Evaluating Cardiotoxicity in Breast Cancer Patients Treated with HER2 Inhibitors: Could a Combination of Radionuclide Ventriculography and Cardiac Biomarkers Predict the Cardiac Impact?
Abstract
Simple Summary
Abstract
1. Introduction
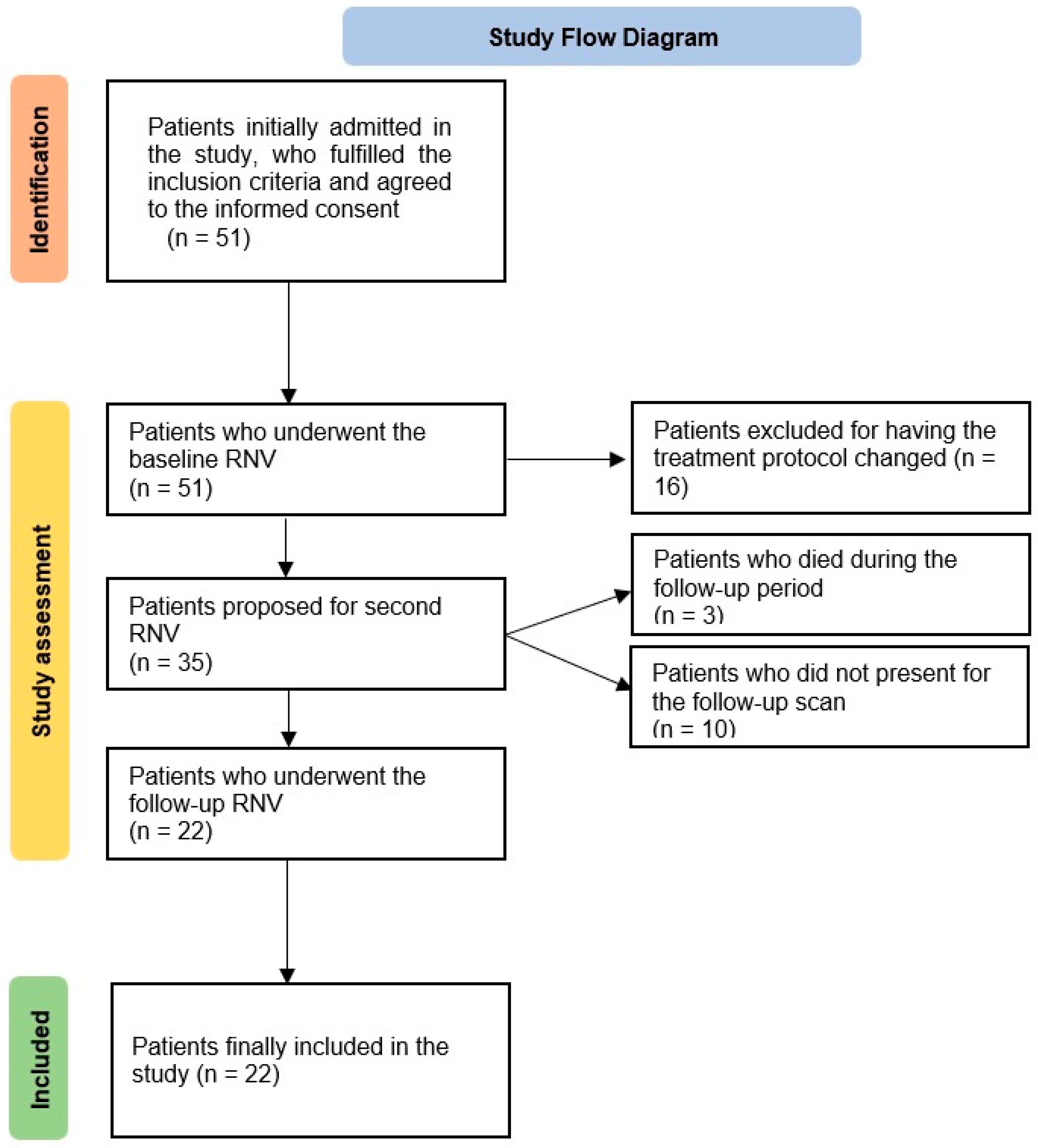
2. Materials and Methods
2.1. Patient Selection
2.2. Erythrocyte Labeling
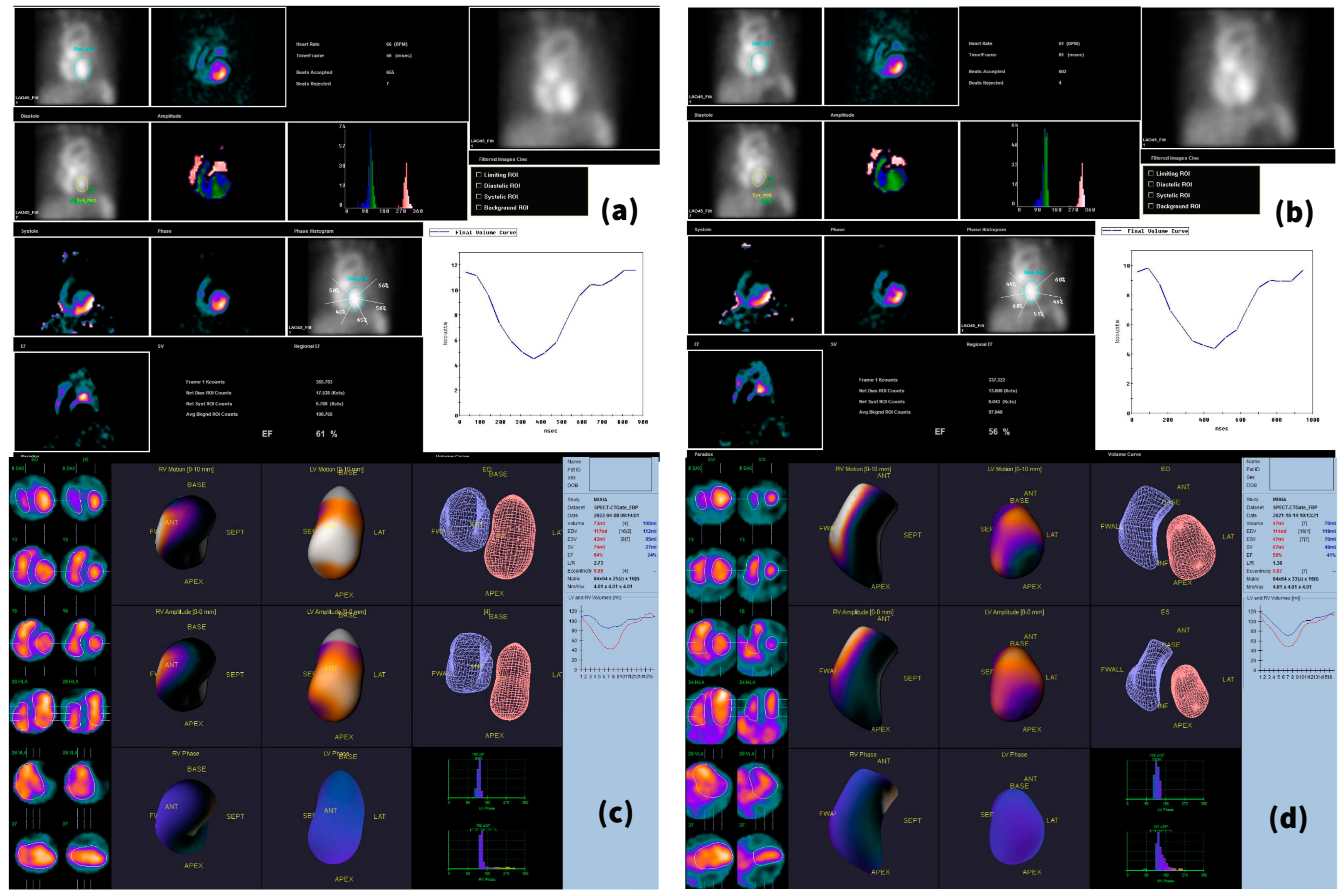
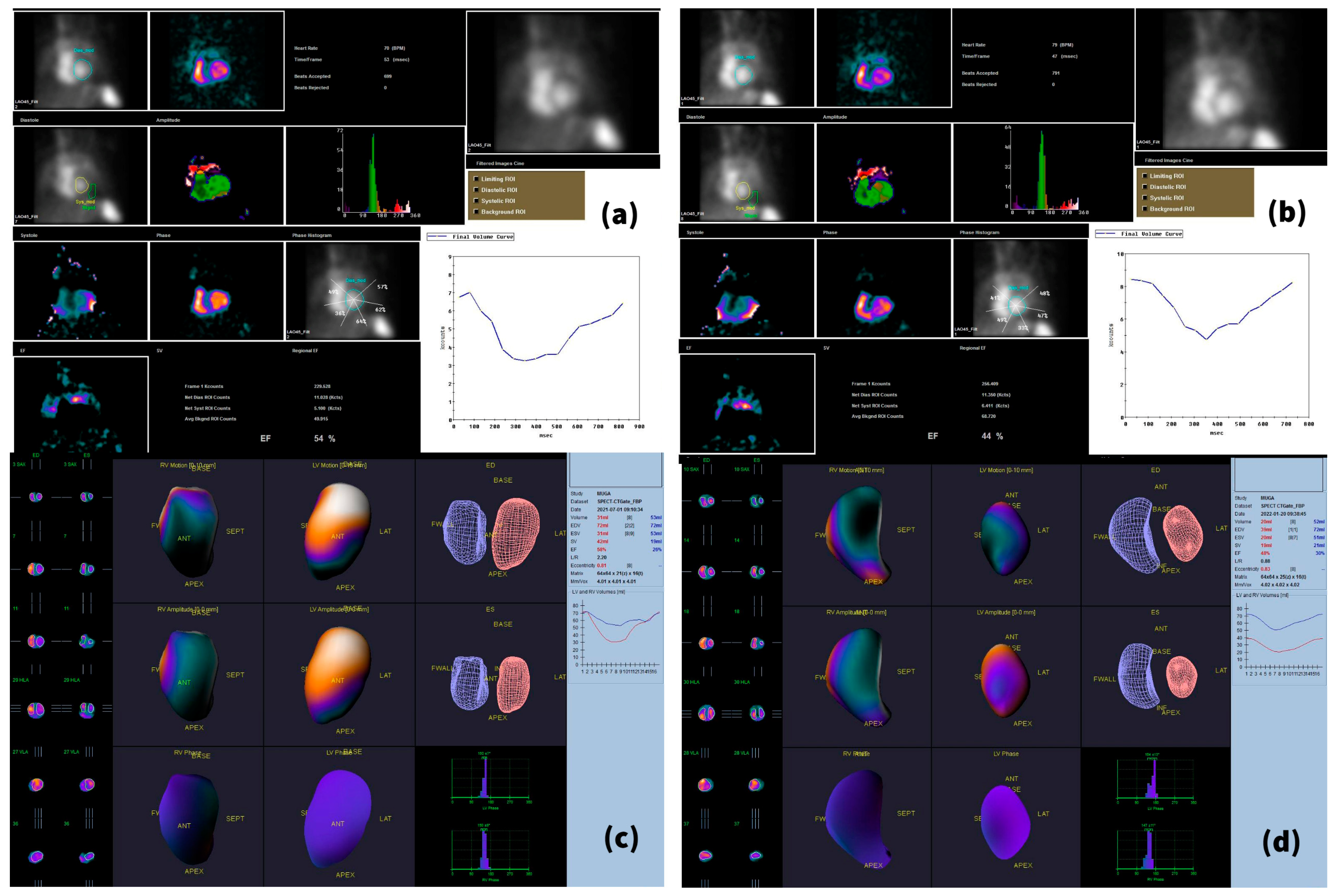
2.3. Imaging Acquisition Technique and Reconstruction
2.4. Biomarker Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ishii, K.; Morii, N.; Yamashiro, H. Pertuzumab in the Treatment of HER2-Positive Breast Cancer: An Evidence-Based Review of Its Safety, Efficacy, and Place in Therapy. Core Evid. 2019, 14, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Gherghe, M.; Mutuleanu, M.-D.; Stanciu, A.E.; Irimescu, I.; Lazar, A.; Bacinschi, X.; Anghel, R.M. Quantitative Analysis of SPECT-CT Data in Metastatic Breast Cancer Patients—The Clinical Significance. Cancers 2022, 14, 273. [Google Scholar] [CrossRef]
- Ben-Dror, J.; Shalamov, M.; Sonnenblick, A. The History of Early Breast Cancer Treatment. Genes 2022, 13, 960. [Google Scholar] [CrossRef] [PubMed]
- Gherghe, M.; Bordea, C.; Blidaru, A. Clinical Significance of the Lymphoscintigraphy in the Evaluation of Non-Axillary Sentinel Lymph Node Localization in Breast Cancer. Chirurgia 2015, 110, 26–32. [Google Scholar] [PubMed]
- Bouwer, N.I.; Jager, A.; Liesting, C.; Kofflard, M.J.M.; Brugts, J.J.; Kitzen, J.J.E.M.; Boersma, E.; Levin, M.-D. Cardiac Monitoring in HER2-Positive Patients on Trastuzumab Treatment: A Review and Implications for Clinical Practice. Breast 2020, 52, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Alhussein, M.M.; Mokbel, A.; Cosman, T.; Aghel, N.; Yang, E.H.; Mukherjee, S.D.; Dent, S.; Ellis, P.M.; Dhesy-Thind, S.; Leong, D.P. Pertuzumab Cardiotoxicity in Patients With HER2-Positive Cancer: A Systematic Review and Meta-Analysis. CJC Open 2021, 3, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Jerusalem, G.; Lancellotti, P.; Kim, S.-B. HER2+ Breast Cancer Treatment and Cardiotoxicity: Monitoring and Management. Breast Cancer Res. Treat. 2019, 177, 237–250. [Google Scholar] [CrossRef]
- Agunbiade, T.A.; Zaghlol, R.Y.; Barac, A. Heart Failure in Relation to Tumor-Targeted Therapies and Immunotherapies. Methodist Debakey Cardiovasc. J. 2019, 15, 250–257. [Google Scholar] [CrossRef]
- Mohan, N.; Jiang, J.; Dokmanovic, M.; Wu, W.J. Trastuzumab-Mediated Cardiotoxicity: Current Understanding, Challenges, and Frontiers. Antib. Ther. 2018, 1, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Y.; Drill, E.; Dang, C.T.; Liu, J.E.; Steingart, R.M.; Yu, A.F. Cardiac Outcomes of Trastuzumab Therapy in Patients with HER2-Positive Breast Cancer and Reduced Left Ventricular Ejection Fraction. Breast Cancer Res. Treat. 2019, 175, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, B.T.; Varga, Z.V.; Wu, W.J.; Pacher, P. Trastuzumab Cardiotoxicity: From Clinical Trials to Experimental Studies. Br. J. Pharmacol. 2017, 174, 3727–3748. [Google Scholar] [CrossRef] [PubMed]
- Tan-Chiu, E.; Yothers, G.; Romond, E.; Geyer, C.E.; Ewer, M.; Keefe, D.; Shannon, R.P.; Swain, S.M.; Brown, A.; Fehrenbacher, L.; et al. Assessment of Cardiac Dysfunction in a Randomized Trial Comparing Doxorubicin and Cyclophosphamide Followed by Paclitaxel, With or Without Trastuzumab As Adjuvant Therapy in Node-Positive, Human Epidermal Growth Factor Receptor 2–Overexpressing Breast Cancer: NSABP B-31. JCO 2005, 23, 7811–7819. [Google Scholar] [CrossRef]
- Dang, C.T.; Yu, A.F.; Jones, L.W.; Liu, J.; Steingart, R.M.; Argolo, D.F.; Norton, L.; Hudis, C.A. Cardiac Surveillance Guidelines for Trastuzumab-Containing Therapy in Early-Stage Breast Cancer: Getting to the Heart of the Matter. JCO 2016, 34, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.; Prout, M.; Geiger, A.M.; Kamineni, A.; Thwin, S.S.; Avila, C.; Silliman, R.A.; Quinn, V.; Yood, M.U. Comorbidities and Cardiovascular Disease Risk in Older Breast Cancer Survivors. Am. J. Manag. Care 2014, 20, 86–92. [Google Scholar]
- Henry, M.L.; Niu, J.; Zhang, N.; Giordano, S.H.; Chavez-MacGregor, M. Cardiotoxicity and Cardiac Monitoring among Chemotherapy-Treated Breast Cancer Patients. JACC Cardiovasc. Imaging 2018, 11, 1084–1093. [Google Scholar] [CrossRef]
- Nowsheen, S.; Aziz, K.; Park, J.Y.; Lerman, A.; Villarraga, H.R.; Ruddy, K.J.; Herrmann, J. Trastuzumab in Female Breast Cancer Patients With Reduced Left Ventricular Ejection Fraction. J. Am. Heart Assoc. 2018, 7, e008637. [Google Scholar] [CrossRef]
- Florescu, M.; Cinteza, M.; Vinereanu, D. Chemotherapy-Induced Cardiotoxicity. Maedica 2013, 8, 59–67. [Google Scholar]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on Cancer Treatments and Cardiovascular Toxicity Developed under the Auspices of the ESC Committee for Practice Guidelines: The Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Ribeiro, M.L.; Jorge, A.J.L.; Nacif, M.S.; Martins, W.d.A. Early Detection and Monitoring of Cancer Chemotherapy-Related Left Ventricular Dysfunction by Imaging Methods. Arq. Bras. Cardiol. 2019, 112, 309–316. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Sachpekidis, V.; Moralidis, E.; Arsos, G. Equilibrium Radionuclide Ventriculography: Still a Clinically Useful Method for the Assessment of Cardiac Function? Hell. J. Nucl. Med. 2018, 21, 213–220. [Google Scholar]
- Alexandre, J.; Cautela, J.; Ederhy, S.; Damaj, G.L.; Salem, J.; Barlesi, F.; Farnault, L.; Charbonnier, A.; Mirabel, M.; Champiat, S.; et al. Cardiovascular Toxicity Related to Cancer Treatment: A Pragmatic Approach to the American and European Cardio-Oncology Guidelines. J. Am. Heart Assoc. 2020, 9, e018403. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of Cardiac Disease in Cancer Patients throughout Oncological Treatment: ESMO Consensus Recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.; Kałużna-Oleksy, M.; Migaj, J.; Straburzyńska-Migaj, E. Clinical Value of Soluble ST2 in Cardiology. Adv. Clin. Exp. Med. 2020, 29, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Dogon, G.; Zeller, M.; Cottin, Y.; Vergely, C. GDF15 and Cardiac Cells: Current Concepts and New Insights. Int. J. Mol. Sci. 2021, 22, 8889. [Google Scholar] [CrossRef] [PubMed]
- Vianello, E.; Dozio, E.; Tacchini, L.; Frati, L.; Corsi Romanelli, M.M. ST2/IL-33 Signaling in Cardiac Fibrosis. Int. J. Biochem. Cell Biol. 2019, 116, 105619. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161. [Google Scholar] [CrossRef]
- Assadi, A.; Zahabi, A.; Hart, R.A. GDF15, an Update of the Physiological and Pathological Roles It Plays: A Review. Pflug. Arch. Eur. J. Physiol. 2020, 472, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.B.; Galt, J.R.; Georgoulias, P.; Malhotra, S.; Pagnanelli, R.; Rischpler, C.; Savir-Baruch, B. SNMMI Procedure Standard/EANM Guideline for Gated Equilibrium Radionuclide Angiography*. J. Nucl. Med. Technol. 2020, 48, 126–135. [Google Scholar] [CrossRef]
- Thygesen, K.; Mair, J.; Mueller, C.; Huber, K.; Weber, M.; Plebani, M.; Hasin, Y.; Biasucci, L.M.; Giannitsis, E.; Lindahl, B.; et al. Recommendations for the Use of Natriuretic Peptides in Acute Cardiac Care†: A Position Statement from the Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care. Eur. Heart J. 2012, 33, 2001–2006. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Sanctis, R.D.; Giordano, L.; D’Antonio, F.; Agostinetto, E.; Marinello, A.; Guiducci, D.; Masci, G.; Losurdo, A.; Zuradelli, M.; Torrisi, R.; et al. Clinical Predictors of Cardiac Toxicity in HER2-Positive Early Breast Cancer Patients Treated with Adjuvant s.c. versus i.v. Trastuzumab. Breast 2021, 57, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Roman, L.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Staroslawska, E.; de la Haba-Rodriguez, J.; Im, S.-A.; et al. Efficacy and Safety of Neoadjuvant Pertuzumab and Trastuzumab in Women with Locally Advanced, Inflammatory, or Early HER2-Positive Breast Cancer (NeoSphere): A Randomised Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2012, 13, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Cortés, J.; Kim, S.-B.; Im, S.-A.; Hegg, R.; Im, Y.-H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A.; et al. Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Baselga, J.; Kim, S.-B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef]
- Swain, S.M.; Ewer, M.S.; Viale, G.; Delaloge, S.; Ferrero, J.-M.; Verrill, M.; Colomer, R.; Vieira, C.; Werner, T.L.; Douthwaite, H.; et al. Pertuzumab, Trastuzumab, and Standard Anthracycline- and Taxane-Based Chemotherapy for the Neoadjuvant Treatment of Patients with HER2-Positive Localized Breast Cancer (BERENICE): A Phase II, Open-Label, Multicenter, Multinational Cardiac Safety Study. Ann. Oncol. 2018, 29, 646–653. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Hegg, R.; Tausch, C.; Seo, J.H.; Tsai, Y.-F.; Ratnayake, J.; et al. Pertuzumab plus Trastuzumab in Combination with Standard Neoadjuvant Anthracycline-Containing and Anthracycline-Free Chemotherapy Regimens in Patients with HER2-Positive Early Breast Cancer: A Randomized Phase II Cardiac Safety Study (TRYPHAENA). Ann. Oncol. 2013, 24, 2278–2284. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Waldron-Lynch, M.; Eng-Wong, J.; Kirk, S.; Cortés, J. Long-Term Efficacy Analysis of the Randomised, Phase II TRYPHAENA Cardiac Safety Study: Evaluating Pertuzumab and Trastuzumab plus Standard Neoadjuvant Anthracycline-Containing and Anthracycline-Free Chemotherapy Regimens in Patients with HER2-Positive Early Breast Cancer. Eur. J. Cancer 2018, 89, 27–35. [Google Scholar] [CrossRef]
- Goel, S.; Simes, R.J.; Beith, J.M. Exploratory Analysis of Cardiac Biomarkers in Women with Normal Cardiac Function Receiving Trastuzumab for Breast Cancer. Asia-Pac. J. Clin. Oncol. 2011, 7, 276–280. [Google Scholar] [CrossRef]
- Sawaya, H.; Sebag, I.A.; Plana, J.C.; Januzzi, J.L.; Ky, B.; Tan, T.C.; Cohen, V.; Banchs, J.; Carver, J.R.; Wiegers, S.E.; et al. Assessment of Echocardiography and Biomarkers for the Extended Prediction of Cardiotoxicity in Patients Treated With Anthracyclines, Taxanes, and Trastuzumab. Circ. Cardiovasc. Imaging 2012, 5, 596–603. [Google Scholar] [CrossRef]
- Kirkham, A.A.; Pituskin, E.; Thompson, R.B.; Mackey, J.R.; Koshman, S.L.; Jassal, D.; Pitz, M.; Haykowsky, M.J.; Pagano, J.J.; Chow, K.; et al. Cardiac and Cardiometabolic Phenotyping of Trastuzumab-Mediated Cardiotoxicity: A Secondary Analysis of the MANTICORE Trial. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 130–139. [Google Scholar] [CrossRef]
- May, B.M.; Pimentel, M.; Zimerman, L.I.; Rohde, L.E. GDF-15 as a Biomarker in Cardiovascular Disease. Arq. Bras. Cardiol. 2021, 116, 494–500. [Google Scholar] [CrossRef]
- Wischhusen, J.; Melero, I.; Fridman, W.H. Growth/Differentiation Factor-15 (GDF-15): From Biomarker to Novel Targetable Immune Checkpoint. Front. Immunol. 2020, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Windrichova, J.; Fuchsova, R.; Kucera, R.; Topolcan, O.; Fiala, O.; Finek, J.; Slipkova, D. MIC1/GDF15 as a Bone Metastatic Disease Biomarker. Anticancer Res. 2017, 37, 1501–1505. [Google Scholar]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Harnden, K.; Mauro, L.; Pennisi, A.; Armitage, M.; Soliman, H. Clinical Practices and Institutional Protocols on Prophylaxis, Monitoring, and Management of Selected Adverse Events Associated with Trastuzumab Deruxtecan. Oncologist 2022, 27, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Acibuca, A.; Sezer, A.; Yilmaz, M.; Sumbul, A.T.; Demircan, S.; Muderrisoglu, I.H.; Ozyilkan, O. Cardiotoxicity of Trastuzumab Emtansine (T-DM1): A Single-Center Experience. J. Int. Med. Res. 2021, 49, 3000605211053755. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Sousa, R.; Tarantino, P.; Tayob, N.; Dang, C.; Yardley, D.A.; Isakoff, S.J.; Valero, V.; Faggen, M.; Mulvey, T.; Bose, R.; et al. Cardiac Outcomes of Subjects on Adjuvant Trastuzumab Emtansine vs Paclitaxel in Combination with Trastuzumab for Stage I HER2-Positive Breast Cancer (ATEMPT) Study (TBCRC033): A Randomized Controlled Trial. NPJ Breast Cancer 2022, 8, 18. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristic | Variable | Value |
|---|---|---|
| Age (Mean ± Standard Deviation) | 55.55 ± 9.89 | |
| Tumor Grading (%) | G2 | 45.5% |
| G3 | 54.5% | |
| PR (%) | Positive | 59.1% |
| Negative | 40.9% | |
| ER (%) | Positive | 54.5% |
| Negative | 45.5% | |
| HER2 Status | 2+ | 40.9% |
| 3+ | 50.1% | |
| Cardiac Risk Factors (%) | Yes | 40.9% |
| No | 59.1% | |
| Metastatic Disease | Yes | 54.5% |
| No | 45.5% | |
| Previous Chemotherapy | Anthracyclines | 40.9% |
| Taxanes | 77.3% |
| Variable | T0 | T1 | p-Value |
|---|---|---|---|
| Planar LVEF (%) | 57.77 ± 6.21 | 53.36 ± 7.30 | 0.003 |
| BP-SPECT LVEF (%) | 57.95 ± 5.89 | 54.95 ± 6.89 | 0.035 |
| NT-proBNP (pmol/L) | 316.69 ± 217.18 | 689.14 ± 688.24 | 0.013 |
| NT-proANP (nmol/L) | 0.69 ± 1.01 | 0.88 ± 1.23 | 0.339 |
| cTnI (pg/mL) | 7.51 ± 15.28 | 13.17 ± 29.37 | 0.141 |
| ST2/IL-33R (ng/mL) | 24.20 ± 20.73 | 23.34 ± 26.74 | 0.882 |
| GDF-15 (ng/mL) | 1522.55 ± 1724.26 | 1236 ± 1132.94 | 0.513 |
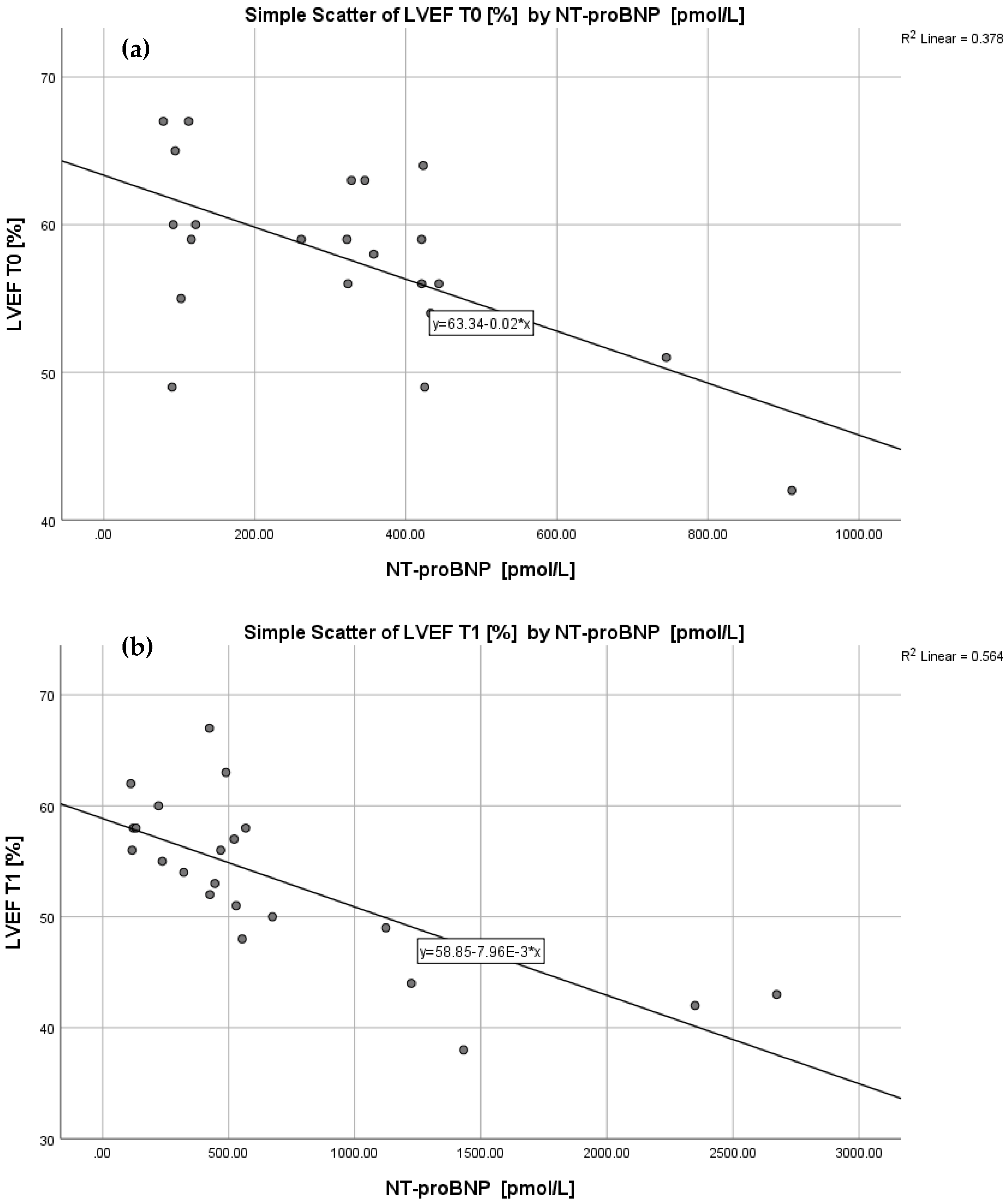
| Biomarker | r Planar LVEF T0 | p | r SPECT LVEF T0 | p | r Planar LVEF T1 | p | r SPECT LVEF T1 | p |
|---|---|---|---|---|---|---|---|---|
| NT-proBNP | −0.615 | 0.002 | −0.679 | 0.001 | −0.751 | 0.000 | −0.785 | 0.000 |
| NT-proANP | 0.280 | 0.207 | 0.175 | 0.436 | 0.417 | 0.053 | 0.258 | 0.246 |
| cTnI | 0.046 | 0.838 | 0.114 | 0.614 | −0.315 | 0.153 | −0.372 | 0.088 |
| ST2/IL-33R | 0.016 | 0.942 | −0.092 | 0.684 | −0.547 | 0.008 | −0.514 | 0.014 |
| GDF-15 | 0.251 | 0.260 | 0.220 | 0.325 | −0.191 | 0.396 | −0.224 | 0.316 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gherghe, M.; Lazar, A.M.; Mutuleanu, M.-D.; Bordea, C.I.; Ionescu, S.; Mihaila, R.I.; Petroiu, C.; Stanciu, A.E. Evaluating Cardiotoxicity in Breast Cancer Patients Treated with HER2 Inhibitors: Could a Combination of Radionuclide Ventriculography and Cardiac Biomarkers Predict the Cardiac Impact? Cancers 2023, 15, 207. https://doi.org/10.3390/cancers15010207
Gherghe M, Lazar AM, Mutuleanu M-D, Bordea CI, Ionescu S, Mihaila RI, Petroiu C, Stanciu AE. Evaluating Cardiotoxicity in Breast Cancer Patients Treated with HER2 Inhibitors: Could a Combination of Radionuclide Ventriculography and Cardiac Biomarkers Predict the Cardiac Impact? Cancers. 2023; 15(1):207. https://doi.org/10.3390/cancers15010207
Chicago/Turabian StyleGherghe, Mirela, Alexandra Maria Lazar, Mario-Demian Mutuleanu, Cristian Ioan Bordea, Sinziana Ionescu, Raluca Ioana Mihaila, Cristina Petroiu, and Adina Elena Stanciu. 2023. "Evaluating Cardiotoxicity in Breast Cancer Patients Treated with HER2 Inhibitors: Could a Combination of Radionuclide Ventriculography and Cardiac Biomarkers Predict the Cardiac Impact?" Cancers 15, no. 1: 207. https://doi.org/10.3390/cancers15010207
APA StyleGherghe, M., Lazar, A. M., Mutuleanu, M.-D., Bordea, C. I., Ionescu, S., Mihaila, R. I., Petroiu, C., & Stanciu, A. E. (2023). Evaluating Cardiotoxicity in Breast Cancer Patients Treated with HER2 Inhibitors: Could a Combination of Radionuclide Ventriculography and Cardiac Biomarkers Predict the Cardiac Impact? Cancers, 15(1), 207. https://doi.org/10.3390/cancers15010207







