Simple Summary
Colorectal cancer (CRC) incidence is increasing worldwide and represents an important health problem.Therapy failure and progression to metastatic disease are major concerns. Among the factors involved in tumor growth, galectin-3 (Gal-3) plays an important role due to its ability to finetune a number of molecular players that act at different levels of cancer-related processes. A clear relationship between Gal-3 and CRC has been demonstrated. Several studies have, indeed, reported a pathogenetic role for this protein in intestinal inflammation and CRC onset/progression. Moreover, some plant-source food-derived bioactive compounds (mostly fibers and polyphenols) can contribute to the control of CRC onset/growth through their capacity to block Gal-3 activities. In this review, we summarize these studies, highlighting the influence of Gal-3 on CRC risk/progression, cancer cell spreading and patient prognosis, as well as the potential of natural food-derived Gal-3 inhibitors as promising candidates for CRC prevention and therapy.
Abstract
Colorectal cancer (CRC) is a leading cause of death worldwide. Despite advances in surgical and therapeutic management, tumor metastases and resistance to therapy still represent major hurdles. CRC risk is highly modifiable by lifestyle factors, including diet, which strongly influences both cancer incidence and related mortality. Galectin-3 (Gal-3) is a multifaceted protein involved in multiple pathophysiological pathways underlying chronic inflammation and cancer. Its versatility is given by the ability to participate in a wide range of tumor-promoting processes, including cell–cell/cell–matrix interactions, cell growth regulation and apoptosis, and the immunosuppressive tumor microenvironment. This review provides an updated summary of preclinical and observational human studies investigating the pathogenetic role of Gal-3 in intestinal inflammation and CRC, as well as the potential of Gal-3 activity inhibition by plant-source food-derived bioactive compounds to control CRC onset/growth. These studies highlight both direct and immuno-mediated effects of Gal-3 on tumor growth and invasiveness and its potential role as a CRC prognostic biomarker. Substantial evidence indicates natural food-derived Gal-3 inhibitors as promising candidates for CRC prevention and therapy. However, critical issues, such as their bioavailability and efficacy, in controlled human studies need to be addressed to translate research progress into clinical applications.
1. Introduction
Colorectal cancer (CRC) is the third-most-common cancer and the second-leading cause of cancer-related mortality worldwide, thus, representing a considerable health issue [1,2] (WHO. Cancer. Available online https://www.who.int/news-room/fact-sheets/detail/cancer, accessed on 3 November 2022). The worldwide increase in CRC incidence and mortality has been associated to the overweight/obesity epidemic and to the increasing adoption of Western lifestyles, with a shift in dietary patterns towards a decreased consumption of plant-source foods and an increased intake of fat, sugar and animal-source foods [3]. Epidemiologic research approximates that more than one-half of colon cancer risk is preventable through lifestyle and dietary measures [4,5], and numerous studies confirmed the association between dietary patterns and CRC risk [6,7] (World Cancer Research Fund /American Institute for Cancer Research, https://www.wcrf.org/wp-content/uploads/2021/02/Colorectal-cancer-report.pdf, accessed on 10 October 2022). In particular, prudent dietary patterns and consumption of fruits and vegetables have been inversely correlated with both the risk of developing CRC and CRC-related mortality [8,9]. Strong evidence has also been provided for an inverse association between CRC risk and consumption of fiber-rich foods [6,10,11,12,13,14,15](World Cancer Research Fund/American Institute for Cancer Research, https://www.wcrf.org/wp-content/uploads/2021/02/Colorectal-cancer-report.pdf, accessed on 10 October 2022).
CRC exhibits a great level of complexity, being characterized by several multi-step disease events linked to the accumulation of genetic/epigenetic alterations, as well as high heterogeneity at all disease stages [16]. Dysregulated immune response and immunosuppression in the tumor microenvironment (TME) strongly contribute to tumor growth and invasion. Surgery is the gold-standard treatment for early-stage disease. However, approximately 25% of patients have metastatic disease already at diagnosis and ~50% of CRC-diagnosed patients will develop metastases, mostly in the liver. Even with the recent advances in surgical and therapeutic options, tumor metastases and therapy resistance are the foremost hurdles in CRC management.
Multiple genes are involved in the biology of CRC, including different galectins. Galectins are glycan-binding proteins, whose importance is due to their ability to control the innate and adaptive immune system in health and disease conditions [17,18]. Sixteen galectins, distinguished into three main groups (prototypal, tandem repeat and chimeric) based on the carbohydrate recognition domain (CRD), have so far been identified in mammals [19]. Galectin- (Gal-) 1, -2, -5, -7, -10, -11, -13, -14, -15 and -16 are “prototype” galectins, characterized by the presence of a single CRD, while Gal-4, -6, -8, -9 and -12 belong to the “tandem-repeat” type with two distinct CRDs connected by a short linker peptide [20,21,22,23].
Gal-3 is the only “chimera-type” galectin, whose unique and intriguing structure is composed of a COOH-terminal domain containing one CRD linked to an extended N-terminal (NT) region [20,21,22,23] (Figure 1A). It is ubiquitously produced and can be found in the cytoplasm, in the nucleus or released in the extracellular space [24]. In humans, Gal-3 is encoded by the LGALS3 gene, located on chromosome 14 locus q21-22 [25], and similarly to several other galectins, is structurally folded as a globule of two antiparallel beta sheets formed by five and six beta strands; the residues involved in carbohydrate binding are part of a pocket formed by four adjacent beta strands [26]. The Gal-3-CRD contains an Asp-Trp-Gly-Arg-motif [27] that is also present in the Bcl-2 family of apoptosis regulators and is responsible for the anti-apoptotic activity of Gal-3 [28]. Its N-terminal region is rich in proline residues (27 in humans), whose functional value is to modulate Gal-3 function [29]. Mutations in each proline within the N-terminal region differentially control NT–CRD interactions, consequently affecting glycan binding, liquid–liquid phase separation and cellular activities [29]. A recent finding has shown that these genetic variants are linked to an increased risk of disorders related to immunity or autoimmunity, such as inflammatory bowel disease (IBD) and type 1 diabetes [30].
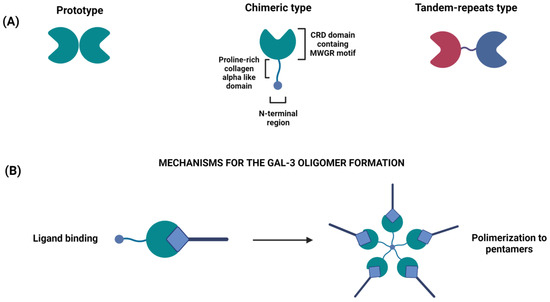
Figure 1.
(A) Classification of the different types of galectins: prototype (Gal-1, -2, -5,-7,- 20,-11, -13, -14, -15 and -16), chimeric type (Gal-3), tandem-repeat type (Gal-4, -6,-8,-9 and -12). Prototype galectins have one CRD that can dimerize; tandem-repeat galectins have two different CRDs (red and blue colors are used to distinguish them) linked by a linker domain; chimera-type galectin (Gal-3) has a single C-terminal CRD and a N-terminal oligomerization domain. (B) Gal-3 oligomerization: upon oligosaccharide binding by the C-terminal domain, the non-lectin domains of Gal-3 oligomerize, thereby cross-linking ligands of the cell surface.
The importance of Gal-3 in regulating the immune response and inflammation as well as the transition from acute to chronic inflammation has been amply recognized. However, its involvement in the pathogenesis of intestinal inflammation has not yet been completely clarified [31,32].
Chronic inflammation plays a key role in colon carcinogenesis, and diet has the potential to influence cancer risk and progression by regulating gut inflammation. A relationship among diet and its components and Gal-3 expression and activity has been higlighted. Pro-inflammatory high fat diets (HFDs) and saturated fatty acids increase Gal-3 levels in exposed animals [33,34,35] and Gal-3 overexpression has been reported in diet-related human disorders, such as obesity and type 2 diabetes [36]. Conversely, some molecules (e.g., fibers, polyphenols) in plant-based food, whose antitumor properties have been extensively proved in the last few decades, are gaining importance for their capacity to suppress Gal-3 expression or to act as competitive inhibitors [37].
This review aims to present insights on the multiple relationships between Gal-3 and CRC and to explore how Gal-3 blocking by food-derived bioactive compounds may contribute to cancer prevention and the development of clinical intervention strategies.
2. Galectin-3 in Colorectal Cancer Development and Progression
The existence of a coordinated network of galectins exhibiting antagonistic roles in intestinal mucosa inflammation is supported by the literature. By exerting anti- or pro-inflammatory activities, galectin family members affect epithelial barrier integrity and gut immune homeostasis. In particular, Gal-3 has been extensively demonstrated to play a pivotal role in intestinal inflammatory diseases as well as in CRC growth and progression [38]. In this section, we will review the human studies reporting this evidence and summarize the main mechanisms through which Gal-3 regulates the activities of cancer and immune cells during CRC onset and progression.
2.1. Role in Human Intestinal Inflammation and Colorectal Cancer
Gal-3 has been reported to play both pro- and anti-inflammatory roles, depending on the body district and subcellular localization [39]. In the context of intestinal mucosa, Gal-3 shows mostly pro-inflammatory functions, even though a regulatory role, limiting the inflammatory process and restoring mucosal homeostasis, has also been described in IBD [32]. This can be explained by its dual role in protecting T cells from apoptosis when present intracellularly [27], while promoting apoptosis when acting from the extracellular space [40,41].
Gal-3 is constitutively expressed within the epithelial compartment of the intestine in both mice [42] and humans [43]. In humans, Gal-3 is expressed in the normal colon in nuclear compartments (Figure 2A), but significant changes in its expression and/or subcellular localization have been observed both in IBD and CRC [38]. As depicted in Figure 2B, a progressive increase in Gal-3 expression is observed in adenoma and during the progression toward advanced cancer, with changes in its subcellular localization from the nucleus in adenomas to cytoplasm in CRC.
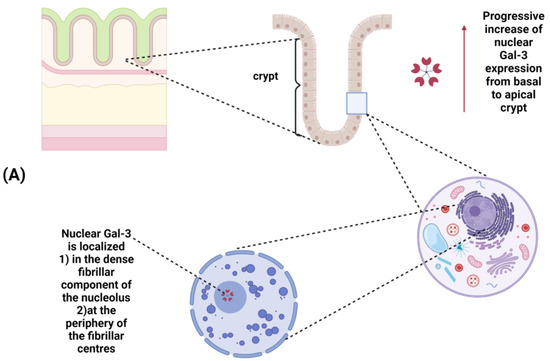
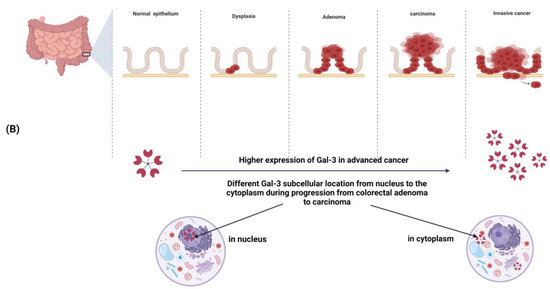
Figure 2.
(A) Galectin-3 expression in normal colonic mucosa: a progressive increase in Gal-3 expression occurs from the basal to the apical side of the crypt; within the nucleus, Gal-3 is localized in the dense fibrillar components of the nucleolus as well as in the periphery of the fibrillar centers.(B) Changes in Gal-3 expression and/or subcellular localization during colorectal cancer progression: Gal-3 expression is increased in advanced cancer. A different subcellular localization (from the nucleus to the cytoplasm) is observed during progression from colorectal adenoma to carcinoma.
Accordingly, serum levels of Gal-3 are significantly elevated in both ulcerative colitis (UC) and Crohn’s disease (CD) patients compared to healthy people [44,45]. Furthermore, enhanced Gal-3 concentration in serum and stool samples of patients with UC correlates with clinically and histologically severe disease [46], thus, representing a valuable biomarker for monitoring UC progression. The results achieved in UC patients are in keeping with data obtained in a murine model of dextran sodium sulphate (DSS)-induced colitis, where Gal-3 expression promotes acute colitis and plays an important pro-inflammatory role in the colonic epithelium [47]. However, the potential use of serum Gal-3 levels as an IBD biomarker is still controversial since recent studies failed to show increased serum levels of Gal-3 in UC and CD patients [48]. Differences in factors that affect the levels of Gal-3, such as age, renal function and co-morbidities, as well as the stage of the disease, could explain the discrepancies observed within IBD studies. Moreover, higher serum and fecal content of Gal-3 was observed in subjects with UC and metabolic syndrome, showing clinically and histologically milder disease compared to subjects suffering from UC only [49], indicating that Gal-3 may also be involved in the mechanism limiting the inflammatory process in UC. Notably, IBD is associated with an increased risk of CRC that appears to raise with the duration, severity and extent of colonic inflammation [50].
Increased Gal-3 expression has been amply recognized in colon carcinogenesis [38,51,52] and is considered a prognostic factor of poor outcome [44]. An interesting study by Tao and colleagues, examining Gal-3 expression in CRC cases in both cancer tissue and adjacent normal tissue via immunohistochemistry, revealed an association between Gal-3 levels and the clinical/pathological characteristics of disease [38]. However, conflicting results emerged on the relationship between the pattern of Gal-3 expression and tumor progression because some studies reported decreased or comparable Gal-3 levels during CRC progression [53,54,55,56,57,58,59]. A possible explanation for this discrepancy comes from the study of Tsuboi and co-workers, who, focusing on Gal-3 expression changes in two different tumor areas, highlighted the presence of a significant number of liver metastases when the expression of Gal-3 was lower at the invasive front of the tumor as compared to its surface [52]. Additionally, emerging evidence reveals that patients with detectable expression of Gal-3 in the tumor have more lymph node and distant metastases, frequent venous invasion and deeper wall invasion in comparison to Gal-3-negative cases [51]. Further, in clinical settings, Gal-3 levels were found to be higher also in feces from patients with severe tumor stage, suggesting the utility of this lectin as a possible biomarker for disease severity and progression [49]. Likewise, the potential use of Gal-3 as a predictive marker of therapy response also emerged from proteomics analysis studies carried out in rectal cancer patients showing Gal-3 protein downregulation in rectal tissues after radiation treatment [60].
Gal-3 is also released into the circulation and a strong expression (up to 5-fold increase) has been reported in the bloodstream of CRC patients [61]. Particularly, circulating Gal-3 has been found to induce the secretion of cytokines contributing to tumor progression, thus, suggesting that Gal-3 could function as a pro-inflammatory mediator during the metastatic cascade [62,63]. Another interesting study evaluated the influence of Gal-3 on postoperative outcomes after gastrointestinal surgery. As shown by Matsuda and colleagues, the high levels of Gal-3 found in the perioperative blood, likely transiently induced by surgical stress, are associated with postoperative complications (POCs) following surgery [64]. Therefore, while, on the one hand, it is unlikely that patients with blood Gal-3 levels below the cut-off value develop POCs, on the other hand, those with higher Gal-3 levels can be considered at risk of POC. Deep insights regarding the clinical value of circulating Gal-3 for young CRC patients have also been reported. It has been recently found that the combined detection of serum Gal-3, Aquaporin-1 (AQP-1) and -3 (AQP-3) (integral membrane proteins that serve as passive channels for water) has potential value for the diagnosis of young patients with colon cancer [65].Specifically, in young patients with colon cancer, pre- and postoperative Gal-3, AQP-1 and AQP-3 serum concentrations were found to be significantly increased, thus demonstrating their importance in evaluating long-term prognosis [66]. A multi-center clinical study was recently undertaken in 13000 asymptomatic individuals to validate Gal-3 and other factors as biomarkers for the early detection of colon cancer [67]. Moreover, clinical research has recently begun to explore the role of Gal-3 in treating cancer and intervention with Gal-3 antagonists is emerging as an attractive option for CRC. A few phase I or II human trials are ongoing in advanced CRC patients with the aim of assessing the safety and efficacy of these inhibitors in combination with standard therapies [67].
2.2. Direct Effects on Tumor Cells
Dysregulated expression of Gal-3 and changes in its localization are involved in regulating multiple processes. Gal-3 synthesis occurs in the cell cytoplasm [24], from where it can be translocated into the nucleus [68] or alternatively secreted into the extracellular space [69]. Changes in its localization (intra- or extracellular) determine its numerous functions. In the intracellular compartment, Gal-3 exhibits dynamic behavior, regulating signal transduction pathways [70], exerting anti-apoptotic activity [71] and contributing to pre-mRNA splicing [72]. In the extracellular space, Gal-3 regulates cell adhesion and is implicated in the organization of the plasma membrane and modulation of tumor invasion and metastasis [73,74]. Other representative examples of extracellular effects include the regulation of immune surveillance [75] and glycoprotein endocytosis [76].
The effect of Gal-3 on apoptosis constitutes the main difference between intracellular and extracellular Gal-3. The intracellular one is anti-apoptotic and is involved in cell proliferation and differentiation, while the extracellular Gal-3 activates inflammatory Th1 and Th17 cells and is involved in target cell apoptosis. Once secreted, Gal-3 interacts with the extracellular matrix and cell surface glycans. In the presence of carbohydrate ligands, it can oligomerize (Figure 1B), forming lectin lattices that act as scaffolds, sustaining the spatial organization of cell surface signaling receptors (e.g., epidermal growth factor receptor, platelet-derived growth factor receptor, fibroblast growth receptor, vascular endothelial growth factor receptor and transforming factor-β receptor) [77,78]. This complex interaction favors the survival of tumor cells in stressed conditions, induces tumor cell detachment and migration, and attracts leukocytes and endothelial cells to the tumor environment, thus, helping angiogenesis [78].
Regardless of its localization, increased levels of Gal-3 are related to increased CRC risk and severity. Particularly, it has been demonstrated that Gal-3 overexpression increases the migration of colon cancer cells through the activation of the K-Ras–Raf–Erk1/2 pathway [79] and that its interaction with extracellular carcinoembryonic antigen (CEA) promotes the migration of cancer cells and the appearance of distal metastases. In addition, synergistic interaction between serum Gal-3 and CEA correlates with poor survival in CRC patients [80]. Wu and co-workers investigated the effect of extracellular Gal-3 on colon cancer cell migration and its correlation with EGFR expression. Their results showed that extracellular Gal-3 increases cancer cell migration, which correlates with EGFR levels. These results suggest that Gal-3 targeting may have a combined effect on EGFR-targeted therapy [81].
Furthermore, by interacting with the oncofetal Thomsen–Friedenreich carbohydrate antigen (Galβ1, 3GalNAcα-, TF) on cancer-associated transmembrane mucin protein 1 (MUC1), circulating Gal-3 induces MUC1 cell surface polarization and exposure of cell adhesion molecules. This leads to the formation of emboli, prolonging survival of disseminating tumor cells in the circulation, thus, favoring the metastatic cascade [82]. Through its direct interaction with MUC1, Gal-3 may activate the MAPK and PI3K/Akt signaling pathways, leading to enhancement in cell proliferation and motility on the cell surface. This Gal-3-triggered MUC1-mediated signaling promotes uncontrolled tumor cell malignancy [83].
Growing evidence indicates that the Wnt/β-catenin pathway is implicated in the maintenance of cancer stem cells in CRC. This finding provided the rationale to investigate the role of Gal-3, known to be involved in Wnt signaling, on the Wnt/beta-catenin pathway in colon cancer cells. The results indicate that Gal-3 regulates β-catenin expression and its nuclear accumulation and activates Wnt signaling by regulating GSK-3β activity via the PI3K/AKT pathway [84,85].
An additional aspect worth deeper investigation is Gal-3’s role in chemo-sensitivity, invasion and metastasis in colon cancer. The data reported by Lu and colleagues unraveled the existence of an miR-128/Gal-3 axis in CRC. In particular, a frequent miR-128 down-regulation, negatively correlating with Gal-3 level, has been shown in CRC. A decreased expression of miR-128 was associated with CRC progression and a worse patient prognosis [86]. The shift in Gal-3 localization is also crucial in the response to chemotherapeutic drugs. Through a complex mechanism, including five steps, Gal-3 inhibits apoptosis induced by anticancer drugs. After a process of phosphorylation and translocation from the nucleus to the cytoplasm in response to chemotherapeutic drugs, Gal-3 activates a pathway that promotes the stabilization of the mitochondrial membrane; this results in the suppression of cytochrome c release and caspase activation, thus, favoring the suppression of cell apoptosis [40].
Gal-3 is also involved in a resistance mechanism of colon cancer cells to TRAIL-induced cell death. TRAIL can induce apoptosis and preferentially kills tumor cells by engaging specific death receptors, i.e., DR4 and DR5. However, tumor cells may resist TRAIL-based therapy in many ways. Findings from Mazurek and co-workers highlighted a mechanism by which Gal-3 inhibits trafficking of death receptors by anchoring them in glycan nano-clusters, thus, blocking the apoptotic signaling execution [87].
2.3. Contribution to Colonic Inflammation and Immunosuppression
In addition to the direct effects on colon cancer cells, Gal-3 regulates immune cell function in both innate and adaptive responses, where it participates in the activation or differentiation of many immunocompetent and inflammatory cells, including neutrophils, monocytes/macrophages, eosinophils, mast and dendritic cells (DCs) and activated T and B cells. Gal-3 interaction with immune cells inhibits the normal functions of the immune system, potentially mediating the immune escape of tumor cells and promoting tumor-driven immunosuppression within the TME, through several mechanisms [88]. In general, Gal-3 has been widely studied in the context of acute inflammatory responses [89] where it provides a powerful pro-inflammatory signal. Through intracellular or extracellular mechanisms, this lectin controls inflammatory responses by modulating cell adhesion, migration, function and survival of various innate immunity cells. The specific effects of Gal-3 on the innate and adaptive immunity are extensively reviewed elsewhere [39,90].
Regarding to intestinal chronic inflammatory conditions and colon carcinogenesis, Gal-3 expression showed different effects on the function of both colon-infiltrated macrophages and T cells, the most important effector immune cells involved in the progression of colon inflammation, as summarized in Table 1.

Table 1.
Studies describing the effects of galectin-3 on the immune system in the context of ulcerative colitis and colorectal cancer.
Specifically, Gal-3 is highly expressed in colonic macrophages in UC patients. In mouse models of experimental colitis, its deficiency inhibits the activation of the NLRP3 inflammasome and the production of inflammatory cytokines in colonic macrophages as well as in mesenteric lymph nodes, resulting in an attenuation of disease severity [47,93]. Likewise, pharmacological inhibition of mesenchymal stem cell (MSC)-produced Gal-3 enhances the capacity of these cells to promote alternative activation of peritoneal macrophages, both in vitro and in vivo [91]. Altogether, these findings indicate that Gal-3 inhibition could be used for improvements in MSC-mediated polarization towards the immunosuppressive M2 phenotype of macrophages in UC.
Conversely, in humans, Muller and colleagues found reduced Gal-3 expression in the inflamed mucosa of CD and UC patients and demonstrated that the inhibition of Gal-3 deeply influences T-cell activity [98]. In line with this evidence, after treatment with recombinant Gal-3, T cells in UC patients developed an immunosuppressive phenotype and were not able to optimally proliferate [92]. Additionally, adoptive transfer of Gal-3-primed T cells significantly reduced bowel inflammation and disease severity by inducing regulatory T cells, which suppressed colon mucosal inflammation [92]. Finally, a recent study by Volarevic and colleagues showed, in a chronic DSS-induced colitis model, that Gal-3 regulates the immunosuppressive capacity of DCs in the gut via a TLR-2-dependent activation of the IDO-1/KYN pathway, promoting the expansion of colon-infiltrated T-regulatory cells, which, consequently, suppress Th1 and Th17 cell-driven colon inflammation [99].
With respect to CRC, several studies have demonstrated that a high level of expression of Gal-3 promotes tumor growth, both in vivo and in vitro. Using a mouse tumor model, Peng and co-workers [94] demonstrated that Gal-3 treatment at high doses abrogates the efficacy of tumor-reactive T cells, induces T-cell apoptosis and promotes tumor immune tolerance. Additionally, Gal-3 null mice showed delayed T-cell, macrophage and DC infiltration into the gut mucosa after inoculation with Citrobacterrodentium, together with a slight delay in the resolution of infection [95]. Gal-3 serum concentration was also examined in patients with untreated CRC in association with interleukin (IL-10, IL-12 and IL-17) production, lymphocyte activation and inflammatory parameters, such as neutrophil/lymphocyte ratio (NLR), white blood cell count (WBC) and C-reactive protein (CRP) [96]. The levels of circulating Gal-3 showed a significant positive correlation with IL-17 and IL-10 production, whereas an inverse correlation was observed with IL-12. Thus, the effect of Gal-3 on the production of effector Th1-promoting cytokines may depress anti-tumor cell-mediated immunity, through Th2-dominant conditions. On the other hand, higher Gal-3 levels were associated with higher inflammatory parameters (NLR, WBC, CRP) and lower lymphocyte stimulation [96], according to the role of chronic systemic inflammation in the suppression of tumor immunity.
In vitro, Gal-3 inhibits the expansion of T cells, promotes CD8 T cell apoptosis, alters macrophage polarization from M1 (anti-tumor) to M2 (pro-tumor) and limits TCR clustering, once secreted by tumor cells [100]. Among the molecular mechanisms accounting for the powerful lymphocyte inhibitory effect of Gal-3 in cancers, this protein modulates the interactions between T cells and antigen-presenting cells, thus, playing a central role in the initial steps of tumor antigen presentation [100]. Specifically, the conditioned medium derived from colon cancer cell lines significantly induces the expression of Gal-3 in THP-1 monocytes and actively influences the phenotype of monocytes, switching their differentiation into a population of non-adherent mixed M1 and M2 cells [97]. These results suggested that colon cancer cells influence monocyte differentiation into suppressive subsets, likely via Gal-3 production. Interestingly, macrophage supernatants modulate the expression and secretion of Gal-3 in colon cancer cells [101], indicating the existence of a bidirectional cross-talk between tumor and immune cells.
Finally, along with the pro-inflammatory and immunoregulatory functions, Gal-3 also exhibits anti-microbial activities. In the small intestine, its increased expression in epithelial cells and macrophages can affect microbiota composition and macrophage activation [102]. Moreover, Gal-3 may interact with commensal bacteria, possibly influencing their colonization capacity [103]. Despite Gal-3 abundance at gastrointestinal sites, little is known yet about its interaction with the microbiota.
3. Galectin-3 Targeting by Bioactive Food Compounds in Colorectal Cancer Prevention and Therapy
Given the prominent role of extra- and intracellular Gal-3 in vital processes of carcinogenesis, the development of efficient Gal-3 inhibitors gained attention in the field of cancer prevention and therapy. Those currently synthetized are predominantly based on competitive blocking of the interaction between Gal-3 CRD and natural carbohydrate binding partners on the cell surface or inside the cells. The most outstanding inhibitors that have moved to the stage of preclinical testing are heterogeneous polysaccharide-based molecules [104]. At the same time, research interest is devoted to the study of natural diet-derived complex carbohydrates or other compounds to be exploited as Gal-3 inhibitors. Indeed, some bioactive food compounds, mostly derived from fruits and vegetables, have been explored for their capacity to negatively regulate Gal-3 expression or to act as competitive inhibitors by targeting the Gal-3 CRD–glycan interaction [105].
In this section, we summarize the studies that have highlighted the role of non-digestible carbohydrates and polyphenols in controlling CRC development or growth through the regulation of Gal-3 activity/expression. Their widespread anti-Gal-3 effects and their role as promising tools for cancer therapy and prevention are discussed.
3.1. Non-Digestible Carbohydrates
Dietary fibers are a heterogeneous group of plant-derived complex carbohydrates that are indigestible for the host and fermented by gut bacteria, the regular consumptionof which supports colonic health and prevents local carcinogenesis [106,107]. Non-digestible carbohydrates (NDCs) consist of complex carbohydrates from the plant cell wall, including pectin and hemicellulose, which vary among foods in structure and composition and, consequently, in physicochemical properties and biological effects. Water-soluble fibers are mainly composed of pectin, which is considered a bioactive polysaccharide due to its beneficial effects on human health and has emerged as a good source for generating high-affinity Gal-3 inhibitors with low toxicity [108]. In addition to being a major component in fruits, vegetables and whole grains, extracted pectin is also used in the food industry as a gelling, thickening, emulsifying and stabilizing agent in a variety of foods [109]. The food source, pectin extraction methods and ripening parameters of fruits are important factors in obtaining functional pectin molecules. Several fruits (apple, plum, apricot, jaboticaba, citrus, lemon and papaya) as well as ginseng, pumpkin, olive, tomato and corn, have been explored for pectin extraction and biological activity studies.
NDCs have the potential to affect cancer risk through different mechanisms, including the influence on the gut microbiota and the regulation of immune responses [110]. By virtue of their capacity to bind the CRD of Gal-3, they interfere with its attachment to glycan-containing surfaces [111] and can inhibit the Gal-3 downstream signaling mechanisms that controls apoptosis and cell cycle in CRC cells as well as the activation of tumor-specific immune responses [112]. Several in vitro and in vivo studies have investigated the interaction between distinct NDC and Gal-3, as well as the therapeutic and chemopreventive effects of this interaction on CRC [37,113] (Table 2).

Table 2.
Studies describing galectin-3-mediated effects of non-digestible carbohydrates in colorectal cancer.
The modified citrus pectin (MCP) has attracted special attention, being the most studied among NDCs. MCP is a preparation derived from the white pith of citrus fruit peels that is modified by high temperature, alterations in pH and/or pectinase treatment, resulting in smaller and less-ramified galactan chains, which have better access to Gal-3 CRD [126]. MCP effectively binds to recombinant Gal-3 and inhibits Gal-3-mediated functions, such as homotypic tumor cell aggregation, binding of tumor to endothelial cells, anchorage-independent growth, angiogenesis and tumor-infiltrating lymphocyte impairment [125].
Early studies reported the beneficial effects of MCP dietary supplementation in animal models of colon cancer [127]. Nangia-Makker and co-authors reported a strong therapeutic effect of oral MCP intake, with inhibition of both primary tumor and liver metastases (LM) growth [114]. MCP was found to reduce tumor growth in vivo by acting as an inhibitor of angiogenesis. In parallel with in vitro assays, it prevented HUVEC migration and capillary tube formation by binding to Gal-3 in the matrix and on the endothelial cells and interfering with receptor recognition. This mechanism was assumed to be responsible for in vivo therapeutic efficacy [114,128]. Accordingly, a dose-dependent inhibitory effect on LM was reported in colon-cancer-bearing mice following oral MCP administration [115]. The therapeutic effect correlated to the high levels of Gal-3 expression found in metastatic lesions, arguing for the blocking of Gal-3 function as the main mechanism of MCP-induced protection [115]. MCP-mediated control of tumor growth was also reported in nude mice engrafted with SW-480 cells where this compound reduced Gal-3 expression and induced apoptosis and reversion of epithelial–mesenchymal transition (EMT) in the engrafted tissue [116].
The chemopreventive activity of alginate polysaccharide from citrus, combined with Lactobacillus acidophilus, was studied in a mouse model of AOM-induced colon carcinogenesis. Alginate MCP dramatically reduced the occurrence of precancerous lesions through Gal-3 and VEGF blocking [117]. Furthermore, oral intake of a modified polysaccharide extracted from apple, mainly including Gal-3 binding galacturonic acid and galactose, protected mice against DMH/DSS-induced colon tumorigenesis [118]. Finally, a recent study demonstrated that SCLP, a pectin-type polysaccharide purified from Smilax china Linn (or China roof), can significantly improve inflammation and the histopathological damage of DSS-induced UC in mice by blocking the interaction of Gal-3 with the NLRP3 inflammasome [3]. The Gal-3-mediated anti-inflammatory activity of this pectin could play a role in CRC prevention, with UC-associated chronic inflammation being a risk factor for colon carcinogenesis.
The antitumor effects of MCP and pectin from other sources have also been explored in in vitro studies. A study by Wu and colleagues showed that extracellular Gal-3 is involved in lamellipodia formation and migration of colon cancer cells and that MCP is able to significantly reduce this effect [81]. Other studies have found that MCP inhibits cancer cell proliferation and aggregation and induces EMT reversion through reductions in Gal-3 interaction with cells and between cells and the extracellular matrix [111,116].
Modified sugar beet, papaya and ginseng pectins have structures composed of neutral (1,4)-β-galactose residues, which were related to Gal-3 interaction and inhibition. As observed for MCP, the binding of these pectins to Gal-3 is associated with strong effects on colon cancer cell proliferation and migration, particularly evident following temperature-, alkali- or enzyme-induced modifications [119,122]. Furthermore, structural studies revealed that the rhamnogalacturonan I-rich fragment RG-I-4 from ginseng pectin alone holds the capacity to inhibit Gal-3-induced hemagglutination and binding to T lymphocytes, and to block colon cancer cell adhesion and aggregation [120]. Likewise, the use of specific enzymes, able to increase the proportion of RG-I regions on apple pectin, equips this molecule with a superior ability to induce apoptosis and ROS production in colon cancer cell lines and to enhance the cytotoxic activity of anticancer drugs [121]. More recently, pectin-rich olive extracts from the byproduct of olive oil production have been tested for their antitumor activity in comparison with MCP. Olive-derived pectin was found to inhibit Gal-3 activity and to reduce colon carcinoma cell proliferation with higher efficiency than MCP [123].
Some fruit flours, consumed as regular flour or incorporated in products, represent an alternative for fiber consumption. The pectin-rich water-soluble fractions of commercial plum, papaya and jaboticaba fruit flours were studied for their inhibitory effect on Gal-3 and for their potential capacity to affect colon cancer cell growth [124]. Only pectin extracted from jaboticaba fruit flour significantly inhibited Gal-3-mediated hemagglutination and decreased the viability of Gal-3 overexpressing HCT116 cells [124]. Notably, the depolymerization of polysaccharides, occurring during heating to produce fruit flours, decreases their beneficial health effects. In keeping with this observation, when pectin extracted from the papaya pulp was tested, inhibition of both Gal-3 activity and colon cancer cell growth was obtained [108,125,129]. The inhibitory effect was also influenced by variations in polysaccharide composition naturally occurring during fruit ripening and was particularly evident at early/intermediate ripening stages [108,110,125], suggesting that the consumption of papayas during intermediate ripening time points could be promising for CRC prevention.
A number of preclinical studies have analyzed the Gal-3-blocking effects of pectins, isolated from different fruits, vegetables and cereals, in other cancer models (melanoma, prostate, breast, bladder, thyroid), as well as in cardiovascular diseases, with promising results. The exceptionally low incidence of toxicities and possible clinical effects of pectin, qualified by the FDA as a GRAS (i.e., generally recognized as safe) food substance, support further testing of these dietary compounds in human studies [113]. Indeed, although studies involving CRC patients are lacking [130], a number of clinical trials are ongoing to assess the therapeutic or preventive value of pectin in cancer and other diseases [131], some with promising results [132,133]. Specifically, in a prospective phase II study using MCP (PectaSol®, P-MCP) in non-metastatic biochemically relapsed prostate cancer patients, a significantly reduced disease progression was observed [132]. Likewise, the use of Belapectin, in association with immune checkpoint inhibitor-based therapy, in a phase I trial involving patients with advanced melanoma or head and neck squamous cell carcinoma, resulted in enhanced anti-tumor immune response [133]. Notably, MCP was approved as a dietary supplement with antioxidant and immunomodulatory properties.
3.2. Polyphenols
Polyphenols are bioactive food compounds mainly found in fruits, vegetables, whole grains and plant-derived beverages (fruit juice, red wine, tea and coffee) [134]. They exert strong anti-inflammatory, antioxidant and immunomodulatory activities, and growing evidence from epidemiological studies has suggested a possible inverse association between polyphenol intake and cancer incidence/mortality [135]. Polyphenols are the most-studied dietary compounds in the field of oncology and have received great attention in CRC research for both chemopreventive and direct anticancer activities, and for their novel role as enhancers of conventional treatments [136,137]. A wide range of clinical trials have been registered, in which some polyphenolic compounds (e.g., curcumin, genistein, fisetin, silybin, epigallocatechin gallate) are being tested as preventive and anti-cancer medicines, mostly in combination with other drugs, in different cancer types, including CRC and precancerous colon adenomatous polyposis [136,138,139].
Some recent studies have highlighted the capacity of these compounds to regulate Gal-3 expression or activity in experimental models of cancer and other pathologies. The results achieved suggest that their capacity to exert inhibitory effects on Gal-3 contributes to their antitumoral activity (Table 3).

Table 3.
Studies describing galectin-3-mediated effects of polyphenols in disease models.
Among polyphenols, resveratrol, a compound attracting considerable interest during the last few decades as an antitumor agent, was found to downregulate Gal-3 expression in ovarian cancer cells through the induction of mir-424-3p [140]. Gal-3 blocking resulted in the inhibition of cancer cell migration and invasion, and in their increased sensitivity to chemotherapy [140]. In a different experimental disease model, HFD-induced atherosclerosis, Li H and colleagues demonstrated that the flavonoid quercetin alleviates atherosclerotic lesions by inhibiting HFD-induced Gal-3 expression and consequent NLRP3 inflammasome activation in macrophages [141]. The negative effect of quercetin on the Gal-3-NLRP3 pathway was also confirmed in in vitro stimulated macrophage cell lines [141]. Accordingly, treatment with berberine suppressed oxidized low-density lipoprotein-induced upregulation of Gal-3 and macrophage activation [142]. Quercetin and berberine were both shown to exert beneficial effects on intestinal inflammation and to inhibit colon tumorigenesis [144,145]. Finally, the effects of xanthohumol and 8-prenylnaringenin, belonging to the prenylflavonoid family, on Gal-3 expression were investigated in a mouse model of type 2 diabetes mellitus [143]. HFD-induced diabetes resulted in overexpression of Gal-3 in the liver and kidney that was associated with oxidative stress. Oral intake of both polyphenols completely suppressed Gal-3 expression and oxidative stress markers [143]. Of note, these compounds have been reported to affect mitochondrial function and oxidative pathways in CRC [146].
Although the effects of polyphenols in regulating Gal-3 activity have been poorly investigated in cancer, the results achieved in different disease models point to Gal-3 inhibition as a novel mechanism underlying the anti-inflammatory and anti-tumoral activity of these compounds. Polyphenol-mediated effects on Gal-3 have not yet been specifically explored in CRC models, but the observation that Gal-3 is highly expressed in CRC tissues, together with its known role in intestinal inflammation and colon carcinogenesis, suggest that the capacity of these compounds to modulate Gal-3 in other disease contexts might also be exploited in the management of CRC, thus, further strengthening the potential of their supplementation for therapeutic and prevention purposes.
4. Conclusions
The mechanisms underlying CRC onset and progression are numerous and complex. Despite the great efforts devoted to the management of CRC, it still represents a leading cause of death worldwide. Although a large choice of targeted treatments is available, more individualized therapies need to be developed to achieve longer survival, fewer adverse reactions and the potential for full recovery.
In this review, we summarized the multiple tumor-promoting and -supporting roles played by Gal-3, from the contrasting effects on apoptosis to TME immunosuppression, tumor angiogenesis and metastasis promotion. Worthy of note, a growing body of evidence highlights the importance of Gal-3–ligand interaction in the pathological processes of colon inflammation and CRC development. Likewise, the immunomodulatory effects of Gal-3, indirectly affecting tumor development/progression, are particularly relevant for CRC and other gastrointestinal cancers whose onset and evolution are deeply influenced by the immune system. Furthermore, the observation that Gal-3 overexpression is significantly related to tumor progression points to this lectin as a potential candidate to predict CRC prognosis. The clinical data currently available, however, are still insufficient to clearly validate the usefulness of Gal-3 as a prognostic biomarker of CRC.
Finally, we focused our attention on the preventive/therapeutic potential of Gal-3 targeting natural food compounds. It is very likely that new benefits will be discovered for these compounds as Gal-3 research continues to identify novel mechanisms underlying Gal-3-mediated disease induction/progression. This will pave the road towards more targeted hypotheses for human study interventions and clinical trials. While surgery and chemo- and radiotherapy interventions continue to represent essential treatments in CRC management, depending on the tumor stage, the experimental evidence reported herein strongly indicates Gal-3 targeting by natural food compounds as a potential intervention strategy, with important implications in the therapeutic management of CRC patients.
5. Future Directions
Fibers and polyphenols exert a broad-spectrum power as anticancer and health-promoting agents. Their Gal-3-independent regulatory effects on inflammation, oxidative stress and microbiota composition have been extensively proved in human studies. The anti-metastatic effects of these compounds associated with Gal-3 blocking hold great promise for the control of CRC and other tumors, given the impact of metastases on therapy efficacy and postoperative prognosis. Thus, more research is needed to definitely prove the connection between their intake and in vivo blocking of Gal-3 activities. The contribution of Gal-3 blocking to the anti-tumoral potential of bioactive food compounds in CRC should be more deeply investigated and considered in future intervention studies. A schematic model on the preventive/therapeutic potential of Gal-3 targeting by these compounds is depicted in Figure 3.
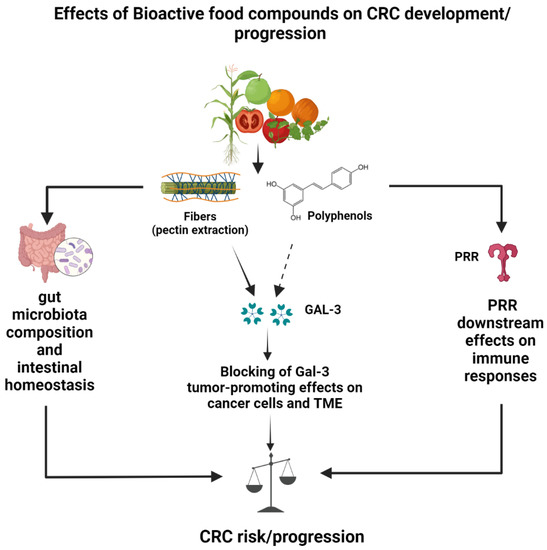
Figure 3.
Schematic model of galectin-3-dependent and -independent effects of dietary fibers and polyphenols potentially leading to reduced CRC risk and progression. Galectin-3-independent protective effects occur through modulation of gut microbiota and attenuation of inflammatory responses induced by pattern recognition receptor (PRR) triggering in immune cells. Galectin-3-dependent protective effects on CRC have been demonstrated for pectin (full arrow) and, so far, only suggested for polyphenols (dotted arrow).
Furthermore, as the effects of these molecules can vary widely according to their structure, administration route and in vivo bioavailability, additional structural composition studies are recommended. Likewise, more attention needs to be paid to still unclear aspects accounting for their systemic and tumor bioavailability following food consumption. Consequently, the recommendations for their intake are expected to change from a quantitative to a qualitative perspective, thus, improving nutritional intake for both healthy individuals and cancer patients.
The exploitation of healthy diets or food-derived bioactive compounds able to target multiple tumor-promoting/supporting pathways represents a desirable goal in cancer prevention and a strategy to enhance the efficacy of conventional therapies. An additional aspect concerns ecological sustainability. As many of the possible sources of pectin, polyphenols and other bioactive molecules are residues and byproducts from agriculture and food-processing industries, their exploitation in clinical studies could lead to a revalorization of materials otherwise discarded.
Author Contributions
All authors (A.A., M.D.C., B.M., S.G. and L.C.) contributed to drafting and revising the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Patterns and Trends in Colorectal Cancer Incidence and Mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer. J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Pan, X.; Wang, H.; Zheng, Z.; Huang, X.; Yang, L.; Liu, J.; Wang, K.; Zhang, Y. Pectic Polysaccharide from Smilax China L. Ameliorated Ulcerative Colitis by Inhibiting the Galectin-3/NLRP3 Inflammasome Pathway. Carbohydr. Polym. 2022, 277, 118864. [Google Scholar] [CrossRef]
- Zhou, E.; Rifkin, S. Colorectal Cancer and Diet: Risk Versus Prevention, is Diet an Intervention? Gastroenterol. Clin. North Am. 2021, 50, 101–111. [Google Scholar] [CrossRef]
- Morrow, L.; Greenwald, B. Healthy Food Choices, Physical Activity, and Screening Reduce the Risk of Colorectal Cancer. Gastroenterol. Nurs. 2022, 45, 113–119. [Google Scholar] [CrossRef]
- Veettil, S.K.; Wong, T.Y.; Loo, Y.S.; Playdon, M.C.; Lai, N.M.; Giovannucci, E.L.; Chaiyakunapruk, N. Role of Diet in Colorectal Cancer Incidence: Umbrella Review of Meta-Analyses of Prospective Observational Studies. JAMA Netw. Open 2021, 4, e2037341. [Google Scholar] [CrossRef]
- Carroll, K.L.; Fruge, A.D.; Heslin, M.J.; Lipke, E.A.; Greene, M.W. Diet as a Risk Factor for Early-Onset Colorectal Adenoma and Carcinoma: A Systematic Review. Front. Nutr. 2022, 9, 896330. [Google Scholar] [CrossRef]
- Morze, J.; Danielewicz, A.; Przybylowicz, K.; Zeng, H.; Hoffmann, G.; Schwingshackl, L. An Updated Systematic Review and Meta-Analysis on Adherence to Mediterranean Diet and Risk of Cancer. Eur. J. Nutr. 2021, 60, 1561–1586. [Google Scholar] [CrossRef]
- Pan, P.; Yu, J.; Wang, L.S. Colon Cancer: What we Eat. Surg. Oncol. Clin. N. Am. 2018, 27, 243–267. [Google Scholar] [CrossRef]
- Bradbury, K.E.; Appleby, P.N.; Key, T.J. Fruit, Vegetable, and Fiber Intake in Relation to Cancer Risk: Findings from the European Prospective Investigation into Cancer and Nutrition (EPIC). Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 394S–398S. [Google Scholar] [CrossRef]
- Leenders, M.; Siersema, P.D.; Overvad, K.; Tjonneland, A.; Olsen, A.; Boutron-Ruault, M.C.; Bastide, N.; Fagherazzi, G.; Katzke, V.; Kuhn, T.; et al. Subtypes of Fruit and Vegetables, Variety in Consumption and Risk of Colon and Rectal Cancer in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2015, 137, 2705–2714. [Google Scholar] [CrossRef]
- Turati, F.; Rossi, M.; Pelucchi, C.; Levi, F.; La Vecchia, C. Fruit and Vegetables and Cancer Risk: A Review of Southern European Studies. Br. J. Nutr. 2015, 113 (Suppl. 2), S102–S110. [Google Scholar] [CrossRef]
- Gianfredi, V.; Salvatori, T.; Villarini, M.; Moretti, M.; Nucci, D.; Realdon, S. Is Dietary Fibre Truly Protective Against Colon Cancer? A Systematic Review and Meta-Analysis. Int. J. Food Sci. Nutr. 2018, 69, 904–915. [Google Scholar] [CrossRef]
- Jochems, S.H.J.; Van Osch, F.H.M.; Bryan, R.T.; Wesselius, A.; van Schooten, F.J.; Cheng, K.K.; Zeegers, M.P. Impact of Dietary Patterns and the Main Food Groups on Mortality and Recurrence in Cancer Survivors: A Systematic Review of Current Epidemiological Literature. BMJ Open 2018, 8, e014530. [Google Scholar] [CrossRef]
- Bamia, C. Dietary Patterns in Association to Cancer Incidence and Survival: Concept, Current Evidence, and Suggestions for Future Research. Eur. J. Clin. Nutr. 2018, 72, 818–825. [Google Scholar] [CrossRef]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A., Jr.; Kinzler, K.W. Cancer Genome Landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a Glance. J. Cell. Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef]
- Liu, F.T.; Rabinovich, G.A. Galectins as Modulators of Tumour Progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef]
- Hirabayashi, J.; Kasai, K. The Family of Metazoan Metal-Independent Beta-Galactoside-Binding Lectins: Structure, Function and Molecular Evolution. Glycobiology 1993, 3, 297–304. [Google Scholar] [CrossRef]
- Cummings, R.D.; Liu, F.T.; Vasta, G.R. Galectins. In Essentials of Glycobiology, 3rd ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Eds.; by The Consortium of Glycobiology Editors, La Jolla, California: Cold Spring Harbor: New York, NY, USA, 2015; pp. 469–480. [Google Scholar]
- Di Lella, S.; Sundblad, V.; Cerliani, J.P.; Guardia, C.M.; Estrin, D.A.; Vasta, G.R.; Rabinovich, G.A. When Galectins Recognize Glycans: From Biochemistry to Physiology and Back Again. Biochemistry 2011, 50, 7842–7857. [Google Scholar] [CrossRef]
- Liu, F.T.; Rabinovich, G.A. Galectins: Regulators of Acute and Chronic Inflammation. Ann. N. Y. Acad. Sci. 2010, 1183, 158–182. [Google Scholar] [CrossRef] [PubMed]
- Thiemann, S.; Baum, L.G. Galectins and Immune Responses-just how do they do those Things they do? Annu. Rev. Immunol. 2016, 34, 243–264. [Google Scholar] [CrossRef] [PubMed]
- Dumic, J.; Dabelic, S.; Flogel, M. Galectin-3: An Open-Ended Story. Biochim. Biophys. Acta 2006, 1760, 616–635. [Google Scholar] [CrossRef]
- Raimond, J.; Zimonjic, D.B.; Mignon, C.; Mattei, M.; Popescu, N.C.; Monsigny, M.; Legrand, A. Mapping of the Galectin-3 Gene (LGALS3) to Human Chromosome 14 at Region 14q21-22. Mamm. Genome 1997, 8, 706–707. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, J.; Kanigsberg, A.; Slaaby, R.; Leffler, H.; Barondes, S.H.; Rini, J.M. X-Ray Crystal Structure of the Human Galectin-3 Carbohydrate Recognition Domain at 2.1-A Resolution. J. Biol. Chem. 1998, 273, 13047–13052. [Google Scholar] [CrossRef]
- Yang, R.Y.; Hsu, D.K.; Liu, F.T. Expression of Galectin-3 Modulates T-Cell Growth and Apoptosis. Proc. Natl. Acad. Sci. USA 1996, 93, 6737–6742. [Google Scholar] [CrossRef] [PubMed]
- Barondes, S.H.; Cooper, D.N.; Gitt, M.A.; Leffler, H. Galectins. Structure and Function of a Large Family of Animal Lectins. J. Biol. Chem. 1994, 269, 20807–20810. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xu, X.; Cheng, H.; Miller, M.C.; He, Z.; Gu, H.; Zhang, Z.; Raz, A.; Mayo, K.H.; Tai, G.; et al. Galectin-3 N-Terminal Tail Prolines Modulate Cell Activity and Glycan-Mediated Oligomerization/Phase Separation. Proc. Natl. Acad. Sci. USA 2021, 118, e2021074118. [Google Scholar] [CrossRef]
- Kovacevic, Z.; Lazarevic, T.; Maksimovic, N.; Grk, M.; Volarevic, V.; GazdicJankovic, M.; Djukic, S.; Janicijevic, K.; MileticKovacevic, M.; Ljujic, B. Galectin 3 (LGALS3) Gene Polymorphisms are Associated with Biochemical Parameters and Primary Disease in Patients with End-Stage Renal Disease in Serbian Population. J. Clin. Med. 2022, 11, 3874. [Google Scholar] [CrossRef]
- Hokama, A.; Mizoguchi, E.; Mizoguchi, A. Roles of Galectins in Inflammatory Bowel Disease. World J. Gastroenterol. 2008, 14, 5133–5137. [Google Scholar] [CrossRef]
- Sundblad, V.; Quintar, A.A.; Morosi, L.G.; Niveloni, S.I.; Cabanne, A.; Smecuol, E.; Maurino, E.; Marino, K.V.; Bai, J.C.; Maldonado, C.A.; et al. Galectins in Intestinal Inflammation: Galectin-1 Expression Delineates Response to Treatment in Celiac Disease Patients. Front. Immunol. 2018, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mao, Y.S.; Chen, F.; Xia, D.X.; Zhao, T.Q. Palmitic Acid Up Regulates Gal-3 and Induces Insulin Resistance in Macrophages by Mediating the Balance between KLF4 and NF-kappaB. Exp. Ther. Med. 2021, 22, 1028. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, F.; Zhong, W.; Jiang, X.; Zhang, F.; Ji, X.; Xue, M.; Qiu, Y.; Yu, J.; Hu, X.; et al. Secretory Galectin-3 Promotes Hepatic Steatosis Via Regulation of the PPARgamma/CD36 Signaling Pathway. Cell. Signal. 2021, 84, 110043. [Google Scholar] [CrossRef] [PubMed]
- Krautbauer, S.; Eisinger, K.; Hader, Y.; Buechler, C. Free Fatty Acids and IL-6 Induce Adipocyte Galectin-3 which is Increased in White and Brown Adipose Tissues of Obese Mice. Cytokine 2014, 69, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Li, X.T.; Yu, L.G.; Wang, L.; Shi, Z.Y.; Guo, X.L. Roles of Galectin-3 in Metabolic Disorders and Tumor Cell Metabolism. Int. J. Biol. Macromol. 2020, 142, 463–473. [Google Scholar] [CrossRef]
- Yue, F.; Xu, J.; Zhang, S.; Hu, X.; Wang, X.; Lu, X. Structural Features and Anticancer Mechanisms of Pectic Polysaccharides: A Review. Int. J. Biol. Macromol. 2022, 209, 825–839. [Google Scholar] [CrossRef]
- Tao, L.; Jin, L.; Dechun, L.; Hongqiang, Y.; Changhua, K.; Guijun, L. Galectin-3 Expression in Colorectal Cancer and its Correlation with Clinical Pathological Characteristics and Prognosis. Open Med. 2017, 12, 226–230. [Google Scholar] [CrossRef]
- Diaz-Alvarez, L.; Ortega, E. The Many Roles of Galectin-3, a Multifaceted Molecule, in Innate Immune Responses against Pathogens. Mediators Inflamm. 2017, 2017, 9247574. [Google Scholar] [CrossRef]
- Fukumori, T.; Takenaka, Y.; Yoshii, T.; Kim, H.R.; Hogan, V.; Inohara, H.; Kagawa, S.; Raz, A. CD29 and CD7 Mediate Galectin-3-Induced Type II T-Cell Apoptosis. Cancer Res. 2003, 63, 8302–8311. [Google Scholar]
- Stillman, B.N.; Hsu, D.K.; Pang, M.; Brewer, C.F.; Johnson, P.; Liu, F.T.; Baum, L.G. Galectin-3 and Galectin-1 Bind Distinct Cell Surface Glycoprotein Receptors to Induce T Cell Death. J. Immunol. 2006, 176, 778–789. [Google Scholar] [CrossRef]
- Nio-Kobayashi, J.; Takahashi-Iwanaga, H.; Iwanaga, T. Immunohistochemical Localization of Six Galectin Subtypes in the Mouse Digestive Tract. J. Histochem. Cytochem. 2009, 57, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Papa Gobbi, R.; De Francesco, N.; Bondar, C.; Muglia, C.; Chirdo, F.; Rumbo, M.; Rocca, A.; Toscano, M.A.; Sambuelli, A.; Rabinovich, G.A.; et al. A Galectin-Specific Signature in the Gut Delineates Crohn’s Disease and Ulcerative Colitis from Other Human Inflammatory Intestinal Disorders. Biofactors 2016, 42, 93–105. [Google Scholar] [PubMed]
- Yu, T.B.; Dodd, S.; Yu, L.G.; Subramanian, S. Serum Galectins as Potential Biomarkers of Inflammatory Bowel Diseases. PLoS ONE 2020, 15, e0227306. [Google Scholar] [CrossRef]
- Frol’ova, L.; Smetana, K., Jr.; Borovska, D.; Kitanovicova, A.; Klimesova, K.; Janatkova, I.; Malickova, K.; Lukas, M.; Drastich, P.; Benes, Z.; et al. Detection of Galectin-3 in Patients with Inflammatory Bowel Diseases: New Serum Marker of Active Forms of IBD? Inflamm. Res. 2009, 58, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Markovic, B.S.; Jankovic, M.G.; Djokovic, B.; Jovicic, N.; Harrell, C.R.; Fellabaum, C.; Djonov, V.; Arsenijevic, N.; Lukic, M.L. Galectin 3 Protects from Cisplatin-Induced Acute Kidney Injury by Promoting TLR-2-Dependent Activation of IDO1/Kynurenine Pathway in Renal DCs. Theranostics 2019, 9, 5976–6001. [Google Scholar] [CrossRef] [PubMed]
- SimovicMarkovic, B.; Nikolic, A.; Gazdic, M.; Bojic, S.; Vucicevic, L.; Kosic, M.; Mitrovic, S.; Milosavljevic, M.; Besra, G.; Trajkovic, V.; et al. Galectin-3 Plays an Important Pro-Inflammatory Role in the Induction Phase of Acute Colitis by Promoting Activation of NLRP3 Inflammasome and Production of IL-1beta in Macrophages. J. Crohns Colitis 2016, 10, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Cibor, D.; Szczeklik, K.; Brzozowski, B.; Mach, T.; Owczarek, D. Serum Galectin 3, Galectin 9 and Galectin 3-Binding Proteins in Patients with Active and Inactive Inflammatory Bowel Disease. J. Physiol. Pharmacol 2019, 70. [Google Scholar] [CrossRef]
- Jovanovic, M.; Gajovic, N.; Zdravkovic, N.; Jovanovic, M.; Jurisevic, M.; Vojvodic, D.; Maric, V.; Arsenijevic, A.; Jovanovic, I. Fecal Galectin-3: A New Promising Biomarker for Severity and Progression of Colorectal Carcinoma. Mediators Inflamm. 2018, 2018, 8031328. [Google Scholar] [CrossRef]
- Nebbia, M.; Yassin, N.A.; Spinelli, A. Colorectal Cancer in Inflammatory Bowel Disease. Clin. Colon Rectal Surg. 2020, 33, 305–317. [Google Scholar] [CrossRef]
- Endo, K.; Kohnoe, S.; Tsujita, E.; Watanabe, A.; Nakashima, H.; Baba, H.; Maehara, Y. Galectin-3 Expression is a Potent Prognostic Marker in Colorectal Cancer. Anticancer Res. 2005, 25, 3117–3121. [Google Scholar]
- Tsuboi, K.; Shimura, T.; Masuda, N.; Ide, M.; Tsutsumi, S.; Yamaguchi, S.; Asao, T.; Kuwano, H. Galectin-3 Expression in Colorectal Cancer: Relation to Invasion and Metastasis. Anticancer Res. 2007, 27, 2289–2296. [Google Scholar] [PubMed]
- Nagy, N.; Legendre, H.; Engels, O.; Andre, S.; Kaltner, H.; Wasano, K.; Zick, Y.; Pector, J.C.; Decaestecker, C.; Gabius, H.J.; et al. Refined Prognostic Evaluation in Colon Carcinoma using Immunohistochemical Galectin Fingerprinting. Cancer 2003, 97, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Itzkowitz, S.H. Galectins: Multipurpose Carbohydrate-Binding Proteins Implicated in Tumor Biology. Gastroenterology 1997, 113, 2003–2005. [Google Scholar] [PubMed]
- Dudas, S.P.; Yunker, C.K.; Sternberg, L.R.; Byrd, J.C.; Bresalier, R.S. Expression of Human Intestinal Mucin is Modulated by the Beta-Galactoside Binding Protein Galectin-3 in Colon Cancer. Gastroenterology 2002, 123, 817–826. [Google Scholar] [CrossRef]
- Nakamura, M.; Inufusa, H.; Adachi, T.; Aga, M.; Kurimoto, M.; Nakatani, Y.; Wakano, T.; Nakajima, A.; Hida, J.I.; Miyake, M.; et al. Involvement of Galectin-3 Expression in Colorectal Cancer Progression and Metastasis. Int. J. Oncol. 1999, 15, 143–148. [Google Scholar] [CrossRef]
- Hegardt, P.; Widegren, B.; Li, L.; Sjogren, B.; Kjellman, C.; Sur, I.; Sjogren, H.O. Nitric Oxide Synthase Inhibitor and IL-18 Enhance the Anti-Tumor Immune Response of Rats Carrying an Intrahepatic Colon Carcinoma. Cancer Immunol. Immunother. 2001, 50, 491–501. [Google Scholar] [CrossRef]
- Kayser, K.; Zink, S.; Andre, S.; Schuring, M.P.; Hecker, E.; Klar, E.; Bovin, N.V.; Kaltner, H.; Gabius, H.J. Primary Colorectal Carcinomas and their Intrapulmonary Metastases: Clinical, Glyco-, Immuno- and Lectin Histochemical, Nuclear and Syntactic Structure Analysis with Emphasis on Correlation with Period of Occurrence of Metastases and Survival. APMIS 2002, 110, 435–446. [Google Scholar] [CrossRef]
- Mazurek, N.; Conklin, J.; Byrd, J.C.; Raz, A.; Bresalier, R.S. Phosphorylation of the Beta-Galactoside-Binding Protein Galectin-3 Modulates Binding to its Ligands. J. Biol. Chem. 2000, 275, 36311–36315. [Google Scholar] [CrossRef]
- Shen, W.Q.; Rong, G.Q.; Wu, Y.; Pu, Y.W.; Ye, Z.Y.; Cao, C.; Yang, X.D.; Xing, C.G. Preliminary Proteomic Analysis of Radiation Response Markers in Rectal Cancer Patients. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8841–8851. [Google Scholar]
- Iurisci, I.; Tinari, N.; Natoli, C.; Angelucci, D.; Cianchetti, E.; Iacobelli, S. Concentrations of Galectin-3 in the Sera of Normal Controls and Cancer Patients. Clin. Cancer Res. 2000, 6, 1389–1393. [Google Scholar]
- Chen, C.; Duckworth, C.A.; Zhao, Q.; Pritchard, D.M.; Rhodes, J.M.; Yu, L.G. Increased Circulation of Galectin-3 in Cancer Induces Secretion of Metastasis-Promoting Cytokines from Blood Vascular Endothelium. Clin. Cancer Res. 2013, 19, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Blair, B.B.; Funkhouser, A.T.; Goodwin, J.L.; Strigenz, A.M.; Chaballout, B.H.; Martin, J.C.; Arthur, C.M.; Funk, C.R.; Edenfield, W.J.; Blenda, A.V. Increased Circulating Levels of Galectin Proteins in Patients with Breast, Colon, and Lung Cancer. Cancers 2021, 13, 4819. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A.; Yamada, M.; Matsumoto, S.; Sakurazawa, N.; Kawano, Y.; Sekiguchi, K.; Yamada, T.; Matsutani, T.; Miyashita, M.; Yoshida, H. Blood Galectin-3 Levels Predict Postoperative Complications After Colorectal Cancer Surgery. J. Nippon Med. Sch. 2019, 86, 142–148. [Google Scholar] [CrossRef]
- Park, J.Y.; Yoon, G. Overexpression of Aquaporin-1 is a Prognostic Factor for Biochemical Recurrence in Prostate Adenocarcinoma. Pathol. Oncol. Res. 2017, 23, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Chen, Z.; Li, N.; Zhang, M. Prognostic Value of Serum Aquaporin-1, Aquaporin-3 and Galectin-3 for Young Patients with Colon Cancer. Ann. Clin. Biochem. 2020, 57, 404–411. [Google Scholar] [CrossRef]
- Shi, Y.; Tang, D.; Li, X.; Xie, X.; Ye, Y.; Wang, L. Galectin Family Members: Emerging Novel Targets for Lymphoma Therapy? Front. Oncol. 2022, 12, 889034. [Google Scholar] [CrossRef]
- Nakahara, S.; Oka, N.; Wang, Y.; Hogan, V.; Inohara, H.; Raz, A. Characterization of the Nuclear Import Pathways of Galectin-3. Cancer Res. 2006, 66, 9995–10006. [Google Scholar] [CrossRef]
- Ochieng, J.; Furtak, V.; Lukyanov, P. Extracellular Functions of Galectin-3. Glycoconj. J. 2002, 19, 527–535. [Google Scholar] [CrossRef]
- Elad-Sfadia, G.; Haklai, R.; Balan, E.; Kloog, Y. Galectin-3 Augments K-Ras Activation and Triggers a Ras Signal that Attenuates ERK but Not Phosphoinositide 3-Kinase Activity. J. Biol. Chem. 2004, 279, 34922–34930. [Google Scholar] [CrossRef]
- Akahani, S.; Nangia-Makker, P.; Inohara, H.; Kim, H.R.; Raz, A. Galectin-3: A Novel Antiapoptotic Molecule with a Functional BH1 (NWGR) Domain of Bcl-2 Family. Cancer Res. 1997, 57, 5272–5276. [Google Scholar]
- Dagher, S.F.; Wang, J.L.; Patterson, R.J. Identification of Galectin-3 as a Factor in Pre-mRNA Splicing. Proc. Natl. Acad. Sci. USA 1995, 92, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Fortuna-Costa, A.; Gomes, A.M.; Kozlowski, E.O.; Stelling, M.P.; Pavao, M.S. Extracellular Galectin-3 in Tumor Progression and Metastasis. Front. Oncol. 2014, 4, 138. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Dong, X.W.; Guo, X.L. Role of the Interaction between Galectin-3 and Cell Adhesion Molecules in Cancer Metastasis. Biomed. Pharmacother. 2015, 69, 179–185. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; van Kooyk, Y.; Cobb, B.A. Glycobiology of Immune Responses. Ann. New York Acad. Sci. 2012, 1253, 1–15. [Google Scholar] [CrossRef]
- Du Toit, A. Endocytosis: Bend it Like Galectin 3. Nat. Rev. Mol. Cell Biol. 2014, 15, 430–431. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Y.; Hill, P.N.; Hsu, D.K.; Liu, F.T. Role of the Carboxyl-Terminal Lectin Domain in Self-Association of Galectin-3. Biochemistry 1998, 37, 4086–4092. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Gabius, H.J.; Andre, S.; Kaltner, H.; Sabesan, S.; Roy, R.; Liu, B.; Macaluso, F.; Brewer, C.F. Galectin-3 Precipitates as a Pentamer with Synthetic Multivalent Carbohydrates and Forms Heterogeneous Cross-Linked Complexes. J. Biol. Chem. 2004, 279, 10841–10847. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.L.; Huang, E.Y.; Jhu, E.W.; Huang, Y.H.; Su, W.H.; Chuang, P.C.; Yang, K.D. Overexpression of Galectin-3 Enhances Migration of Colon Cancer Cells Related to Activation of the K-Ras-Raf-Erk1/2 Pathway. J. Gastroenterol. 2013, 48, 350–359. [Google Scholar] [CrossRef]
- Wu, K.L.; Huang, E.Y.; Yeh, W.L.; Hsiao, C.C.; Kuo, C.M. Synergistic Interaction between Galectin-3 and Carcinoembryonic Antigen Promotes Colorectal Cancer Metastasis. Oncotarget 2017, 8, 61935–61943. [Google Scholar] [CrossRef][Green Version]
- Wu, K.L.; Kuo, C.M.; Huang, E.Y.; Pan, H.M.; Huang, C.C.; Chen, Y.F.; Hsiao, C.C.; Yang, K.D. Extracellular Galectin-3 Facilitates Colon Cancer Cell Migration and is Related to the Epidermal Growth Factor Receptor. Am. J. Transl. Res. 2018, 10, 2402–2412. [Google Scholar]
- Barrow, H.; Rhodes, J.M.; Yu, L.G. The Role of Galectins in Colorectal Cancer Progression. Int. J. Cancer 2011, 129, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Akita, K.; Yashiro, M.; Sawada, T.; Hirakawa, K.; Murata, T.; Nakada, H. Binding of Galectin-3, a Beta-Galactoside-Binding Lectin, to MUC1 Protein Enhances Phosphorylation of Extracellular Signal-Regulated Kinase 1/2 (ERK1/2) and Akt, Promoting Tumor Cell Malignancy. J. Biol. Chem. 2015, 290, 26125–26140. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Tang, J.W.; Owusu, L.; Sun, M.Z.; Wu, J.; Zhang, J. Galectin-3 in Cancer. Clin. Chim. Acta 2014, 431, 185–191. [Google Scholar] [CrossRef]
- Song, S.; Mazurek, N.; Liu, C.; Sun, Y.; Ding, Q.Q.; Liu, K.; Hung, M.C.; Bresalier, R.S. Galectin-3 Mediates Nuclear Beta-Catenin Accumulation and Wnt Signaling in Human Colon Cancer Cells by Regulation of Glycogen Synthase Kinase-3beta Activity. Cancer Res. 2009, 69, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Wang, J.; Yang, G.; Yu, N.; Huang, Z.; Xu, H.; Li, J.; Qiu, J.; Zeng, X.; Chen, S.; et al. Posttranscriptional Regulation of Galectin-3 by miR-128 Contributes to Colorectal Cancer Progression. Oncotarget 2017, 8, 15242–15251. [Google Scholar] [CrossRef]
- Mazurek, N.; Byrd, J.C.; Sun, Y.; Hafley, M.; Ramirez, K.; Burks, J.; Bresalier, R.S. Cell-Surface Galectin-3 Confers Resistance to TRAIL by Impeding Trafficking of Death Receptors in Metastatic Colon Adenocarcinoma Cells. Cell Death Differ. 2012, 19, 523–533. [Google Scholar] [CrossRef]
- Guo, Y.; Shen, R.; Yu, L.; Zheng, X.; Cui, R.; Song, Y.; Wang, D. Roles of Galectin3 in the Tumor Microenvironment and Tumor Metabolism (Review). Oncol. Rep. 2020, 44, 1799–1809. [Google Scholar]
- Henderson, N.C.; Sethi, T. The Regulation of Inflammation by Galectin-3. Immunol. Rev. 2009, 230, 160–171. [Google Scholar] [CrossRef]
- Farhad, M.; Rolig, A.S.; Redmond, W.L. The Role of Galectin-3 in Modulating Tumor Growth and Immunosuppression within the Tumor Microenvironment. Oncoimmunology 2018, 7, e1434467. [Google Scholar] [CrossRef]
- SimovicMarkovic, B.; Nikolic, A.; Gazdic, M.; Nurkovic, J.; Djordjevic, I.; Arsenijevic, N.; Stojkovic, M.; Lukic, M.L.; Volarevic, V. Pharmacological Inhibition of Gal-3 in Mesenchymal Stem Cells Enhances their Capacity to Promote Alternative Activation of Macrophages in Dextran Sulphate Sodium-Induced Colitis. Stem Cells Int. 2016, 2016, 2640746. [Google Scholar]
- Tsai, H.F.; Wu, C.S.; Chen, Y.L.; Liao, H.J.; Chyuan, I.T.; Hsu, P.N. Galectin-3 Suppresses Mucosal Inflammation and Reduces Disease Severity in Experimental Colitis. J. Mol. Med. 2016, 94, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Lippert, E.; Stieber-Gunckel, M.; Dunger, N.; Falk, W.; Obermeier, F.; Kunst, C. Galectin-3 Modulates Experimental Colitis. Digestion 2015, 92, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Wang, H.Y.; Miyahara, Y.; Peng, G.; Wang, R.F. Tumor-Associated Galectin-3 Modulates the Function of Tumor-Reactive T Cells. Cancer Res. 2008, 68, 7228–7236. [Google Scholar] [CrossRef]
- Curciarello, R.; Steele, A.; Cooper, D.; MacDonald, T.T.; Kruidenier, L.; Kudo, T. The Role of Galectin-1 and Galectin-3 in the Mucosal Immune Response to CitrobacterRodentium Infection. PLoS ONE 2014, 9, e107933. [Google Scholar] [CrossRef] [PubMed]
- Shimura, T.; Shibata, M.; Gonda, K.; Nakajima, T.; Chida, S.; Noda, M.; Suzuki, S.; Nakamura, I.; Ohki, S.; Takenoshita, S. Association between Circulating Galectin-3 Levels and the Immunological, Inflammatory and Nutritional Parameters in Patients with Colorectal Cancer. Biomed. Rep. 2016, 5, 203–207. [Google Scholar] [CrossRef]
- Sawa-Wejksza, K.; Dudek, A.; Lemieszek, M.; Kalawaj, K.; Kandefer-Szerszen, M. Colon Cancer-Derived Conditioned Medium Induces Differentiation of THP-1 Monocytes into a Mixed Population of M1/M2 Cells. Tumour Biol. 2018, 40, 1010428318797880. [Google Scholar] [CrossRef]
- Muller, S.; Schaffer, T.; Flogerzi, B.; Fleetwood, A.; Weimann, R.; Schoepfer, A.M.; Seibold, F. Galectin-3 Modulates T Cell Activity and is Reduced in the Inflamed Intestinal Epithelium in IBD. Inflamm. Bowel Dis. 2006, 12, 588–597. [Google Scholar] [CrossRef]
- Volarevic, V.; Zdravkovic, N.; Harrell, C.R.; Arsenijevic, N.; Fellabaum, C.; Djonov, V.; Lukic, M.L.; SimovicMarkovic, B. Galectin-3 Regulates Indoleamine-2,3-Dioxygenase-Dependent Cross-Talk between Colon-Infiltrating Dendritic Cells and T Regulatory Cells and may Represent a Valuable Biomarker for Monitoring the Progression of Ulcerative Colitis. Cells 2019, 8, 709. [Google Scholar] [CrossRef] [PubMed]
- Laderach, D.J.; Compagno, D. Unraveling how Tumor-Derived Galectins Contribute to Anti-Cancer Immunity Failure. Cancers 2021, 13, 4529. [Google Scholar] [CrossRef]
- Dumont, P.; Berton, A.; Nagy, N.; Sandras, F.; Tinton, S.; Demetter, P.; Mascart, F.; Allaoui, A.; Decaestecker, C.; Salmon, I. Expression of Galectin-3 in the Tumor Immune Response in Colon Cancer. Lab. Invest. 2008, 88, 896–906. [Google Scholar] [CrossRef]
- deKivit, S.; Kraneveld, A.D.; Garssen, J.; Willemsen, L.E. Glycan Recognition at the Interface of the Intestinal Immune System: Target for Immune Modulation Via Dietary Components. Eur. J. Pharmacol. 2011, 668 (Suppl. 1), S124–S132. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, D.; Kane, M.; Joshi, L.; Hickey, R.M. Detection of Galectin-3 Interaction with Commensal Bacteria. Appl. Environ. Microbiol. 2013, 79, 3507–3510. [Google Scholar] [CrossRef] [PubMed]
- Filipova, M.; Bojarova, P.; Rodrigues Tavares, M.; Bumba, L.; Elling, L.; Chytil, P.; Gunar, K.; Kren, V.; Etrych, T.; Janouskova, O. Glycopolymers for Efficient Inhibition of Galectin-3: In Vitro Proof of Efficacy using Suppression of T Lymphocyte Apoptosis and Tumor Cell Migration. Biomacromolecules 2020, 21, 3122–3133. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, K.; Francuz, T.; Gallego-Colon, E.; Ruiz-Agamez, N.; Kubeczko, M.; Grochola, I.; Wojnar, J. Galectin Targeted Therapy in Oncology: Current Knowledge and Perspectives. Int. J. Mol. Sci. 2018, 19, 210. [Google Scholar] [CrossRef]
- Beukema, M.; Faas, M.M.; de Vos, P. The Effects of Different Dietary Fiber Pectin Structures on the Gastrointestinal Immune Barrier: Impact Via Gut Microbiota and Direct Effects on Immune Cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef]
- Bishehsari, F.; Engen, P.A.; Preite, N.Z.; Tuncil, Y.E.; Naqib, A.; Shaikh, M.; Rossi, M.; Wilber, S.; Green, S.J.; Hamaker, B.R.; et al. Dietary Fiber Treatment Corrects the Composition of Gut Microbiota, Promotes SCFA Production, and Suppresses Colon Carcinogenesis. Genes 2018, 9, 102. [Google Scholar] [CrossRef]
- Prado, S.B.R.D.; Ferreira, G.F.; Harazono, Y.; Shiga, T.M.; Raz, A.; Carpita, N.C.; Fabi, J.P. Ripening-Induced Chemical Modifications of Papaya Pectin Inhibit Cancer Cell Proliferation. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef]
- Moslemi, M. Reviewing the Recent Advances in Application of Pectin for Technical and Health Promotion Purposes: From Laboratory to Market. Carbohydr. Polym. 2021, 254, 117324. [Google Scholar] [CrossRef]
- do Prado, S.B.R.; Castro-Alves, V.C.; Ferreira, G.F.; Fabi, J.P. Ingestion of Non-Digestible Carbohydrates from Plant-Source Foods and Decreased Risk of Colorectal Cancer: A Review on the Biological Effects and the Mechanisms of Action. Front. Nutr. 2019, 6, 72. [Google Scholar] [CrossRef]
- Gunning, A.P.; Bongaerts, R.J.; Morris, V.J. Recognition of Galactan Components of Pectin by Galectin-3. FASEB J. 2009, 23, 415–424. [Google Scholar] [CrossRef]
- Kamili, N.A.; Arthur, C.M.; Gerner-Smidt, C.; Tafesse, E.; Blenda, A.; Dias-Baruffi, M.; Stowell, S.R. Key Regulators of Galectin-Glycan Interactions. Proteomics 2016, 16, 3111–3125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, P.; Zhang, H. Pectin in cancer therapy: A review. Trends Food Sci. Technol. 2015, 44, 258–271. [Google Scholar] [CrossRef]
- Nangia-Makker, P.; Hogan, V.; Honjo, Y.; Baccarini, S.; Tait, L.; Bresalier, R.; Raz, A. Inhibition of Human Cancer Cell Growth and Metastasis in Nude Mice by Oral Intake of Modified Citrus Pectin. J. Natl. Cancer Inst. 2002, 94, 1854–1862. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Huang, Z.L.; Yang, G.H.; Lu, W.Q.; Yu, N.R. Inhibitory Effect of Modified Citrus Pectin on Liver Metastases in a Mouse Colon Cancer Model. World J. Gastroenterol. 2008, 14, 7386–7391. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, P.; Lu, S.M.; Ling, Z.Q. Chemoprevention of Low-Molecular-Weight Citrus Pectin (LCP) in Gastrointestinal Cancer Cells. Int. J. Biol. Sci. 2016, 12, 746–756. [Google Scholar] [CrossRef]
- Odun-Ayo, F.; Mellem, J.; Naicker, T.; Reddy, L. Chemoprevention of Azoxymethane-Induced Colonic Carcinogenesis in Balb/C Mice using a Modified Pectin Alginate Probiotic. Anticancer Res. 2015, 35, 4765–4775. [Google Scholar]
- Li, Y.; Liu, L.; Niu, Y.; Feng, J.; Sun, Y.; Kong, X.; Chen, Y.; Chen, X.; Gan, H.; Cao, S.; et al. Modified Apple Polysaccharide Prevents Against Tumorigenesis in a Mouse Model of Colitis-Associated Colon Cancer: Role of Galectin-3 and Apoptosis in Cancer Prevention. Eur. J. Nutr. 2012, 51, 107–117. [Google Scholar] [CrossRef]
- Cheng, H.; Li, S.; Fan, Y.; Gao, X.; Hao, M.; Wang, J.; Zhang, X.; Tai, G.; Zhou, Y. Comparative Studies of the Antiproliferative Effects of Ginseng Polysaccharides on HT-29 Human Colon Cancer Cells. Med. Oncol. 2011, 28, 175–181. [Google Scholar] [CrossRef]
- Gao, X.; Zhi, Y.; Sun, L.; Peng, X.; Zhang, T.; Xue, H.; Tai, G.; Zhou, Y. The Inhibitory Effects of a Rhamnogalacturonan I (RG-I) Domain from Ginseng Pectin on Galectin-3 and its Structure-Activity Relationship. J. Biol. Chem. 2013, 288, 33953–33965. [Google Scholar] [CrossRef]
- Maksymowicz, J.; Palko-Labuz, A.; Sobieszczanska, B.; Chmielarz, M.; Ferens-Sieczkowska, M.; Skonieczna, M.; Wikiera, A.; Wesolowska, O.; Sroda-Pomianek, K. The use of Endo-Cellulase and Endo-Xylanase for the Extraction of Apple Pectins as Factors Modifying their Anticancer Properties and Affecting their Synergy with the Active Form of Irinotecan. Pharmaceuticals 2022, 15, 732. [Google Scholar] [CrossRef]
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Modified Sugar Beet Pectin Induces Apoptosis of Colon Cancer Cells Via an Interaction with the Neutral Sugar Side-Chains. Carbohydr. Polym. 2016, 136, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Oria, A.; Rodriguez-Gutierrez, G.; Alaiz, M.; Vioque, J.; Giron-Calle, J.; Fernandez-Bolanos, J. Pectin-Rich Extracts from Olives Inhibit Proliferation of Caco-2 and THP-1 Cells. Food Funct. 2019, 10, 4844–4853. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, R.S.; Pedrosa, L.F.; Diethelm, L.T.H.; Souza, T.; Shiga, T.M.; Fabi, J.P. The Purification of Pectin from Commercial Fruit Flours Results in a Jaboticaba Fraction that Inhibits Galectin-3 and Colon Cancer Cell Growth. Food Res. Int. 2020, 137, 109747. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, L.F.; Lopes, R.G.; Fabi, J.P. The Acid and Neutral Fractions of Pectins Isolated from Ripe and Overripe Papayas Differentially Affect Galectin-3 Inhibition and Colon Cancer Cell Growth. Int. J. Biol. Macromol. 2020, 164, 2681–2690. [Google Scholar] [CrossRef] [PubMed]
- Gunning, A.P.; Pin, C.; Morris, V.J. Galectin 3-Beta-Galactobiose Interactions. Carbohydr. Polym. 2013, 92, 529–533. [Google Scholar] [CrossRef]
- Hayashi, A.; Gillen, A.C.; Lott, J.R. Effects of Daily Oral Administration of Quercetin Chalcone and Modified Citrus Pectin on Implanted Colon-25 Tumor Growth in Balb-C Mice. Altern. Med. Rev. 2000, 5, 546–552. [Google Scholar]
- Bresalier, R.S.; Mazurek, N.; Sternberg, L.R.; Byrd, J.C.; Yunker, C.K.; Nangia-Makker, P.; Raz, A. Metastasis of Human Colon Cancer is Altered by Modifying Expression of the Beta-Galactoside-Binding Protein Galectin 3. Gastroenterology 1998, 115, 287–296. [Google Scholar] [CrossRef]
- Pedrosa, L.F.; Raz, A.; Fabi, J.P. The Complex Biological Effects of Pectin: Galectin-3 Targeting as Potential Human Health Improvement? Biomolecules 2022, 12, 289. [Google Scholar] [CrossRef]
- Donadio, J.L.S.; Prado, S.B.R.; Rogero, M.M.; Fabi, J.P. Effects of Pectins on Colorectal Cancer: Targeting Hallmarks as a Support for Future Clinical Trials. Food Funct. 2022, 13, 11438–11454. [Google Scholar] [CrossRef]
- Eliaz, I.; Raz, A. Pleiotropic Effects of Modified Citrus Pectin. Nutrients 2019, 11, 2619. [Google Scholar] [CrossRef]
- Keizman, D.; Frenkel, M.; Peer, A.; Kushnir, I.; Rosenbaum, E.; Sarid, D.; Leibovitch, I.; Mano, R.; Yossepowitch, O.; Margel, D.; et al. Modified Citrus Pectin Treatment in Non-Metastatic Biochemically Relapsed Prostate Cancer: Results of a Prospective Phase II Study. Nutrients 2021, 13, 4295. [Google Scholar] [CrossRef] [PubMed]
- Curti, B.D.; Koguchi, Y.; Leidner, R.S.; Rolig, A.S.; Sturgill, E.R.; Sun, Z.; Wu, Y.; Rajamanickam, V.; Bernard, B.; Hilgart-Martiszus, I.; et al. Enhancing Clinical and Immunological Effects of Anti-PD-1 with Belapectin, a Galectin-3 Inhibitor. J. Immunother. Cancer 2021, 9, e002371. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Mileo, A.M.; Nistico, P.; Miccadei, S. Polyphenols: Immunomodulatory and Therapeutic Implication in Colorectal Cancer. Front. Immunol. 2019, 10, 729. [Google Scholar] [CrossRef]
- Bracci, L.; Fabbri, A.; Del Corno, M.; Conti, L. Dietary Polyphenols: Promising Adjuvants for Colorectal Cancer Therapies. Cancers 2021, 13, 4499. [Google Scholar] [CrossRef]
- Amintas, S.; Dupin, C.; Boutin, J.; Beaumont, P.; Moreau-Gaudry, F.; Bedel, A.; Krisa, S.; Vendrely, V.; Dabernat, S. Bioactive Food Components for Colorectal Cancer Prevention and Treatment: A Good Match. Crit. Rev. Food Sci. Nutr. 2022, 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Del Bo’, C.; Martini, D.; Porrini, M.; Riso, P. A Review of Registered Clinical Trials on Dietary (Poly)Phenols: Past Efforts and Possible Future Directions. Foods 2020, 9, 1606. [Google Scholar] [CrossRef]
- Alam, M.N.; Almoyad, M.; Huq, F. Polyphenols in Colorectal Cancer: Current State of Knowledge Including Clinical Trials and Molecular Mechanism of Action. Biomed. Res. Int. 2018, 2018, 4154185. [Google Scholar] [CrossRef]
- El-Kott, A.F.; Shati, A.A.; Ali Al-Kahtani, M.; Alharbi, S.A. The Apoptotic Effect of Resveratrol in Ovarian Cancer Cells is Associated with Downregulation of Galectin-3 and Stimulating miR-424-3p Transcription. J. Food Biochem. 2019, 43, e13072. [Google Scholar] [CrossRef]
- Li, H.; Xiao, L.; He, H.; Zeng, H.; Liu, J.; Jiang, C.; Mei, G.; Yu, J.; Chen, H.; Yao, P.; et al. Quercetin Attenuates Atherosclerotic Inflammation by Inhibiting Galectin-3-NLRP3 Signaling Pathway. Mol. Nutr. Food Res. 2021, 65, e2000746. [Google Scholar] [CrossRef]
- Pei, C.; Zhang, Y.; Wang, P.; Zhang, B.; Fang, L.; Liu, B.; Meng, S. Berberine Alleviates Oxidized Low-Density Lipoprotein-Induced Macrophage Activation by Downregulating Galectin-3 Via the NF-kappaB and AMPK Signaling Pathways. Phytother. Res. 2019, 33, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Luis, C.; Costa, R.; Rodrigues, I.; Castela, A.; Coelho, P.; Guerreiro, S.; Gomes, J.; Reis, C.; Soares, R. Xanthohumol and 8-Prenylnaringenin Reduce Type 2 Diabetes-Associated Oxidative Stress by Downregulating Galectin-3. Porto Biomed. J. 2018, 4, e23. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Jiang, Z.; Jiang, M.; Sun, Y. Berberine as a Potential Agent for the Treatment of Colorectal Cancer. Front. Med. 2022, 9, 886996. [Google Scholar] [CrossRef]
- Chen, H.; Yin, S.; Xiong, Z.; Li, X.; Zhang, F.; Chen, X.; Guo, J.; Xie, M.; Mao, C.; Jin, L.; et al. Clinicopathologic Characteristics and Prognosis of Synchronous Colorectal Cancer: A Retrospective Study. BMC Gastroenterol. 2022, 22, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, L.; Li, G.; Gao, Z. Xanthohumol Protects Against Azoxymethane-Induced Colorectal Cancer in Sprague-Dawley Rats. Environ. Toxicol. 2020, 35, 136–144. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).