Protoporphyrin IX (PpIX) Fluorescence during Meningioma Surgery: Correlations with Histological Findings and Expression of Heme Pathway Molecules
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Sample Collection
2.2. Immunohistochemistry
2.3. Quantitative Real-Time PCR (qPCR)
2.4. Statistical Analyses
3. Results
3.1. Capability of FGR to Identify Neoplastic Tissue during Meningioma Surgery Varies
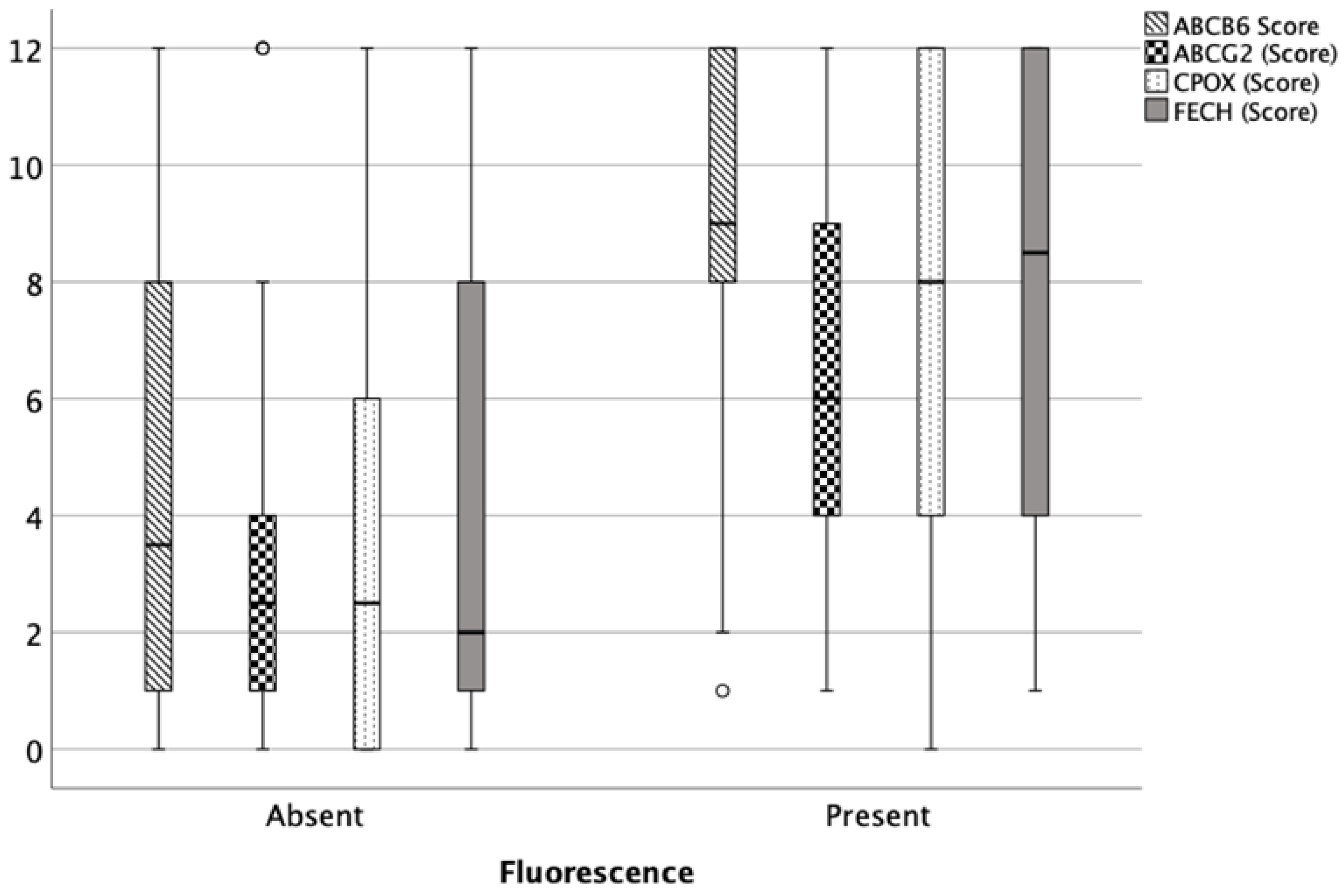
3.2. Protein Expression of ABCB6, ABCG2, FECH and CPOX
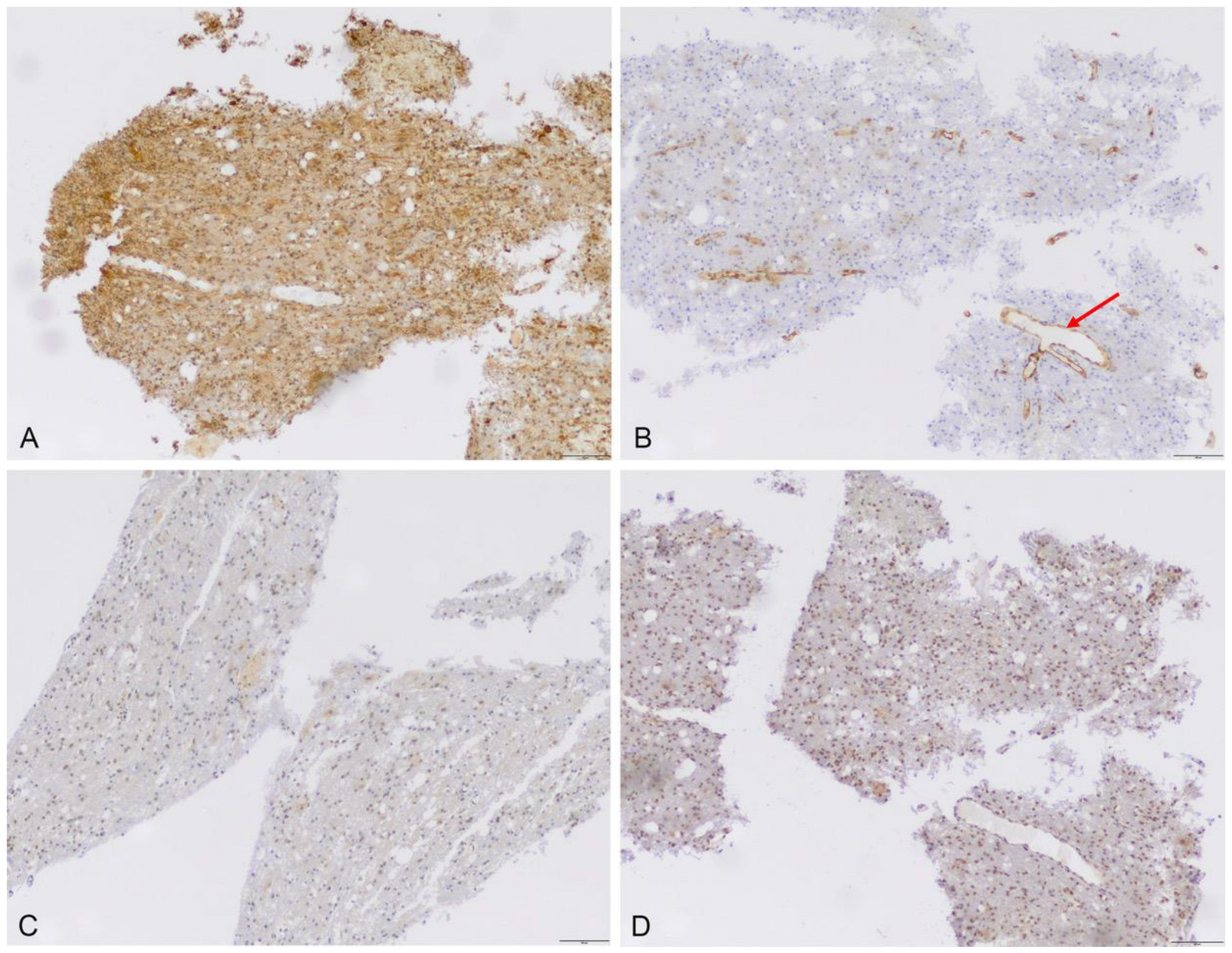
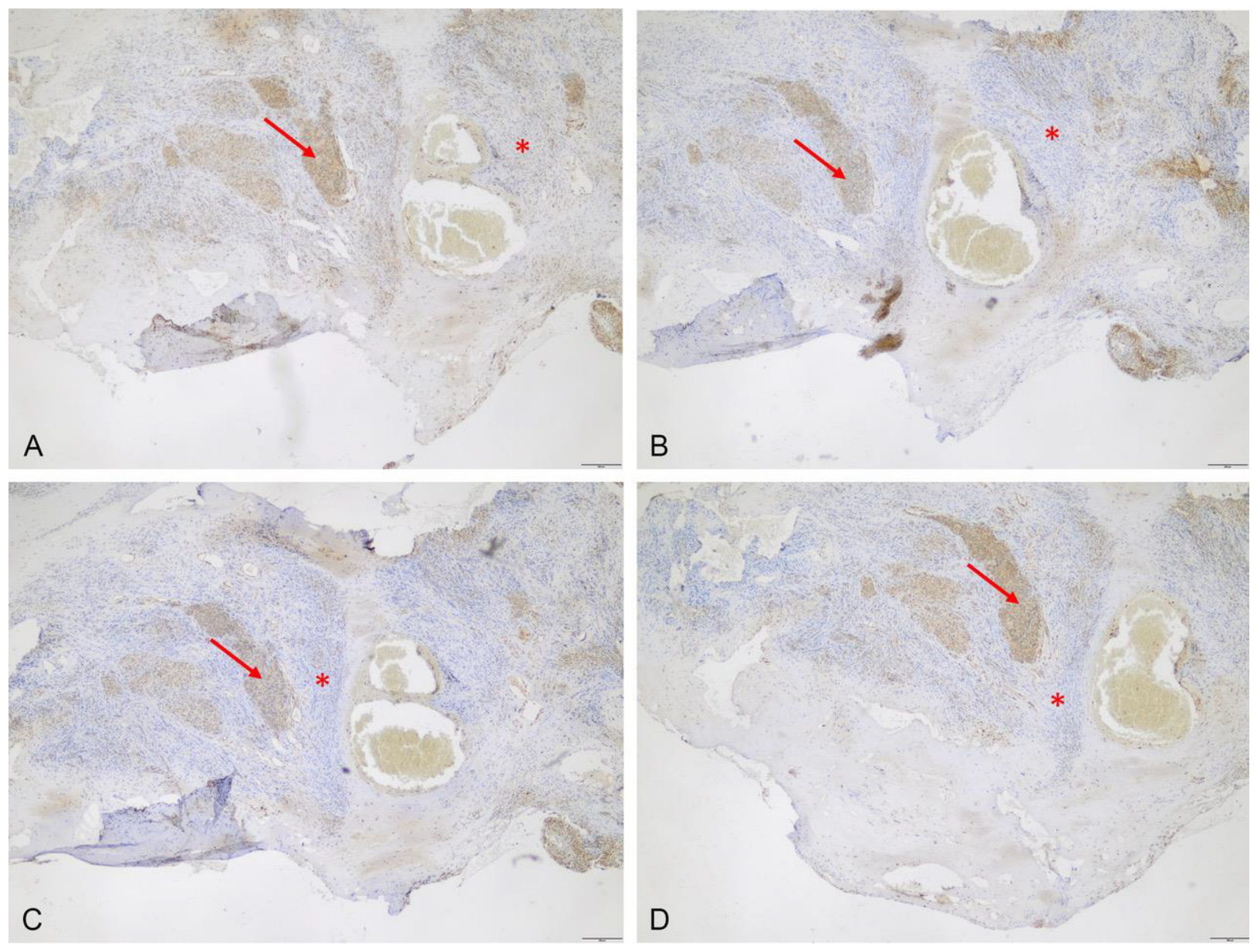
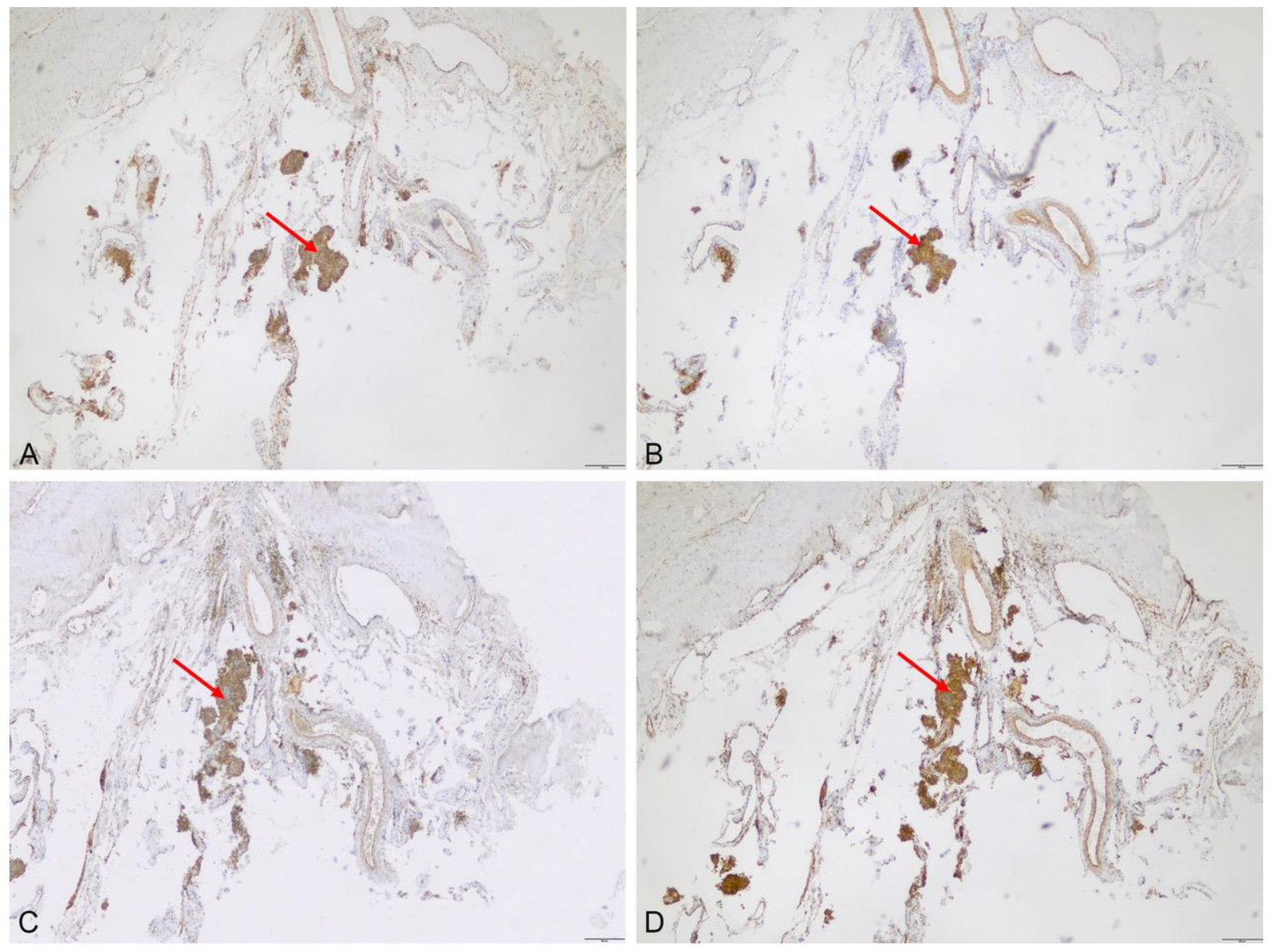
3.2.1. Immunhistochemistry
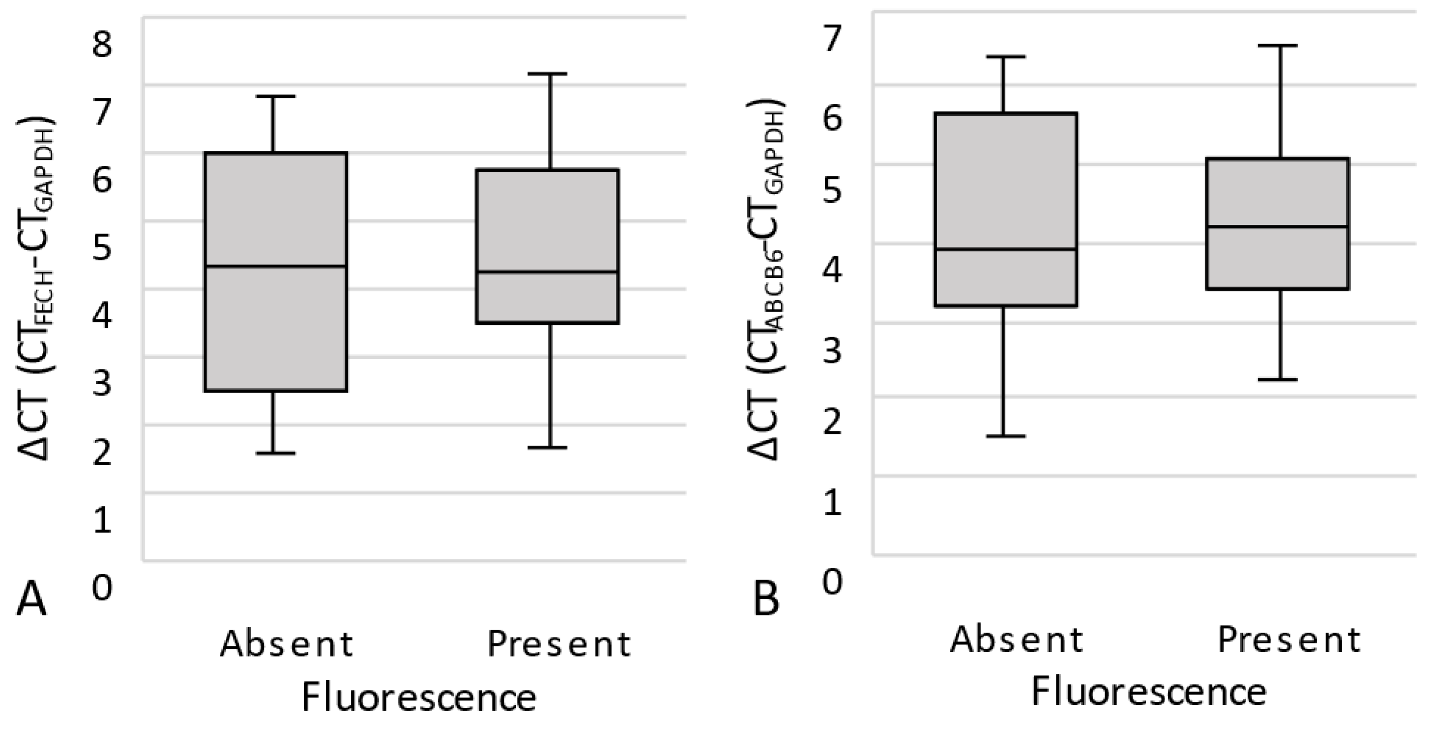
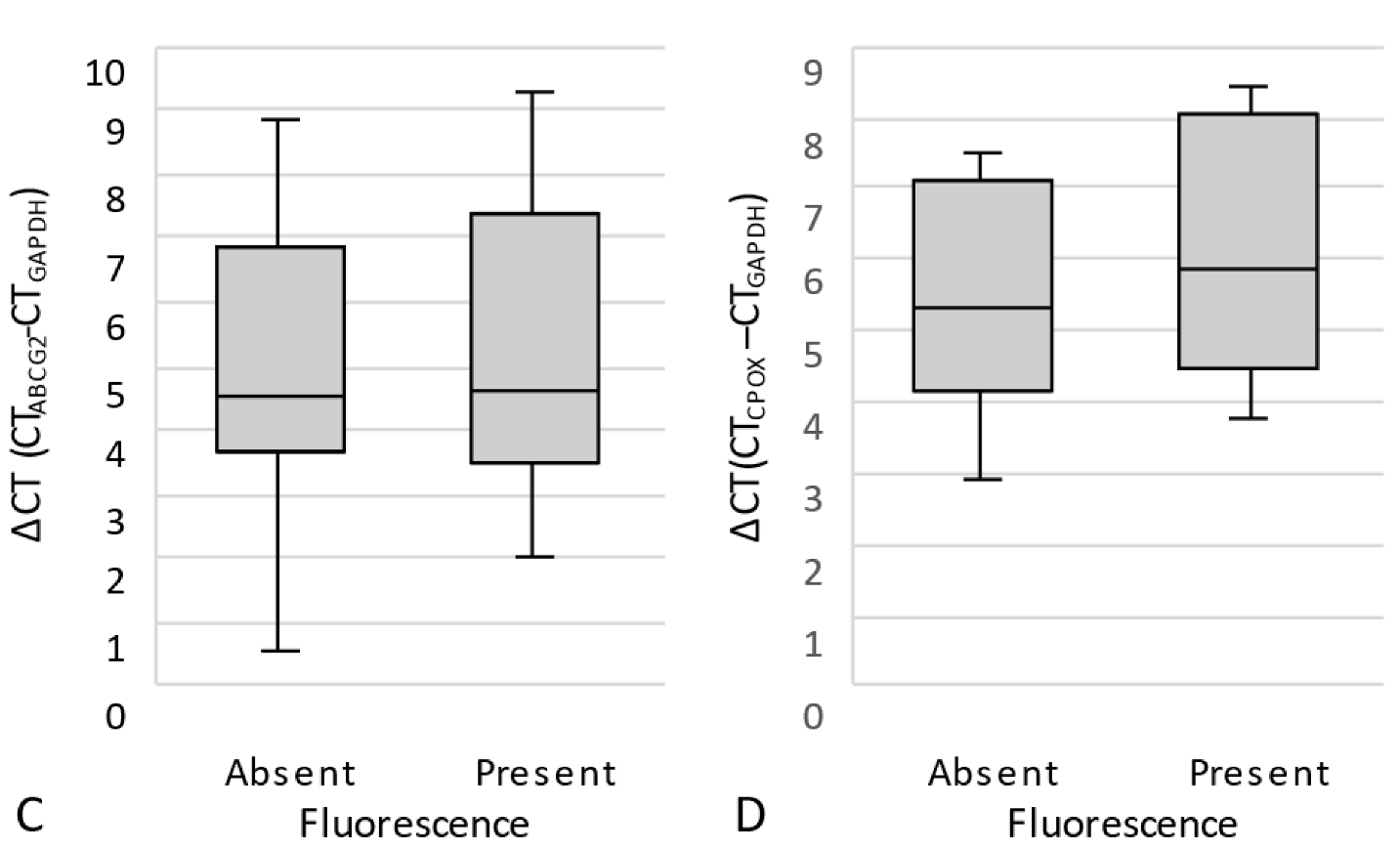
3.2.2. Quantitative Real-Time PCR (qPCR)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldbrunner, R.; Stavrinou, P.; Jenkinson, M.D.; Sahm, F.; Mawrin, C.; Weber, D.C.; Preusser, M.; Minniti, G.; Lund-Johansen, M.; Lefranc, F.; et al. EANO guideline on the diagnosis and management of meningiomas. Neuro Oncol. 2021, 23, 1821–1834. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D. The recurrence of intracranial meningiomas after surgical treatment. J. Neurol. Neurosurg. Psychiatry 1957, 20, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, N.J.; Michael, A.P. 5-Aminolevulinic acid radiodynamic therapy for treatment of high-grade gliomas: A systematic review. Clin. Neurol. Neurosurg. 2021, 201, 106430. [Google Scholar] [CrossRef]
- Gerritsen, J.K.W.; Broekman, M.L.D.; De Vleeschouwer, S.; Schucht, P.; Nahed, B.V.; Berger, M.S.; Vincent, A. Safe surgery for glioblastoma: Recent advances and modern challenges. Neurooncol. Pract. 2022, 9, 364–379. [Google Scholar] [CrossRef] [PubMed]
- Traylor, J.I.; Pernik, M.N.; Sternisha, A.C.; McBrayer, S.K.; Abdullah, K.G. Molecular and Metabolic Mechanisms Underlying Selective 5-Aminolevulinic Acid-Induced Fluorescence in Gliomas. Cancers 2021, 13, 580. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kajimoto, Y.; Inoue, Y.; Ikegami, Y.; Kuroiwa, T. Critical role of ABCG2 in ALA-photodynamic diagnosis and therapy of human brain tumor. Adv. Cancer Res. 2015, 125, 197–216. [Google Scholar] [CrossRef]
- Jin, Y.; Bin, Z.Q.; Qiang, H.; Liang, C.; Hua, C.; Jun, D.; Dong, W.A.; Qing, L. ABCG2 is related with the grade of glioma and resistance to mitoxantone, a chemotherapeutic drug for glioma. J. Cancer Res. Clin. Oncol. 2009, 135, 1369–1376. [Google Scholar] [CrossRef]
- Pustogarov, N.; Panteleev, D.; Goryaynov, S.A.; Ryabova, A.V.; Rybalkina, E.Y.; Revishchin, A.; Potapov, A.A.; Pavlova, G. Hiding in the Shadows: CPOX Expression and 5-ALA Induced Fluorescence in Human Glioma Cells. Mol. Neurobiol. 2017, 54, 5699–5708. [Google Scholar] [CrossRef]
- Sun, W.; Kajimoto, Y.; Inoue, H.; Miyatake, S.; Ishikawa, T.; Kuroiwa, T. Gefitinib enhances the efficacy of photodynamic therapy using 5-aminolevulinic acid in malignant brain tumor cells. Photodiagnosis Photodyn. Ther. 2013, 10, 42–50. [Google Scholar] [CrossRef]
- Takahashi, K.; Ikeda, N.; Nonoguchi, N.; Kajimoto, Y.; Miyatake, S.; Hagiya, Y.; Ogura, S.; Nakagawa, H.; Ishikawa, T.; Kuroiwa, T. Enhanced expression of coproporphyrinogen oxidase in malignant brain tumors: CPOX expression and 5-ALA-induced fluorescence. Neuro Oncol. 2011, 13, 1234–1243. [Google Scholar] [CrossRef]
- Teng, L.; Nakada, M.; Zhao, S.G.; Endo, Y.; Furuyama, N.; Nambu, E.; Pyko, I.V.; Hayashi, Y.; Hamada, J.I. Silencing of ferrochelatase enhances 5-aminolevulinic acid-based fluorescence and photodynamic therapy efficacy. Br. J. Cancer 2011, 104, 798–807. [Google Scholar] [CrossRef]
- Zhao, S.G.; Chen, X.F.; Wang, L.G.; Yang, G.; Han, D.Y.; Teng, L.; Yang, M.C.; Wang, D.Y.; Shi, C.; Liu, Y.H.; et al. Increased expression of ABCB6 enhances protoporphyrin IX accumulation and photodynamic effect in human glioma. Ann. Surg. Oncol. 2013, 20, 4379–4388. [Google Scholar] [CrossRef] [PubMed]
- Motekallemi, A.; Jeltema, H.R.; Metzemaekers, J.D.; van Dam, G.M.; Crane, L.M.; Groen, R.J. The current status of 5-ALA fluorescence-guided resection of intracranial meningiomas-a critical review. Neurosurg. Rev. 2015, 38, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Valdes, P.A.; Bekelis, K.; Harris, B.T.; Wilson, B.C.; Leblond, F.; Kim, A.; Simmons, N.E.; Erkmen, K.; Paulsen, K.D.; Roberts, D.W. 5-Aminolevulinic acid-induced protoporphyrin IX fluorescence in meningioma: Qualitative and quantitative measurements in vivo. Neurosurgery 2014, 10 (Suppl. S1), 74–82; discussion 82–73. [Google Scholar] [CrossRef] [PubMed]
- Valdes, P.A.; Millesi, M.; Widhalm, G.; Roberts, D.W. 5-aminolevulinic acid induced protoporphyrin IX (ALA-PpIX) fluorescence guidance in meningioma surgery. J. Neurooncol. 2019, 141, 555–565. [Google Scholar] [CrossRef]
- Millesi, M.; Kiesel, B.; Mischkulnig, M.; Martinez-Moreno, M.; Wohrer, A.; Wolfsberger, S.; Knosp, E.; Widhalm, G. Analysis of the surgical benefits of 5-ALA-induced fluorescence in intracranial meningiomas: Experience in 204 meningiomas. J. Neurosurg. 2016, 125, 1408–1419. [Google Scholar] [CrossRef]
- Brokinkel, B.; Kroger, S.; Senner, V.; Jeibmann, A.; Karst, U.; Stummer, W. Visualizing protoporphyrin IX formation in the dura tail of meningiomas by mass spectrometry imaging. Acta Neurochir. 2018, 160, 1433–1437. [Google Scholar] [CrossRef]
- Knipps, J.; Beseoglu, K.; Kamp, M.; Fischer, I.; Felsberg, J.; Neumann, L.M.; Steiger, H.J.; Cornelius, J.F. Fluorescence Behavior and Dural Infiltration of Meningioma Analyzed by 5-Aminolevulinic Acid-Based Fluorescence: Operating Microscope Versus Mini-Spectrometer. World Neurosurg. 2017, 108, 118–127. [Google Scholar] [CrossRef]
- Coluccia, D.; Fandino, J.; Fujioka, M.; Cordovi, S.; Muroi, C.; Landolt, H. Intraoperative 5-aminolevulinic-acid-induced fluorescence in meningiomas. Acta Neurochir. 2010, 152, 1711–1719. [Google Scholar] [CrossRef]
- Perry, A.; Louis, D.N.; von Deimling, A.; Sahm, F.; Rushing, E.J.; Mawrin, C.; Claus, E.B.; Loeffler, J.; Sadetzki, S. Meningiomas. In WHO Classification of Tumors of the Central Nervous System; Louis, D.N., Ohgaki, H., Wiestler, O.D., Cavenee, W.K., Ellison, D.W., Figarella-Branger, D., Perry, A., Reifenberger, G., von Deimlig, A., Eds.; International Agency on Cancer Research: Lyon, France, 2016; pp. 232–245. [Google Scholar]
- Wadiura, L.I.; Millesi, M.; Makolli, J.; Wais, J.; Kiesel, B.; Mischkulnig, M.; Mercea, P.A.; Roetzer, T.; Knosp, E.; Rossler, K.; et al. High Diagnostic Accuracy of Visible 5-ALA Fluorescence in Meningioma Surgery According to Histopathological Analysis of Tumor Bulk and Peritumoral Tissue. Lasers Surg. Med. 2021, 53, 300–308. [Google Scholar] [CrossRef]
- Morofuji, Y.; Matsuo, T.; Hayashi, Y.; Suyama, K.; Nagata, I. Usefulness of intraoperative photodynamic diagnosis using 5-aminolevulinic acid for meningiomas with cranial invasion: Technical case report. Neurosurgery 2008, 62, 102–103; discussion 103–104. [Google Scholar] [CrossRef] [PubMed]
- Whitson, W.J.; Valdes, P.A.; Harris, B.T.; Paulsen, K.D.; Roberts, D.W. Confocal microscopy for the histological fluorescence pattern of a recurrent atypical meningioma: Case report. Neurosurgery 2011, 68, E1768–E1772; discussion E1763–E1772. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Abramov, I.; Belykh, E.; Mignucci-Jimenez, G.; Park, M.T.; Eschbacher, J.M.; Preul, M.C. Characterization of ex vivo and in vivo intraoperative neurosurgical confocal laser endomicroscopy imaging. Front. Oncol. 2022, 12, 979748. [Google Scholar] [CrossRef] [PubMed]
- Mehidine, H.; Refregiers, M.; Jamme, F.; Varlet, P.; Juchaux, M.; Devaux, B.; Abi Haidar, D. Molecular changes tracking through multiscale fluorescence microscopy differentiate Meningioma grades and non-tumoral brain tissues. Sci. Rep. 2021, 11, 3816. [Google Scholar] [CrossRef]
- Cornelius, J.F.; Slotty, P.J.; Kamp, M.A.; Schneiderhan, T.M.; Steiger, H.J.; El-Khatib, M. Impact of 5-aminolevulinic acid fluorescence-guided surgery on the extent of resection of meningiomas--with special regard to high-grade tumors. Photodiagnosis Photodyn. Ther. 2014, 11, 481–490. [Google Scholar] [CrossRef]
- Lau, D.; Hervey-Jumper, S.L.; Chang, S.; Molinaro, A.M.; McDermott, M.W.; Phillips, J.J.; Berger, M.S. A prospective Phase II clinical trial of 5-aminolevulinic acid to assess the correlation of intraoperative fluorescence intensity and degree of histologic cellularity during resection of high-grade gliomas. J. Neurosurg. 2016, 124, 1300–1309. [Google Scholar] [CrossRef]
- Liu, H.G.; Pan, Y.F.; You, J.; Wang, O.C.; Huang, K.T.; Zhang, X.H. Expression of ABCG2 and its significance in colorectal cancer. Asian Pac. J. Cancer Prev. 2010, 11, 845–848. [Google Scholar]
- Nedeljkovic, M.; Tanic, N.; Prvanovic, M.; Milovanovic, Z.; Tanic, N. Friend or foe: ABCG2, ABCC1 and ABCB1 expression in triple-negative breast cancer. Breast Cancer 2021, 28, 727–736. [Google Scholar] [CrossRef]
- Freitag, D.; McLean, A.L.; Simon, M.; Koch, A.; Grube, S.; Walter, J.; Kalff, R.; Ewald, C. NANOG overexpression and its correlation with stem cell and differentiation markers in meningiomas of different WHO grades. Mol. Carcinog. 2017, 56, 1953–1964. [Google Scholar] [CrossRef]
- Hefti, M.; Holenstein, F.; Albert, I.; Looser, H.; Luginbuehl, V. Susceptibility to 5-aminolevulinic acid based photodynamic therapy in WHO I meningioma cells corresponds to ferrochelatase activity. Photochem. Photobiol. 2011, 87, 235–241. [Google Scholar] [CrossRef]
- Mischkulnig, M.; Roetzer-Pejrimovsky, T.; Lotsch-Gojo, D.; Kastner, N.; Bruckner, K.; Prihoda, R.; Lang, A.; Martinez-Moreno, M.; Furtner, J.; Berghoff, A.; et al. Heme Biosynthesis Factors and 5-ALA Induced Fluorescence: Analysis of mRNA and Protein Expression in Fluorescing and Non-fluorescing Gliomas. Front. Med. 2022, 9, 907442. [Google Scholar] [CrossRef] [PubMed]
- Bunk, E.C.; Wagner, A.; Stummer, W.; Senner, V.; Brokinkel, B. 5-ALA kinetics in meningiomas: Analysis of tumor fluorescence and PpIX metabolism in vitro and comparative analyses with high-grade gliomas. J. Neurooncol. 2021, 152, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Brokinkel, B.; Suero Molina, E.; Warneke, N.; Holling, M.; Bunk, E.C.; Hess, K.; Senner, V.; Paulus, W.; Stummer, W. Real-time in vivo kinetics of protoporphyrin IX after administration of 5-aminolevulinic acid in meningiomas and comparative analyses with glioblastomas. Acta Neurochir. 2020, 162, 2197–2202. [Google Scholar] [CrossRef] [PubMed]

| Antigene | Manufacturer | Order# | Dilution | Positive Control | Host Species |
|---|---|---|---|---|---|
| ABCB6 | Invitrogen | PA5-78693 | 1:100 | Mamma carcinoma | Rabbit |
| CPOX | Invitrogen | PA5-97613 | 1:200 | Liver | Rabbit |
| FECH | Abcam | ab219349 | 1:288 | Kidney | Rabbit |
| ABCG2 | Abcam | ab229193 | 1:2000 | Placenta | Rabbit |
| Gene of Interest | TaqMan Assay (Thermo Fisher) |
|---|---|
| ABCB6 | Hs00180568-m1 |
| CPOX | Hs01071019-m1 |
| FECH | Hs00164616-m1 |
| ABCG2 | Hs01053790-m1 |
| GAPDH (Reference) | Hs02786624-g1 |
| Variable | N | (n%) |
|---|---|---|
| Age (median) | 61 years (19–85) | |
| Females | 27 | 61 |
| Males | 17 | 39 |
| Tumor location | ||
| Convexity/parasagittal | 34 | 77 |
| Skull base | 10 | 23 |
| Indication | ||
| Primary diagnosis | 36 | 82 |
| Recurrence | 8 | 18 |
| Previous therapy | ||
| Previous microsurgery | 1 | 2 |
| Previous microsurgery and irradiation | 4 | 9 |
| Previous irradiation | 1 | 2 |
| Previous microsurgery, irradiation and PRRT | 1 | 2 |
| Extent of resection | ||
| Simpson I–III | 37 | 84 |
| Simpson IV–V | 7 | 16 |
| WHO Grade | ||
| 1 | 37 | 84 |
| 2/3 | 7 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spille, D.C.; Bunk, E.C.; Thomas, C.; Özdemir, Z.; Wagner, A.; Akkurt, B.H.; Mannil, M.; Paulus, W.; Grauer, O.M.; Stummer, W.; et al. Protoporphyrin IX (PpIX) Fluorescence during Meningioma Surgery: Correlations with Histological Findings and Expression of Heme Pathway Molecules. Cancers 2023, 15, 304. https://doi.org/10.3390/cancers15010304
Spille DC, Bunk EC, Thomas C, Özdemir Z, Wagner A, Akkurt BH, Mannil M, Paulus W, Grauer OM, Stummer W, et al. Protoporphyrin IX (PpIX) Fluorescence during Meningioma Surgery: Correlations with Histological Findings and Expression of Heme Pathway Molecules. Cancers. 2023; 15(1):304. https://doi.org/10.3390/cancers15010304
Chicago/Turabian StyleSpille, Dorothee C., Eva C. Bunk, Christian Thomas, Zeynep Özdemir, Andrea Wagner, Burak H. Akkurt, Manoj Mannil, Werner Paulus, Oliver M. Grauer, Walter Stummer, and et al. 2023. "Protoporphyrin IX (PpIX) Fluorescence during Meningioma Surgery: Correlations with Histological Findings and Expression of Heme Pathway Molecules" Cancers 15, no. 1: 304. https://doi.org/10.3390/cancers15010304
APA StyleSpille, D. C., Bunk, E. C., Thomas, C., Özdemir, Z., Wagner, A., Akkurt, B. H., Mannil, M., Paulus, W., Grauer, O. M., Stummer, W., Senner, V., & Brokinkel, B. (2023). Protoporphyrin IX (PpIX) Fluorescence during Meningioma Surgery: Correlations with Histological Findings and Expression of Heme Pathway Molecules. Cancers, 15(1), 304. https://doi.org/10.3390/cancers15010304






