Results from a Phase 1b/2 Study of Ibrutinib Combination Therapy in Advanced Urothelial Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Assessments and Analyses
2.3. Statistical Considerations and Analysis Populations
2.4. Data Availability
3. Results
3.1. Phase 1b
Baseline Characteristics and Patient Disposition
3.2. Phase 1b/2 at RP2D
Baseline Characteristics and Patient Disposition
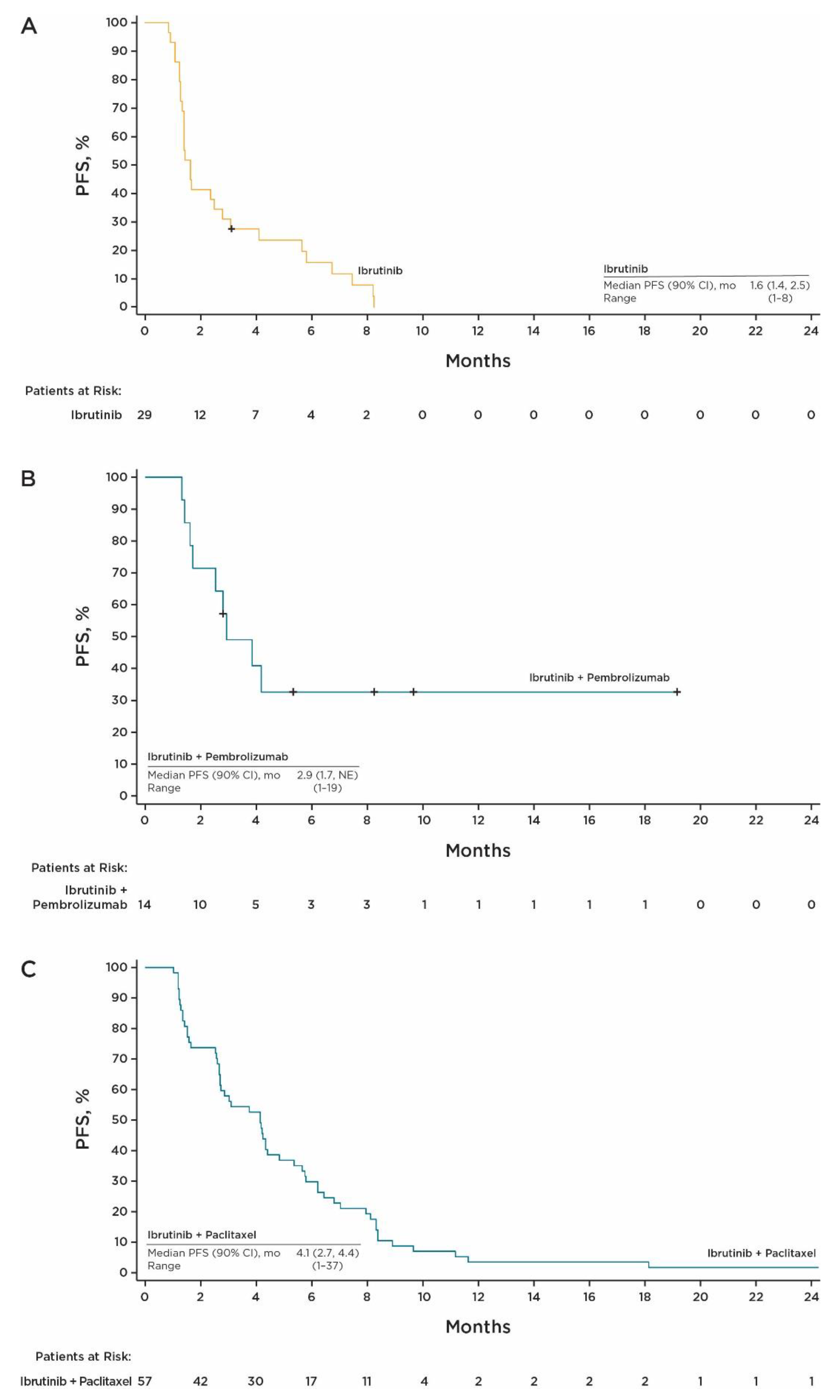
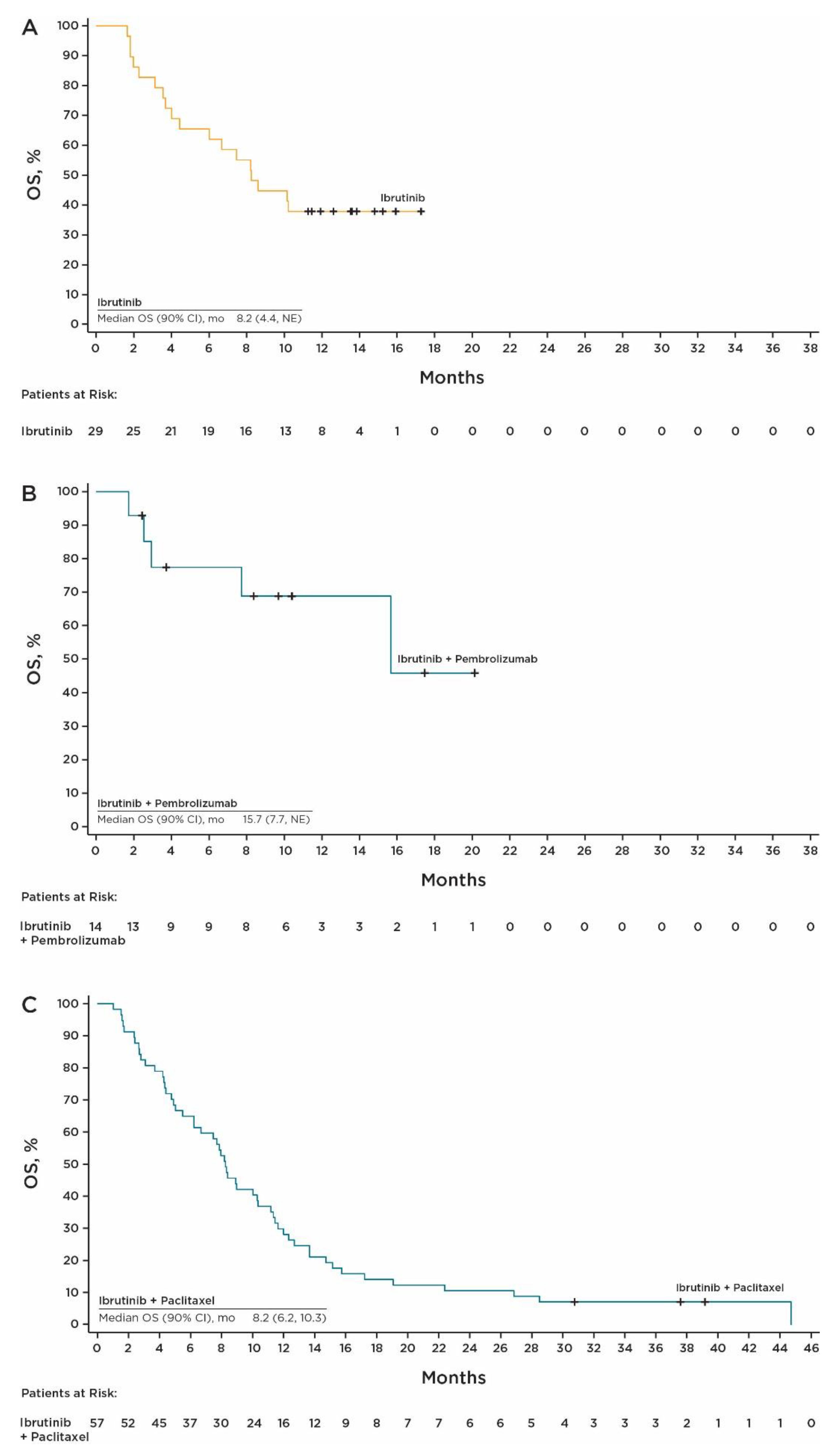
3.3. Efficacy at RP2D
3.4. Phase 1b Safety
3.5. Safety at RP2D
3.6. Pharmacokinetics
3.7. Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer Cancer. Data Visualization Tools for Exploring the Global Cancer Burden in 2020. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/30-Bladder-fact-sheet.pdf (accessed on 3 January 2023).
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of bladder cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, B.; Srinivas, S. Urothelial carcinoma: The evolving landscape of immunotherapy for patients with advanced disease. Res. Rep. Urol. 2018, 10, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Hepp, Z.; Shah, S.N.; Smoyer, K.; Vadagam, P. Epidemiology and treatment patterns for locally advanced or metastatic urothelial carcinoma: A systematic literature review and gap analysis. J. Manag. Care Spec. Pharm. 2021, 27, 240–255. [Google Scholar] [CrossRef]
- Fang, W.; Yang, Z.Y.; Chen, T.Y.; Shen, X.F.; Zhang, C. Ethnicity and survival in bladder cancer: A population-based study based on the SEER database. J. Transl. Med. 2020, 18, 145. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef]
- Imbruvica (Ibrutinib) [Prescribing Information]; Pharmacyclics LLC: San Francisco, CA, USA, 2022.
- Sagiv-Barfi, I.; Kohrt, H.E.; Czerwinski, D.K.; Ng, P.P.; Chang, B.Y.; Levy, R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc. Natl. Acad. Sci. USA 2015, 112, E966–E972. [Google Scholar] [CrossRef]
- Campbell, R.; Chong, G.; Hawkes, E.A. Novel indications for Bruton’s tyrosine kinase inhibitors, beyond hematological malignancies. J. Clin. Med. 2018, 7, 62. [Google Scholar] [CrossRef]
- Szklener, K.; Michalski, A.; Żak, K.; Piwoński, M.; Mańdziuk, S. Ibrutinib in the treatment of solid tumors: Current state of knowledge and future directions. Cells 2022, 11, 1338. [Google Scholar] [CrossRef]
- Guo, S.; Sun, F.; Guo, Z.; Li, W.; Alfano, A.; Chen, H.; Magyar, C.E.; Huang, J.; Chai, T.; Qiu, S.; et al. Tyrosine kinase ETK/BMX is up-regulated in bladder cancer and predicts poor prognosis in patients with cystectomy. PLoS ONE 2011, 6, e17778. [Google Scholar] [CrossRef]
- Dubovsky, J.A.; Beckwith, K.A.; Natarajan, G.; Woyach, J.A.; Jaglowski, S.; Zhong, Y.; Hessler, J.D.; Liu, T.-M.; Chang, B.Y.; Larkin, K.M.; et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 2013, 122, 2539–2549. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v4.03. 14 June 2010. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 3 January 2023).
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Bladder Cancer (Version 2.2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf (accessed on 3 January 2023).
- Raggi, D.; Miceli, R.; Sonpavde, G.; Giannatempo, P.; Mariani, L.; Galsky, M.D.; Bellmunt, J.; Necchi, A. Second-line single-agent versus doublet chemotherapy as salvage therapy for metastatic urothelial cancer: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 49–61. [Google Scholar] [CrossRef]
- Bellmunt, J.; De Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Fradet, Y.; Bellmunt, J.; Vaughn, D.J.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; Necchi, A.; et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: Results of >2 years of follow-up. Ann. Oncol. 2019, 30, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Durán, I.; Van Der Heijden, M.S.; Loriot, Y.; Vogelzang, N.J.; De Giorgi, U.; Oudard, S.; Retz, M.M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757. [Google Scholar] [CrossRef]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef]
- Galsky, M.D.; Arija, J.Á.A.; Bamias, A.; Davis, I.D.; De Santis, M.; Kikuchi, E.; Garcia-del-Muro, X.; De Giorgi, U.; Mencinger, M.; Izumi, K.; et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1547–1557. [Google Scholar] [CrossRef]
- Powles, T.; Csőszi, T.; Özgüroğlu, M.; Matsubara, N.; Géczi, L.; Cheng, S.Y.-S.; Fradet, Y.; Oudard, S.; Vulsteke, C.; Barrera, R.M.; et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Harrison, M.R.; O’Donnell, P.H.; Alva, A.S.; Hahn, N.M.; Appleman, L.J.; Cetnar, J.; Burke, J.M.; Fleming, M.T.; Milowsky, M.I.; et al. A randomized phase 2 trial of pembrolizumab versus pembrolizumab and acalabrutinib in patients with platinum-resistant metastatic urothelial cancer. Cancer 2020, 126, 4485–4497. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Harrington, B.; O’brien, S.; Jones, J.A.; Schuh, A.; Devereux, S.; Chaves, J.; Wierda, W.G.; Awan, F.T.; Brown, J.R.; et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2016, 374, 323–332. [Google Scholar] [CrossRef]
- Mhibik, M.; Gaglione, E.M.; Eik, D.; Kendall, E.K.; Blackburn, A.; Keyvanfar, K.; Baptista, M.J.; Ahn, I.E.; Sun, C.; Qi, J.; et al. BTK inhibitors, irrespective of ITK inhibition, increase efficacy of a CD19/CD3-bispecific antibody in CLL. Blood 2021, 138, 1843–1854. [Google Scholar] [CrossRef]
- Vaughn, D.J.; Broome, C.M.; Hussain, M.; Gutheil, J.C.; Markowitz, A.B. Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J. Clin. Oncol. 2002, 20, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Joly, F.; Houédé, N.; Noal, S.; Chevreau, C.; Priou, F.; Chinet-Charrot, P.; Rolland, F.; Fléchon, A.; Henry-Amar, M.; Culine, S. Do patients with advanced urothelial carcinoma benefit from weekly paclitaxel chemotherapy? A GETUG phase II study. Clin. Genitorin. Cancer 2009, 7, E28–E33. [Google Scholar] [CrossRef]
- Papamichael, D.; Gallagher, C.J.; Oliver, R.T.; Johnson, P.W.; Waxman, J. Phase II study of paclitaxel in pretreated patients with locally advanced/metastatic cancer of the bladder and ureter. Br. J. Cancer 1997, 75, 606–607. [Google Scholar] [CrossRef] [PubMed]
- Sideris, S.; Aoun, F.; Zanaty, M.; Martinez, N.C.; Latifyan, S.; Awada, A.; Gil, T. Efficacy of weekly paclitaxel treatment as a single agent chemotherapy following first-line cisplatin treatment in urothelial bladder cancer. Mol. Clin. Oncol. 2016, 4, 1063–1067. [Google Scholar] [CrossRef]
- McCaffrey, J.A.; Hilton, S.; Mazumdar, M.; Sadan, S.; Kelly, W.K.; Scher, H.I.; Bajorin, D.F. Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J. Clin. Oncol. 1997, 15, 1853–1857. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Flaig, T.W.; Friedlander, T.W.; Milowsky, M.I.; Srinivas, S.; Petrylak, D.P.; Merchan, J.R.; Bilen, M.A.; Carret, A.-S.; Yuan, N.; et al. Study EV-103: Durability results of enfortumab vedotin plus pembrolizumab for locally advanced or metastatic urothelial carcinoma. J. Clin. Oncol. 2020, 38 (Suppl. S15), 5044. [Google Scholar]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef]
- Yu, E.Y.; Petrylak, D.P.; O’Donnell, P.H.; Lee, J.L.; van der Heijden, M.S.; Loriot, Y.; Stein, M.N.; Necchi, A.; Kojima, T.; Harrison, M.R.; et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2021, 22, 872–882. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Balar, A.V.; Petrylak, D.P.; Kalebasty, A.R.; Loriot, Y.; Fléchon, A.; Jain, R.K.; Agarwal, N.; Bupathi, M.; Barthelemy, P.; et al. TROPHY-U-01: A phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J. Clin. Oncol. 2021, 39, 2474–2485. [Google Scholar] [CrossRef] [PubMed]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef] [PubMed]

| Ibrutinib 840 mg N = 35 b | Ibrutinib 560 mg + Pembrolizumab N = 18 | Ibrutinib 840 mg + Paclitaxel N = 59 | |
|---|---|---|---|
| Median age (range), years | 71 (52–88) | 70 (52–84) | 68 (48–90) |
| >65 years, n (%) | 24 (69) | 12 (67) | 36 (61) |
| Male, n (%) | 26 (74) | 13 (72) | 51 (86) |
| Race, n (%) | |||
| White | 30 (86) | 16 (89) | 47 (80) |
| Black or African American | 0 | 1 (6) | 0 |
| Asian | 5 (14) | 0 | 12 (20) |
| American Indian/Alaska Native | 0 | 1 (6) | 0 |
| ECOG performance status, n (%) | |||
| 0 | 8 (23) | 7 (39) | 9 (15) |
| 1 | 27 (77) | 11 (61) | 50 (85) |
| Time from initial diagnosis to start of treatment, median (range), months | 38 (15–129) | 17 (2–85) | 20 (5–161) |
| Metastatic sites of disease, n (%) | |||
| 0 | 0 | 1 (6) | 1 (2) |
| 1 | 9 (26) | 4 (22) | 13 (22) |
| 2 | 15 (43) | 7 (39) | 24 (41) |
| >2 | 11 (31) | 6 (33) | 21 (36) |
| Sites of metastasis, n (%) | |||
| With metastases | 34 (97) | 17 (94) | 56 (95) |
| Bone | 11 (31) | 7 (39) | 14 (24) |
| Liver | 13 (37) | 5 (28) | 12 (20) |
| Lung | 16 (46) | 10 (56) | 25 (42) |
| Lymph node | 25 (71) | 10 (56) | 46 (78) |
| Peritoneal | 6 (17) | 3 (17) | 5 (8) |
| Number of prior regimens, n (%) | |||
| 0 | N/A | 5 (28) | N/A |
| 1 | 5 (14) | 11 (61) | 28 (47) |
| 2 | 30 (86) | 2 (11) | 31 (53) |
| Treatment duration, ibrutinib, median (range), months | 1.4 (0.1–6.3) | 2.2 (0.1–19.9) | 2.3 (0.2–38.0) |
| Treatment duration of partner drug, median (range), months | N/A | 1.7 (0.0–19.4) | 1.6 (0.0–5.6) |
| Ibrutinib treatment disposition, n (%) | |||
| Still on treatment | 0 | 0 | 0 |
| Discontinued treatment | 35 (100) | 18 (100) | 59 (100) |
| Primary reason for ibrutinib discontinuation, n (%) | |||
| Disease progression | 21 (60) | 6 (33) | 36 (61) |
| Clinical deterioration | 4 (11) | 0 | 4 (7) |
| Adverse events unrelated to PD or TCC | 5 (14) | 3 (17) | 9 (15) |
| Death | 2 (6) | 3 (17) | 1 (2) |
| Withdrawal of consent | 3 (9) | 4 (22) | 7 (12) |
| Investigator decision | 0 | 0 | 2 (3) |
| Study terminated by sponsor | 0 | 2 (11) | 0 |
| Companion drug treatment disposition, n (%) | |||
| Still on treatment | N/A | 0 | 0 |
| Discontinued treatment | N/A | 18 (100) | 59 (100) |
| Primary reason for discontinuation of companion drug, n (%) | |||
| Disease progression | N/A | 6 (33) | 27 (46) |
| Clinical deterioration | N/A | 0 | 1 (2) |
| Adverse events unrelated to PD | N/A | 1 (6) | 24 (41) |
| Death | N/A | 3 (17) | 0 |
| Withdrawal of consent | N/A | 6 (33) | 7 (12) |
| Investigator decision | N/A | 0 | 0 |
| Study terminated by sponsor | N/A | 2 (11) | 0 |
| Ibrutinib 840 mg N = 29 | Ibrutinib 560 mg + Pembrolizumab N = 14 | Ibrutinib 840 mg + Paclitaxel N = 57 | |
|---|---|---|---|
| Median PFS (range), months | 1.6 (0.9–8.2) | 2.9 (1.3–19.2+) | 4.1 (1.0–37.4+) |
| Best confirmed ORR, % (90% CI) | 6.9 (1.2, 20.2) | 35.7 (15.3, 61.0) | 26.3 (17.0, 37.6) |
| Best overall response, n (%) | |||
| CR | 0 | 0 | 2 (3.5) |
| PR | 2 (6.9) | 5 (35.7) | 13 (22.8) |
| SD | 12 (41.4) | 5 (35.7) | 23 (40.4) |
| PD | 15 (51.7) | 4 (28.6) | 12 (21.1) |
| Not evaluable | 0 | 0 | 1 (1.8) |
| Unknown/missing | 0 | 0 | 6 (10.5) |
| DCR, % (90% CI) | 48.3 (32.0, 64.8) | 71.4 (46.0, 89.6) | 66.7 (55.0, 77.0) |
| Median OS, (90% CI), months | 8.2 (4.4, NE) | 15.7 (7.7, NE) | 8.2 (6.2, 10.3) |
| Ibrutinib 840 mg N = 35 | Ibrutinib 560 mg + Pembrolizumab N = 18 | Ibrutinib 840 mg + Paclitaxel N = 59 | ||||
|---|---|---|---|---|---|---|
| TEAE a | Any Grade (≥20%) | Grade ≥3 (≥10%) | Any Grade (≥20%) | Grade ≥3 (≥10%) | Any Grade (≥20%) | Grade ≥3 (≥10%) |
| Diarrhea | 10 (29) | 2 (6) | 8 (44) | 0 | 44 (75) | 6 (10) |
| Asthenia | 13 (37) | 1 (3) | 31 (53) | 13 (22) | ||
| Decreased appetite | 12 (34) | 0 | 25 (42) | 2 (3) | ||
| Anemia | 7 (20) | 2 (6) | 24 (41) | 10 (17) | ||
| Nausea | 16 (46) | 1 (3) | 6 (33) | 0 | 23 (39) | 1 (2) |
| Fatigue | 10 (29) | 2 (6) | 8 (44) | 1 (6) | 19 (32) | 4 (7) |
| Constipation | 9 (26) | 0 | 7 (39) | 0 | 19 (32) | 0 |
| Alopecia | 18 (31) | 1 (2) | ||||
| Stomatitis | 4 (22) | 0 | 17 (29) | 1 (2) | ||
| Vomiting | 10 (29) | 0 | 17 (29) | 1 (2) | ||
| Peripheral sensory neuropathy | 16 (27) | 6 (10) | ||||
| Edema peripheral | 4 (22) | 1 (6) | 15 (25) | 0 | ||
| Urinary tract infection | 7 (20) | 2 (6) | 13 (22) | 7 (12) | ||
| Arthralgia | 6 (33) | 0 | 13 (22) | 1 (2) | ||
| Hematuria | 6 (33) | 1 (6) | 14 (24) | 1 (2) | ||
| Dizziness | 9 (26) | 0 | 4 (22) | 0 | ||
| Rash maculopapular | 5 (28) | 1 (6) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mar, N.; Zakharia, Y.; Falcon, A.; Morales-Barrera, R.; Mellado, B.; Duran, I.; Oh, D.-Y.; Williamson, S.K.; Gajate, P.; Arkenau, H.-T.; et al. Results from a Phase 1b/2 Study of Ibrutinib Combination Therapy in Advanced Urothelial Carcinoma. Cancers 2023, 15, 2978. https://doi.org/10.3390/cancers15112978
Mar N, Zakharia Y, Falcon A, Morales-Barrera R, Mellado B, Duran I, Oh D-Y, Williamson SK, Gajate P, Arkenau H-T, et al. Results from a Phase 1b/2 Study of Ibrutinib Combination Therapy in Advanced Urothelial Carcinoma. Cancers. 2023; 15(11):2978. https://doi.org/10.3390/cancers15112978
Chicago/Turabian StyleMar, Nataliya, Yousef Zakharia, Alejandro Falcon, Rafael Morales-Barrera, Begona Mellado, Ignacio Duran, Do-Youn Oh, Stephen K. Williamson, Pablo Gajate, Hendrik-Tobias Arkenau, and et al. 2023. "Results from a Phase 1b/2 Study of Ibrutinib Combination Therapy in Advanced Urothelial Carcinoma" Cancers 15, no. 11: 2978. https://doi.org/10.3390/cancers15112978
APA StyleMar, N., Zakharia, Y., Falcon, A., Morales-Barrera, R., Mellado, B., Duran, I., Oh, D.-Y., Williamson, S. K., Gajate, P., Arkenau, H.-T., Jones, R. J., Teo, M. Y., Turan, T., McLaughlin, R. T., Peltier, H. M., Chong, E., Atluri, H., Dean, J. P., & Castellano, D. (2023). Results from a Phase 1b/2 Study of Ibrutinib Combination Therapy in Advanced Urothelial Carcinoma. Cancers, 15(11), 2978. https://doi.org/10.3390/cancers15112978





