.J. Targeting FLT3 mutations in AML: Review of current knowledge and evidence. Leukemia 2019, 33, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740. [Google Scholar] [CrossRef]
- Perl, A.E.; Larson, R.A.; Podoltsev, N.A.; Strickland, S.; Wang, E.S.; Atallah, E.; Schiller, G.J.; Martinelli, G.; Neubauer, A.; Sierra, J.; et al. Follow-up of patients with R/R FLT3-mutation–positive AML treated with gilteritinib in the phase 3 ADMIRAL trial. Blood 2022, 139, 3366–3375. [Google Scholar] [CrossRef]
- Perl, A.E.; Hosono, N.; Montesinos, P.; Podoltsev, N.; Martinelli, G.; Panoskaltsis, N.; Recher, C.; Smith, C.C.; Levis, M.J.; Strickland, S.; et al. Clinical outcomes in patients with relapsed/refractory FLT3-mutated acute myeloid leukemia treated with gilteritinib who received prior midostaurin or sorafenib. Blood Cancer J. 2022, 12, 84. [Google Scholar] [CrossRef]
- Mayer, R.J.; Davis, R.B.; Schiffer, C.A.; Berg, D.T.; Powell, B.L.; Schulman, P.; Omura, G.A.; Moore, J.O.; McIntyre, O.R.; Frei, E., 3rd. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N. Engl. J. Med. 1994, 331, 896–903. [Google Scholar] [CrossRef]
- Burnett, A.K.; Russell, N.H.; Hills, R.K.; Hunter, A.E.; Kjeldsen, L.; Yin, J.; Gibson, B.E.; Wheatley, K.; Milligan, D. Optimization of chemotherapy for younger patients with acute myeloid leukemia: Results of the medical research council AML15 trial. J. Clin. Oncol. 2013, 31, 3360–3368. [Google Scholar] [CrossRef]
- Jaramillo, S.; Benner, A.; Krauter, J.; Martin, H.; Kindler, T.; Bentz, M.; Salih, H.R.; Held, G.; Köhne, C.H.; Götze, K.; et al. Condensed versus standard schedule of high-dose cytarabine consolidation therapy with pegfilgrastim growth factor support in acute myeloid leukemia. Blood Cancer J. 2017, 7, e564. [Google Scholar] [CrossRef]
- Huls, G.; Chitu, D.A.; Havelange, V.; Jongen-Lavrencic, M.; van de Loosdrecht, A.A.; Biemond, B.J.; Sinnige, H.; Hodossy, B.; Graux, C.; Kooy, R.V.M.; et al. Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood 2019, 133, 1457–1464. [Google Scholar] [CrossRef]
- Wei, A.H.; Döhner, H.; Pocock, C.; Montesinos, P.; Afanasyev, B.; Dombret, H.; Ravandi, F.; Sayar, H.; Jang, J.H.; Porkka, K.; et al. Oral Azacitidine Maintenance Therapy for Acute Myeloid Leukemia in First Remission. N. Engl. J. Med. 2020, 383, 2526–2537. [Google Scholar] [CrossRef] [PubMed]
- Burchert, A.; Bug, G.; Fritz, L.V.; Finke, J.; Stelljes, M.; Röllig, C.; Wollmer, E.; Wäsch, R.; Bornhäuser, M.; Berg, T.; et al. Sorafenib Maintenance After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia with FLT3-Internal Tandem Duplication Mutation (SORMAIN). J. Clin. Oncol. 2020, 38, 2993–3002. [Google Scholar] [CrossRef] [PubMed]

| Induction Regimens for Newly Diagnosed Acute Myeloid Leukemia (AML) | |
|---|---|
| Drugs | Schedules |
| “7 + 3” | Anthracycline IV × 3 days Cytarabine IV continuous dose 100–200 mg/m2 Day 1–7 |
| “5 + 2” | Anthracycline IV × 2 days Cytarabine IV continuous dose 100–200 mg/m2 Day 1–5 |
| Vyxeos | (7 + 3) Vyxeos 100 units/m2 IV on Days 1, 3 and 5 (5 + 2) Vyxeos 65 units/m2 IV on Days 1 and 3 |
| Gemtuzumab Ozogamicin with 7 + 3 | Gemtuzumab 3 mg/m2 on Days 1, 4 and 7 “7 + 3” |
| Gemtuzumab Ozogamicin | Gemtuzumab 6 mg/m2 on Day 1 and 3 mg/m2 on Day 8 Cycle 1 Followed by up to 8 doses of 2 mg/m2 Day 1 every 28 days |
| Midostaurin with Daunorubicin and Cytarabine | DAUNORUBICIN 60 mg/m2 IV day 1–3 Midostaurin 50 mg BID PO Days 8–21 Cytarabine 200 mg/m2 IV Day 1–7 |
| Venetoclax + Azacitidine | Azacitidine 75 mg/m2 IV or subQ Day 1–7 every 28 days Venetoclax 400 mg PO once daily Day 1–28. (Dose ramped up as 100 mg on Day 1, 200 mg on Day 2 and 400 mg on Day 3 during cycle 1) |
| Venetoclax + Low dose cytarabine | Low dose cytarabine 20 mg/m2 SubQ Day 1–10 every 28 days Venetoclax 600 mg PO once daily Days 1–28 (Dose ramped up as 100 mg on Day 1, 200 mg on Day 2, 400 mg on Day 3 and 600 mg on Day 4) |
| Glasdegib + Low dose cytarabine | Low dose cytarabine 20 mg BID SubQ Day 1–10 every 28 days Glasdegib 100 mg PO daily Day 1–28 |
| Ivosidenib + Azacitidine | Azacitidine 75 mg/m2 subQ Days 1–7 every 28 days Ivosidenib 500 mg PO once daily Day 1–28. |
| Regimens for R/R AML | |
| Gemtuzumab | Gemtuzumab 3 mg/m2 on Days 1, 4 and 7 |
| Enasidenib | Enasidenib 100 mg PO daily |
| Ivosidenib | Ivosidenib 500 mg PO daily |
| Olutasidenib | Olutasidenib 150 mg PO BID |
| Gilteritinib | Gilteritinib 120 mg PO daily |
| Post Remission Therapy (Consolidation) | |
| Cytarabine | Cytarabine 1.5–3 g/m2 given BID on Days 1, 3 and 5 [total 6 doses] |
| Gemtuzumab + cytarabine | Gemtuzumab 3 g/m2 on Day 1 Cytarabine 1.5–3 g/m2 given BID on Day 1, 3 and 5 |
| Maintenance | |
| Oral azacitidine | Azacitidine 300 mg PO daily Day 1–14 every 28 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Premnath, N.; Madanat, Y.F. Paradigm Shift in the Management of Acute Myeloid Leukemia—Approved Options in 2023. Cancers 2023, 15, 3002. https://doi.org/10.3390/cancers15113002
Premnath N, Madanat YF. Paradigm Shift in the Management of Acute Myeloid Leukemia—Approved Options in 2023. Cancers. 2023; 15(11):3002. https://doi.org/10.3390/cancers15113002
Chicago/Turabian StylePremnath, Naveen, and Yazan F. Madanat. 2023. "Paradigm Shift in the Management of Acute Myeloid Leukemia—Approved Options in 2023" Cancers 15, no. 11: 3002. https://doi.org/10.3390/cancers15113002
APA StylePremnath, N., & Madanat, Y. F. (2023). Paradigm Shift in the Management of Acute Myeloid Leukemia—Approved Options in 2023. Cancers, 15(11), 3002. https://doi.org/10.3390/cancers15113002







